Abstract
Here, a PLLCL-on-chip platform was developed by direct electrospinning of poly (L-lactide-co-ε-caprolactone) (PLLCL) on polymethyl methacrylate (PMMA) microfluidic chips. Designed microchip provides the electrospinning of free-standing aligned PLLCL fibers which eliminates limitations of conventional electrospinning. Besides, aligned fiber structure favors cell alignment through contactless manipulation. Average fiber diameter, and fiber alignment was evaluated by SEM analyses, then, leakage profile of microchip was investigated. 3D cell culture studies were conducted using HeLa and NIH-3T3 cells, and nearly 85% cell viability was observed in PLLCL-on-chip for 15 days, while cell viability of 2D control started to decrease after 7 days based on Live dead and Alamar Blue analyses. These findings emphasize biocompatibility of PLLCL-on-chip platform for 3D cell culture and its ability to mimic extracellular matrix (ECM). Immunostaining results prove that PLLCL-on-chip platform favors the secretion of ECM proteins compared to control groups, and cytoskeletons of cells were in aligned orientation in PLLCL-on-chip, while they were in random orientation in control groups. Overall, these results demonstrate that the developed platform is suitable for the formation of various 3D cell culture models and a potential candidate for cell alignment studies.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, the incorporation of microfabrication methodologies with tissue engineering perspective, especially 3D cell culture models, has given rise to innovative platforms known as “organ-on-chip” or “tissue-on-chip” systems (Heintz et al. 2017; Christoffersson et al. 2017; Toudeshkchoui et al. 2019; Moon et al. 2023). These systems provide a unique opportunity to mimic the structural and functional characteristics of tissues or organs in laboratory conditions (Zhang et al. 2018; Seidi et al. 2022; Kammala et al. 2023). Also, they enable the investigation of disease modeling and progression (Skardal et al. 2016, 2020; Li et al. 2023b); development of drugs (Polini et al. 2014; Skardal et al. 2020; De Stefano et al. 2022), therapeutic and personalized medicine strategies (Yang et al. 2020; Subia et al. 2021) in a controlled and physiologically relevant microenvironment (Agarwal et al. 2017). One of the 3D cell culture formation techniques used in combination with microfluidic systems is electrospinning. Electrospinning is a tunable and controllable microfabrication technique that allows the production of micro/nanometer range fibers in an ECM-like architecture (Sun et al. 2007; Shie Karizmeh et al. 2022; Xu et al. 2023). ECM is a dynamic 3D fibrillar network of macromolecules where it provides structural support for cells embedded in this structure (Karamanos et al. 2021). Besides, physical, and biochemical signals can be conducted by ECM. In addition to the advantages of the electrospinning method in mimicking the 3D ECM structure, it also provides several advantages such as a high surface-to-volume ratio, interconnected pores, and the ability to tune mechanical and physical properties (Boudriot et al. 2006; Xu et al. 2022). Electrospun fibers on microfluidic systems enable the creation of 3D cell culture in an adjustable way with the aforementioned advantages and provide a dynamic culture while simulating flow microenvironment during 3D cell culture formation (Castiaux et al. 2019; Collins et al. 2021).
In literature, there are varied studies combining the electrospinning method with microfluidics (Park and Kim 2015; Park et al. 2018; Vasconcelos et al. 2022; Su et al. 2022). Incorporation of electrospun fibers with microfluidic chips can be done by collecting fibers on a metal surface before transferring them into a microfluidic chip and it results in the formation of randomly oriented fibers (Su et al. 2022). Detachment of electrospun material from the metal surface results in distortions in fiber structures, loss of structural integrity (Wallin et al. 2012; Park et al. 2018), and sometimes it might not be possible to fully remove the material from the surface (Su et al. 2022). These obstacles can be overcome by directly electrospinning fibers onto a microfluidic chip; however due to contact with a collector surface, fiber morphology changes unexpectedly. On the other hand, free-standing electrospun mats can also overcome these limitations while preventing direct contact of fibers with the surface (Su et al. 2022). Park et al. presented a new technique for creating patterned nanofiber mats on dielectric polymers without the need for a metal collector. Although this technique provides direct electrospinning of polymer onto a microfluidic chip, it is unable to form free-standing fibers (Park et al. 2018). In another study, Su et al. used a polyelectrolyte as a collector instead of metal. Even though electrospun fibers were fabricated with tunable properties, fibers were in contact with the surface and not produced in the free-standing form (Su et al. 2022). Hence, developing a new methodology that provides direct electrospinning of polymer onto a microfluidic chip that enables the fabrication of free-standing fibers combined with chip structure is important. Besides, fibers with this free-standing feature will facilitate nutrient and oxygen diffusion and increase the surface-to-volume ratio, providing more area for cells to adhere, thus increasing cell viability and proliferation.
