Abstract
Fluid flow through the osteocyte canaliculi network is widely believed to be a main factor that controls bone adaptation. The difficulty of in vivo measurement of this flow within cortical bone makes computational models an appealing alternative to estimate it. We present in this paper a finite element dual porosity macroscopic model that can contribute to evaluate the interstitial fluid flow induced by mechanical loads in large pieces of bone. This computational model allows us to predict the macroscopic fluid flow at both vascular and canalicular porosities in a whole loaded bone. Our results confirm that the general trend in the fluid flow field predicted is similar to the one obtained with previous microscopic models, and that in a whole bone model it is able to estimate the zones with higher bone remodeling.











Similar content being viewed by others
References
Aiffantis E. C. (1977). Introducing a multi-porous media. Develop. Mech. 8:209–211
Aiffantis E. C. (1979). On the response of fissured rocks. Develop. Mech. 10:249–253
Anderson E. J., Kaliyamoorthy S., Alexander J. I. D., Knothe Tate M. L. (2005). Nano-microscale models of periosteocytic flow show differences in stresses imparted to cell body processes. Ann. Biomed. Eng. 33(1):52–62
Bacabac R. G., Smit T. H., Mullender M. G., Van Loon J. J. W. A., Klein-Nulend J. (2005). Initial stress-kick is required for fluid shear stress-induced rate dependent activation bone cells. Ann. Biomed. Eng. 33(1):104–110
Beno T., Yoon Y., Cowin S. C., Fritton S. P. (2006). Estimation of bone permeability using accurate microstructural measurements. J. Biomech. 39:2378–2387
Biot M. A. (1941). General theory of three-dimensional consolidation. J. Appl. Phys. 12:155–164
Burger E. H., Klein-Nulend J., Smit T. H. (2003). Strain-derived canalicular fluid flow regulates osteoclast activity in a remodelling osteon – a proposal. J. Biomech. 36:1453–1459
Cowin S. C. (1999). Bone poroelasticity. J. Biomech. 32:217–238
Cowin S. C. (2002). Mechanosensation and fluid transport in living bone. J. Muskuloske Neuron. Interact. 2(3):256–260
Duguid, J. O. Flow in fractured porous media. PhD Thesis. New Jersey: Princeton University, 1973.
Duguid, J. O., and J. Abel. Finite element Galerkin method for flow in fractured porous media. In: Finite Element Methods in Flow Problems, edited by Gallagher et al. Huntsville: UAH Press, 1974, pp. 559–615.
Duguid J. O., Lee P. C. Y. (1977). Flow in fractured porous media. Water Resour. Res. 13:558–566
Fritton S. P., McLeod K. J., Rubin C. T. (2000). Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J. Biomech. 33:317–325
Gururaja S., Kim H. J., Swan C. C., Brand R. A., Lakes R. S. (2005). Modeling deformation-induced fluid flow in cortical bone’s canalicular-lacunar system. Ann Biomed Eng 33(1):7–25
Khalili-Naghadesh N., Valliappan S. (1996). Unified theory of flow and deformation in double porous media. Eur. J. Mech. A/Solid 15:321–336
Knothe Tate M. L. (1989). Interstitial fluid flow. In: Cowin S. C. (ed) Bone Mechanics Handbook. CRC Press, Boca Raton
Knothe Tate M. L., Niederer P., Knothe U. (1998). In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone 22(2):107–117
Li G. P., Bronk J. T., An K. N., Kelly P. J. (1987). Permeability of cortical bone of canine tibiae. Microvasc. Res. 34:302–310
Mak A. F. T., Huang D. T., Zhang J. D., Tong P. (1997). Deformation-induced hierarchical flows and drag forces in bone canaliculi and matrix microporosity. J. Biomech. 30:11–18
Hibbit, Karlsson and Sorensen, Inc. Abaqus user’s Manual, v. 6.3. HKS inc. Pawtucket, RI, USA, 2002.