In this study, a novel platform was developed by direct electrospinning of poly (L-Lactide-co-ε-caprolactone) (PLLCL) as free-standing fibers onto the microfluidic chip. Additionally, the developed microfluidic platform is advantageous in terms of its ability to allow fiber alignment. Aligned fibers guide cells, and affect their morphology and orientation (Zhong et al. 2006). Cell alignment is the oriented organization of cells (Zhu et al. 2005) and plays a crucial role especially in fibroblastic (Zhong et al. 2006), cardiac and neural tissues (Babaliari et al. 2021). The orientation of cells can be guided by tuning the fiber alignment and it facilitates the formation of highly organized 3D tissue structures (Yan et al. 2012).
Here, the PLLCL-on-chip platform was developed and introduced. First, a microfluidic chip was designed and fabricated. Then, PLLCL was electrospun and collected directly on a microfluidic chip as free-standing aligned fibers. Characterization of the PLLCL-on-chip platform was conducted by morphological analysis, leakage test, and protein adsorption assay. Following the characterization, 3D cell culture studies were done using NIH-3T3 and HeLa as model cell lines in this proof-of-concept study. Cell viability and proliferation profiles were evaluated by Live/Dead and Alamar Blue assays for 15 days. Finally, cellular, and extracellular components were analyzed by immunostaining on day 1, 7, and 15.
2 Experimental
2.1 Microfluidic chip fabrication
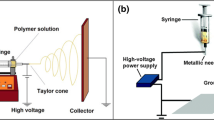
The microfluidic chip was designed using graphic design software (CorelDRAW X7) and was fabricated via laser ablation (Zing 16, Epilog) from Poly methyl methacrylate (PMMA) sheets (2 mm, Netpleksi, Turkey). The designed microfluidic chip has a reservoir where the electrospun Poly (L-Lactide-co-ε-caprolactone) (PLLCL) is directly collected on it as free-standing fibers (Fig. 1). Fabricated PMMA layers were bonded by double-sided adhesive (DSA) (Li et al. 2023a). Before electrospinning, microchips were placed on the collector.
2.2 Scaffold production and characterization
PLLCL scaffold was fabricated by electrospinning methodology (Inovenso NE300, Turkey). 10% PLLCL solution (Evonik Industries, Germany) was prepared as described elsewhere (Türker et al. 2019) where PLLCL was dissolved in Dichloromethane (DCM, Sigma Aldrich) and Dimethylformamide (DMF, Sigma Aldrich) ( 9:1 DCM: DMF ratio). 25 kV, 180 mm tip-to-collector distance, 20G nozzle, 3 mL/h flow rate, and 500 rpm rotating speed were used for electrospinning of PLLCL. Following the 8-hour electrospinning, PLLCL free-standing fibers were collected on top of the microfluidic chip reservoir by the guidance of aluminum film and then they were stored at 4 °C for further characterization and 3D cell culture studies.
Morphology of electrospun PLLCL was observed by scanning electron microscopy (SEM; FEI Quanta 250 FEG). Electrospun PLLCL was coated with a thin gold layer through argon gas (Emitech K550X). Fiber diameter, alignment angle, and % alignment were calculated using ImageJ Software (NIH) (Allan et al. 2021). Briefly, fiber diameters were measured using the “Measure” tool of ImageJ, and the number of fibers (count) versus diameter intervals was plotted using OriginPro software. Then, the average fiber diameter was calculated using the equation; “sum of fiber diameters/fiber counts”.
A leakage test was performed to control the bonding efficiency of the microfluidic chip with and without PLLCL. A microfluidic chip which has no electrospun PLLCL fibers was used as the control group. Red food dye (Ponceau 4r E124) was injected into the microfluidic system by a syringe pump at a flow rate of 100 µl/min. Leakage profiles were investigated and visualized following the 24-hour incubation.
Protein adsorption of PLLCL-on-chip was determined by Bicinchoninic acid (BCA) assay (Thermo Scientific) (Walker 2009) in the range of 0–2000 µg/mL BSA (Bovine Serum Albumin). Absorbance values were measured by UV/Vis spectrophotometer (Fisher Scientific™ accuSkan™ GO).
2.3 3D cell culture and characterization
HeLa (Human epithelial cervix adenocarcinoma, ATCC® CCL-2) and NIH-3T3 (Mouse fibroblast cell line, ATCC® CRL-1658) cell lines were cultured with standard cell culture techniques. Briefly, cells were cultured in high glucose DMEM (GIBCO, Thermo Fischer Scientific) supplemented with 10% Fetal Bovine Serum (GIBCO, Thermo Fischer Scientific) and 1% Penicillin/Streptomycin (GIBCO, Thermo Fischer Scientific). Cells were expanded and cultured until 80–90% confluency was reached. Then, they were harvested by trypsinization and used for further 3D cell culture studies.
A 3D model was fabricated by culturing 10 × 103 cells directly on PLLCL-on-chip where TCPS (Tissue culture polystyrene) and electrospun PLLCL were used as 2D and 3D controls, respectively. Cell viability was evaluated by Live/Dead (AAT Bioquest) and Alamar Blue (ChemCruz) assays for 15 days. Live/Dead assay was carried out using %0.1 Calcein and Propidium Iodide, while %1 Alamar Blue was used to evaluate cell viability. In addition, cell morphology on PLLCL-on-chip was visualized by SEM during long-term culture (45 days). For the characterization of 3D model, Phalloidin, DAPI, and Collagen Type-І staining was performed to observe F-actin, nuclei, and Collagen type-I, respectively. On day 1, 7, and 15, fixation of cells was done with 4% Paraformaldehyde, then cells were permeabilized by 0.1% Triton X-100 and blocked by 1% BSA. TRITC-conjugated Phalloidin (Sigma-Aldrich), DAPI (Sigma-Aldrich), and Anti-Collagen Type-I FITC (Sigma-Aldrich) staining were carried out and visualized using a fluorescence microscope (Zeiss Observer Z1).
2.4 Statistical analysis
Measurement of fiber diameter, alignment degree, protein adsorption, and cell viability analyses were done using 3 independent data sets and given as mean value ± standard deviation (SD). Cell viability analyses were also carried out using ANOVA by GraphPad Prism (GraphPad Prism, Inc., USA), and the significance level was accepted as p < .05 in all analyses.
3 Result and discussion
3.1 Fabrication and characterization of PLLCL-on-chip platform
Microfluidic chip fabrication was done by laser ablation methodology and PMMA layers were bonded by DSA (Fig. 1a). Layers 1 and 4 provide support and isolation for the microfluidic platform, while layer 2 has engraved areas beside the reservoir to enable the placing of aluminum film which allows the collection of free-standing fibers in aligned orientation. In addition, layer 3 provides extra volume for 3D cell culture studies (Figure S1). Prior to the electrospinning process, layers 1, and 2 were assembled, then the PLLCL scaffold was collected as free-standing aligned fibers on top of the reservoir part (Fig. 1b) with a cover area of 0.32 cm2. Later, layer 3 was bonded which increased the volume of the microchip and allowed diffusion of nutrients and oxygen in addition to the removal of metabolites in and out of the microenvironment for 3D cell culture (Fig. 1c).