Nur A., Byerlee J. D. (1971). An exact effective stress law for elastic deformation of rocks with fluids. J. Geophys. Res. 76:6414–6419
Rubin C. T., Lanyon L. E. (1984). Regulation of bone formation by applied dynamic loads. J. Bone Join Sur. 66A:397–415
Smit T. H., Burger E. H., Huyghe J. M. (2002). A case for strain-induced fluid flow as regulator of BMU-coupling and osteonal alignment. J. Bone Miner. Res. 17(11):2021–2029
Srinivasan S., Agans S. C., King K. A., Moy N. Y., Poliachik S. L., Gross T. S. (2003). Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. Bone 33:946–955
Starkebaum W., Pollack S. R., Korostoff E. (1979). Microelectrode studies of stress-generated potential in four-point bending of bone. J. Biomed. Mater. Res. 13:729–751
Steck R., Niederer P., Knothe Tate M. L. (2003). A Finite Element analisis for the prediction of load-induced fluid flow and mechanochemical transduction in bone. J. Theor. Biol. 220:249–259
Swan C. C., Lakes R. S., Brand R. A., Stewart K. J. (2003). Micromechanically based poroelastic modeling of fluid flow in haversian bone. J. Biomech. Eng. 125:25–37
Valliappan S., Khalili-Naghadesh N. (1990). Flow through fissured porous media with deformable matrix. Int. J. Numerical Methods Eng. 29:1079–1094
You J., Yellowley C. E., Donahue H. J., Zhang Y., Chen Q., Jacobs C. R. (2000). Substrate deformation levels associated with routine physical activity are less stimulatory to bones cells relative to loading-induced oscillatory fluid flow. J Biomech Eng 122:387–393
Wang L., Fritton S. P., Cowin S. C., Weinbaum S. (1999). Fluid pressure relaxation depends upon osteon microstructure: modelling an oscillatory bending experiment. J. Biomech. 32:663–672
Wolff J. (1892). Das gesetz der transformation der knochen. Hirschwald, Berlin
Zhang D., Weinbaum S., Cowin S. C. (1998). On the calculation of bone pore water pressure due to mechanical loading. Int. J. Solids Structures 35:4981–4997
Zhang D., Weinbaum S., Cowin S. C. (1998). Estimates of the peak pressures in bone pore water. J. Biomech. Eng. 120:697–703
Zienkiewicz O. C., Shiomi T. (1984). Dynamic behaviour of porous media; the generalized Biot formulation and its numerical solution. Int. J. Num. Anal. Meth. Geomech. 8(1):71–96
Acknowledgments
First, the authors would like to thanks Dr. Sundar Srinivasan for his kindness supplying the rat tibia geometry. We would like also to thanks Dr. Stephen C. Cowin, Dr. Susannah P. Fritton and Dr. Liyun Wang for helping to develop this job with their comments. Last, we gratefully acknowledge the financial support of the Spanish Ministry of Science and Technology through the research project DPI2006-09692.
Author information
Authors and Affiliations
Corresponding author
Appendix A: Dual Porosity Model
Appendix A: Dual Porosity Model
For saturated porous media (like cortical bone tissue), the equilibrium equations can be written as
where F i is the body force per unit volume and τ ij is the total stress that can be expressed as
being σ ij the effective stress, p 1 and p 2 the pore fluid pressures in the two levels of porosity (here PV and PLC), α1 and α2 the effective stress coefficients and δ ij the Kronecker’s delta. For an elastic and isotropic bone matrix, the effective stress can be written as
with ɛ ij the global strain tensor and λ and G the so called Lame’s constants, that are expressed in terms of the Young’s modulus E and the Poisson’s ratio ν as λ = Eν/(1 + ν)(1 − 2ν) and G = E/2(1 + ν).