Electrospinning of PLLCL was carried out and collected on top of the reservoir part of the PMMA microfluidic chip. Electrospun PLLCL fibers-on-chip was morphologically analyzed by SEM (Fig. 2a). Fibers aligned during electrospinning and their alignment was visible on SEM images while no bead formation was observed. Fiber diameters and alignment angles were measured using ImageJ and their distribution was given in Fig. 2b-c. The average fiber diameter was 1.58 ± 0.68 μm and results were consistent with literature, and there was no remarkable difference between electrospun PLLCL fibers on-chip and on metal collector in terms of fiber diameter (Türker et al. 2019). In addition to fiber diameter, alignment angles were measured using ImageJ, and their distribution was given in Fig. 2c. The alignment of fibers can be interpreted as alignment angle (Cooper et al. 2011a; Sankar et al. 2021), and % alignment (Harper et al. 2009; Cooper et al. 2011b). The alignment angle of fibers was calculated as 27.10° ± 1.31° (Fig. 2c), and the alignment percentage was 90.6%. These results prove that direct electrospinning on a microfluidic chip using the optimized parameters (25 kV voltage, 3mL/h flow rate, 500 rpm rotating speed, and 180 mm tip-to-collector distance) provided aligned fibers with a high percentage.
Dye solution was injected into the microchip with a 100 µl/min flow rate to control the bonding efficiency and leakage profiles of microchips. Microchip without PLLCL (control), and PLLCL-on-chip were observed for 24 h, and their images are given in Fig. 3a-b. During 24 h, no leakage was observed either in the control (Fig. 3a) or PLLCL-on-chip groups (Fig. 3b). Presence of electrospun PLLCL fibers did not prevent the bonding of PMMA layers. In addition to the leakage test, volume of the microchip was calculated by measuring the time between the entrance and leaving time points of the dye solution, and it was calculated as 300 µl.
Cell adhesion capability onto scaffolds can be followed by protein adsorption, therefore BCA assay was performed before cell culture studies. The amount of adsorbed protein on PLLCL-on-chip was calculated as 244.5 µg/mL at maximum (Fig. 3c), which is higher than the amount of adsorbed protein on PLLCL fibers collected on metal collector (3D control) (Türker et al. 2019). Due to the free-standing geometry of the electrospun fibers and increased reservoir area within the microchip, the total available volume for diffusion and adsorption was increased significantly.
3.2 3D cell culture studies
In tissue engineering and 3D cell culture studies, scaffolds are expected to favor cell adhesion, proliferation, and viability. Evaluation of cell adhesion and viability was done by long-term culture of HeLa cells on PLLCL-on-chip, where TCPS (Tissue culture polystyrene) and electrospun PLLCL were used as 2D and 3D controls, respectively (Fig. 4). Cell adhesion on PLLCL-on-chip had started on day 1, where rapid adaptation of cells to 3D microenvironment was achieved on day 3 (Fig. 4a). Following the adaptation period, higher cell viability and proliferation were observed in PLLCL-on-chip compared to 2D and 3D controls, besides, cells homogenously covered the scaffolds with high viability at the end of 15 days. Cells on 2D control had high viability until day 5 and starting from day 7 the viability had decreased due to overconfluency and contact inhibition. This phenomenon is well-known in literature; long-term culture in 2D is challenging and results in low cell viability (Bonnier et al. 2015; Duval et al. 2017), which supports these findings. The adaptation of cells in the 3D control group was longer than PLLCL-on-chip; cell adhesion started on day 3 and proliferated on day 5. Although high cell viability was observed in 3D control, the number of dead cells was higher on day 15. Overall, compared to 2D and 3D controls, on-chip PLLCL enables a long-term culture of cells with high cell adhesion and viability while shortening adaptation time (Fig. 4a).
Alamar blue assay which is a colorimetric assay that responds to cellular metabolic activity (O’Brien et al. 2000) was also performed to quantitatively analyze cell viability. Cell viability in the PLLCL-on-chip group increased through culturing time and reached nearly 85% on day 15, which is consistent with the live-dead assay results. However, cell viability in the control group increased until day 7 and then decreased up to day 15 (Fig. 4b). These results suggest that the PLLCL-on-chip platform promotes cell viability and potentially provides a more favorable microenvironment for cell growth compared to conventional methods. Also, electrospinning of free-standing fiber onto the microchip platform increased the surface-to-volume ratio of the scaffold and overcame the diffusion limitations while promoting adhesion and proliferation of cells.
3.3 Characterization of 3D cell culture
Adhesion of HeLa cells on scaffold and their morphology on the PLLCL-on-chip platform were observed by SEM. Cell proliferation was increasing through 45 days; where cells spread and stretched over electrospun PLLCL fibers-on-chip (Fig. 5a-b). Since electrospun fibers mimic native ECM structure, cells easily spread and proliferate on fiber structure, and following the adaptation period, they have gained their natural morphology. Further, cells started to form 3D cellular clusters and on day 45, thick filopodia formation was observed between HeLa cells and PLLCL fibers (Fig. 5b), which indicates the formation of 3D cellular structures and their corresponding microenvironment (Szewczyk et al. 2019). Besides, increased cell number during long-term culture highlights that PLLCL-on-chip platform favors cell proliferation, which is correlated with cell viability results. Overall, these results demonstrated that developed PLLCL-on-chip platform provides a suitable microenvironment and has good biocompatibility for 3D cell culture studies.
Cells are found in aligned orientation in muscle tissues, such as smooth and cardiac muscles, hence the recapitulation of cell alignment is essential for mimicking these muscle tissues. Therefore, NIH-3T3 fibroblast cells are generally preferred model cell line for the formation of 3D muscle models (Bashur et al. 2006; Fallahi et al. 2020; McGarry et al. 2023). In this study, alignment of NIH-3T3 cells was also investigated in addition to HeLa cells. Here, cellular, and extracellular components of 3D cell culture were analyzed by immunostaining of F-actin and Collagen type-I. As shown in Fig. 6, the cytoskeletons of HeLa (Fig. 6a) and NIH-3T3 (Fig. 6b) cells were visualized through F-actin immunostaining and aligned orientations were observed in accordance with fiber alignment, while cytoskeletons of 2D and 3D control were in random orientation (Figure S2). At the end of 15 days, the fluorescence intensity of F-actin for HeLa cells in PLLCL-on-chip was 2.3- and 1.24-fold higher than 2D and 3D controls, respectively; while for NIH 3T3 cells, 1.19- and 1.1-fold higher F-actin fluorescence intensity was observed in PLLCL-on-chip than in 2D and 3D controls, respectively.
In addition to F-actin immunostaining, secretion of Collagen type-I was also investigated. Collagen type-I secretion was visualized on day 7 for HeLa cells in PLLCL-on-chip (Fig. 6a), while it was observed on day 1 for NIH-3T3 cells (Fig. 6b). Then, Collagen type-I secretion increased for both cell lines and reached a maximum on day 15. Collagen Type-I secretion was 5.84- and 1.99-fold higher for HeLa cells in PLLCL-on-chip compared to 2D and 3D controls on day 15, respectively, while for NIH-3T3 cells, 4.73- and 1.06-fold higher Collagen Type-I secretion was calculated in PLLCL-on-chip than 2D, and 3D controls, respectively (Figure S2). As expected the secretion of Collagen Type-I was considerably low since cell-cell and cell-ECM interaction was limited in 2D control, even after 15 days (Duval et al. 2017; Picollet-D’hahan et al. 2016). In 3D control and PLLCL-on-chip platform, electrospun fibers serve as an ECM analog, favoring cell-cell, and cell-ECM interactions, which induce the secretion of ECM proteins, such as Collagen (Rosso et al. 2004). These results demonstrate that free-standing electrospun PLLCL-on-chip leads to the fabrication of aligned fibers, which cannot be obtained easily by conventional electrospinning, thereby affecting the orientation of cells to be cultured on this platform. Hence it eliminates the disadvantages of contactless manipulation methods applied in the literature, such as cell damage, inhomogeneous alignment, complexity, and equipment requirement. As a result, this platform can be used to obtain aligned cells without cell damage in 3D structure, especially in muscle, neural, and cardiac tissue engineering (Li et al. 2014).