The governing equation for the solid phase is obtained combining Eqs. (4), (5) and (6) getting:
For small deformations, strains are related to displacements by the well-known Cauchy strain tensor
with \(\user2{u}\) the displacement vector. Substituting Eq. (8) into (7), the governing equation of the solid phase can we written as a function of displacements as
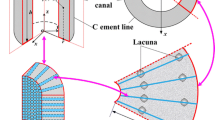
The definition of the effective stress parameters α1 and α2 can be determined in terms of physically measurable parameters. Following the procedure of Khalili and Valliappan,15 a representative planar volume of cortical bone is subjected to external principal stresses σ ii and to internal vascular pressure p 1 and lacuno-canalicular pressure p 2. These stresses can be decomposed in four components, as it is shown in Fig. 12: (I) equal PV, PLC and external hydrostatic pressure p 1; (II) zero PV pressure and equal PLC and external hydrostatic pressure (p 2 − p 1); (III) external hydrostatic pressure \((\overline{\sigma} -p_2)\), being \(\overline{\sigma} =\sigma_{ii}/3\); and (IV) external deviator stress \(\widetilde{\sigma_{ij}}=\sigma_{ij}-\overline{\sigma}\delta_{ij}\). The total volumetric strain of this representative bone volume may be calculated, according to Nur and Byerlee,21 as
being
where K s, K v and K are the drained bulk modulus of the bone matrix, the vascular porosity and the cortical bone, respectively. Introducing Eq. (11) into (10) and rearranging, the volumetric strain results
Applying now the definition of effective stress, it can also be written as
Comparing Eqs. (12) and (13) yields
Note that when the lacuno-canalicular porosity is reduced to zero (i.e. K v = K), the stress effective coefficients are those used in single porosity models (α1 = 1 − K/K s, α2 = 0).
The governing equations for fluid flow in PV and PLC can be obtained from the mass conservation equations for the fluid. Darcy’s law has been considered here as the constitutive equation, that is,
being k the permeability of the pore network and μ the viscosity of the fluid. Subindex α takes the value 1 for the PV and 2 for the PLC, and v αi represents the relative fluid velocity in each network with respect to the bone matrix, that can be expressed in terms of the porosity ϕα, the absolute velocity v αif and the velocity of the bone matrix v is as
Mass conservation of the fluid in the PV and PLC networks can be written as
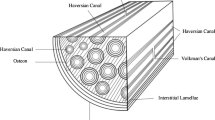
where Γ corresponds to the leakage term that represents the rate of flow between canaliculi and Haversian canals. Substitution of Eq. (16) into (17) yields
which, introducing the Lagrangian material derivative relative to the solid (d s()/dt = ∂()/∂t + v is∂()/∂x i ) can be rearranged as
According to the definition of compressibility one can write for the fluid
Substituting Eqs. (15) and (20) into (19) divided by ρf it results in
Equations (9) and (21) conform the governing equations system for cortical bone. However, in order to completely solve these equations another relationship is needed to establish the term d sϕα/dt. This relationship can be derived from the definition of porosities
where V is a representative volume of cortical bone, and V 1 and V 2 are the volumes of PV and PLC in that representative volume. Differentiation of Eq. (22) yields
The last term in Eq. (23) is actually related to the volumetric strain of the matrix
and, for the first terms in the right-hand side of Eq. (23), which correspond to the variation in fluid content of each porosity ζα, Khalili and Valliappan15 showed that they can be expressed as
Applying (23) in (21) and considering that, in general, v is∂()/∂x i ≪ ∂()/∂t and then d s()/dt ≈ ∂()/∂t, it results into
and recovering Eq. (9) the complete set of governing equations for the bone cortical tissue becomes
where γ is the so-called leakage parameter, which modulates the regulating role that bone lining cells perform on bone surfaces controlling the flux of fluid between compartments and the interstitial fluid pressure.16 The rest of the parameters are defined as
Rights and permissions
About this article
Cite this article
Fornells, P., García-Aznar, J. & Doblaré, M. A Finite Element Dual Porosity Approach to Model Deformation-Induced Fluid Flow in Cortical Bone. Ann Biomed Eng 35, 1687–1698 (2007). https://doi.org/10.1007/s10439-007-9351-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-007-9351-5