4 Conclusion
Integration of electrospinning methodology with microfluidic systems overcomes the problems and limitations of conventional techniques for the formation of 3D cell cultures. In this study, an on-chip platform suitable for 3D cell culture was developed; a PMMA microfluidic system was designed, and scaffolds were fabricated by direct electrospinning of free-standing PLLCL fibers which mimic native ECM structure owing to its fibrillar structure and aligned orientation. Electrospun fibers were characterized, where the alignment angle was calculated as 27.10° with a 90.6% alignment. The leakage profile of PLLCL-on-chip was investigated, and no leakage was observed for prolonged incubation time. Then, the protein adsorption capacity of this platform was evaluated and calculated as 244.5 µg/mL at maximum, which is higher than the 3D control. Following the characterization of the on-chip platform, 3D cell culture studies were conducted using HeLa and NIH-3T3 cells. High cell viability was achieved in PLLCL-on-chip through 15 days, while cell viability of control groups was lower. Cell morphology was characterized by SEM analysis, acquired images showed that cells covered the scaffold, and filopodia formation was observed between cells and fibers, which contributes to the formation of 3D structure. Cellular and extracellular component analysis was done by immunostaining, F-actin staining confirmed the aligned cytoskeleton that validated the aligned orientation of cells. The fluorescence intensity of F-actin in PLLCL-on-chip was higher than in control groups for both cell lines. Collagen secretion of cells cultured in PLLCL-on-chip increased through culture time and showed a higher secretion ratio compared to control groups of both cell lines. These findings highlight the biocompatibility and suitability of the PLLCL-on-chip platform for 3D cell culture, as well as its ability to mimic the ECM. Fabrication of aligned free-standing fibers favors the culture of aligned cells, which is important in 3D cell culture studies of some tissues; such as muscle, neural, and cardiac tissues. In conclusion, the developed PLLCL-on-chip platform offers a unique combination of electrospinning methodology and microfluidic system, which provides aligned fiber structure thereby cell alignment, and is a promising tool for 3D cell culture research.
Data availability
Data is available on request from the authors.
References
Agarwal P, Wang H, Sun M et al (2017) Microfluidics enabled Bottom-Up Engineering of 3D vascularized Tumor for Drug Discovery. ACS Nano 11:6691–6702. https://doi.org/10.1021/acsnano.7b00824
Allan SJ, Ellis MJ, De Bank PA (2021) Decellularized grass as a sustainable scaffold for skeletal muscle tissue engineering. J Biomed Mater Res - Part A 109:2471–2482. https://doi.org/10.1002/jbm.a.37241
Babaliari E, Kavatzikidou P, Mitraki A et al (2021) Combined effect of shear stress and laser-patterned topography on Schwann cell outgrowth: synergistic or antagonistic? Biomater Sci 9:1334–1344. https://doi.org/10.1039/d0bm01218a
Bashur CA, Dahlgren LA, Goldstein AS (2006) Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(d,l-lactic-co-glycolic acid) meshes. Biomaterials 27:5681–5688. https://doi.org/10.1016/j.biomaterials.2006.07.005
Bonnier F, Keating ME, Wróbel TP et al (2015) Cell viability assessment using the Alamar blue assay: a comparison of 2D and 3D cell culture models. Toxicol Vitr 29:124–131. https://doi.org/10.1016/j.tiv.2014.09.014
Boudriot U, Dersch R, Greiner A, Wendorff JH (2006) Electrospinning approaches toward scaffold engineering - A brief overview. Artif Organs 30:785–792. https://doi.org/10.1111/j.1525-1594.2006.00301.x
Castiaux AD, Spence DM, Martin RS (2019) Review of 3D cell culture with analysis in microfluidic systems. Anal Methods 11:4220–4232. https://doi.org/10.1039/c9ay01328h
Christoffersson J, Van Noort D, Mandenius CF (2017) Developing organ-on-a-chip concepts using bio-mechatronic design methodology. Biofabrication 9:025023. https://doi.org/10.1088/1758-5090/aa71ca
Collins T, Pyne E, Christensen M et al (2021) Spheroid-on-chip microfluidic technology for the evaluation of the impact of continuous flow on metastatic potential in cancer models in vitro. Biomicrofluidics 15. https://doi.org/10.1063/5.0061373
Cooper A, Bhattarai N, Zhang M (2011a) Fabrication and cellular compatibility of aligned chitosan-PCL fibers for nerve tissue regeneration. Carbohydr Polym 85:149–156. https://doi.org/10.1016/j.carbpol.2011.02.008
Cooper A, Bhattarai N, Zhang M (2011b) Fabrication and cellular compatibility of aligned chitosan-PCL fibers for nerve tissue regeneration. Carbohydr Polym 85:149–156. https://doi.org/10.1016/j.carbpol.2011.02.008
De Stefano P, Bianchi E, Dubini G (2022) The impact of microfluidics in high-throughput drug-screening applications. Biomicrofluidics 16:31501. https://doi.org/10.1063/5.0087294
Duval K, Grover H, Han LH et al (2017) Modeling physiological events in 2D vs. 3D cell culture. Physiology 32:266–277. https://doi.org/10.1152/physiol.00036.2016
Fallahi A, Yazdi IK, Serex L et al (2020) Customizable composite fibers for Engineering skeletal muscle models. ACS Biomater Sci Eng 6:1112–1123. https://doi.org/10.1021/acsbiomaterials.9b00992
Harper LT, Turner TA, Martin JRB, Warrior NA (2009) Fiber alignment in directed carbon fiber preforms - a feasibility study. J Compos Mater 43:57–74. https://doi.org/10.1177/0021998308098151
Heintz KA, Mayerich D, Slater JH (2017) Image-guided, laser-based fabrication of vascular-derived microfluidic networks. J Vis Exp 2017:55101. https://doi.org/10.3791/55101
Kammala AK, Richardson LS, Radnaa E et al (2023) Microfluidic technology and simulation models in studying pharmacokinetics during pregnancy. Front Pharmacol 14:1–15. https://doi.org/10.3389/fphar.2023.1241815
Karamanos NK, Theocharis AD, Piperigkou Z et al (2021) A guide to the composition and functions of the extracellular matrix. FEBS J 288:6850–6912. https://doi.org/10.1111/febs.15776
Li Y, Huang G, Zhang X et al (2014) Engineering cell alignment in vitro. Biotechnol Adv 32:347–365. https://doi.org/10.1016/j.biotechadv.2013.11.007
Li Y, Wang X, Wang Y, Fan Y (2023a) Low-cost hybrid bonding between thermoplastics and PDMS with differential adhesive tape for microfluidic devices. J Mater Sci Mater Electron 34. https://doi.org/10.1007/s10854-023-09998-0
Li Z, Li Q, Zhou C et al (2023b) Organoid-on-a-chip: current challenges, trends, and future scope toward medicine. Biomicrofluidics 17. https://doi.org/10.1063/5.0171350
McGarry K, Sefat E, Suh TC et al (2023) Comparison of NIH 3T3 Cellular Adhesion on Fibrous scaffolds constructed from natural and synthetic polymers. https://doi.org/10.3390/biomimetics8010099. Biomimetics 8:
Moon HR, Surianarayanan N, Singh T, Han B (2023) Microphysiological systems as reliable drug discovery and evaluation tools: evolution from innovation to maturity. Biomicrofluidics 17:61504. https://doi.org/10.1063/5.0179444
O’Brien J, Wilson I, Orton T, Pognan F (2000) Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267:5421–5426. https://doi.org/10.1046/j.1432-1327.2000.01606.x
Park SM, Kim DS (2015) Electrolyte-assisted electrospinning for a self-assembled, free-standing nanofiber membrane on a curved surface. Adv Mater 27:1682–1687. https://doi.org/10.1002/adma.201404741
Park SM, Eom S, Choi D et al (2018) Direct fabrication of spatially patterned or aligned electrospun nanofiber mats on dielectric polymer surfaces. Chem Eng J 335:712–719. https://doi.org/10.1016/j.cej.2017.11.018
Picollet-D’hahan N, Dolega ME, Liguori L et al (2016) A 3D toolbox to enhance physiological relevance of human tissue models. Trends Biotechnol 34:757–769. https://doi.org/10.1016/j.tibtech.2016.06.012
Polini A, Prodanov L, Bhise NS et al (2014) Organs-on-a-chip: a new tool for drug discovery. Expert Opin Drug Discov 9:335–352. https://doi.org/10.1517/17460441.2014.886562
Rosso F, Giordano A, Barbarisi M, Barbarisi A (2004) From Cell-ECM interactions to tissue Engineering. J Cell Physiol 199:174–180. https://doi.org/10.1002/jcp.10471
Sankar D, Mony U, Jayakumar R (2021) Combinatorial effect of plasma treatment, fiber alignment and fiber scale of poly (ε-caprolactone)/collagen multiscale fibers in inducing tenogenesis in non-tenogenic media. Mater Sci Eng C. https://doi.org/10.1016/j.msec.2021.112206. 127:
Seidi S, Eftekhari A, Khusro A et al (2022) Simulation and modeling of physiological processes of vital organs in organ-on-a-chip biosystem. J King Saud Univ - Sci 34:101710. https://doi.org/10.1016/j.jksus.2021.101710
Shie Karizmeh M, Poursamar SA, Kefayat A et al (2022) An in vitro and in vivo study of PCL/chitosan electrospun mat on polyurethane/propolis foam as a bilayer wound dressing. Biomater Adv 135:112667. https://doi.org/10.1016/j.msec.2022.112667
Skardal A, Shupe T, Atala A (2016) Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov Today 21:1399–1411. https://doi.org/10.1016/j.drudis.2016.07.003
Skardal A, Aleman J, Forsythe S et al (2020) Drug compound screening in single and integrated multi-organoid body-on-a-chip systems. Biofabrication 12:025017. https://doi.org/10.1088/1758-5090/ab6d36
Su W, Zhang M, Wei W et al (2022) Microfluidics-assisted electrospinning of aligned nanofibers for modeling intestine barriers. PeerJ 10:e13513. https://doi.org/10.7717/peerj.13513
Subia B, Dahiya UR, Mishra S et al (2021) Breast tumor-on-chip models: from disease modeling to personalized drug screening. J Control Release 331:103–120. https://doi.org/10.1016/j.jconrel.2020.12.057
Sun T, Norton D, McKean RJ et al (2007) Development of a 3D cell culture system for investigating cell interactions with electrospun fibers. Biotechnol Bioeng 97:1318–1328. https://doi.org/10.1002/bit.21309
Szewczyk PK, Metwally S, Karbowniczek JE et al (2019) Surface-potential-controlled cell proliferation and Collagen Mineralization on Electrospun Polyvinylidene Fluoride (PVDF) Fiber scaffolds for bone regeneration. ACS Biomater Sci Eng 5:582–593. https://doi.org/10.1021/acsbiomaterials.8b01108
Toudeshkchoui MG, Rabiee N, Rabiee M et al (2019) Microfluidic devices with gold thin film channels for chemical and biomedical applications: a review. Biomed Microdevices 21. https://doi.org/10.1007/s10544-019-0439-0
Türker E, Yildiz ÜH, Arslan Yildiz A (2019) Biomimetic hybrid scaffold consisting of co-electrospun collagen and PLLCL for 3D cell culture. Int J Biol Macromol 139:1054–1062. https://doi.org/10.1016/j.ijbiomac.2019.08.082
Vasconcelos F, Lima AC, Bonani W et al (2022) Microfluidic-assisted electrospinning, an alternative to coaxial, as a controlled dual drug release system to treat inflammatory arthritic diseases. Biomater Adv 134. https://doi.org/10.1016/j.msec.2021.112585
Walker JM (2009) The Bicinchoninic Acid (BCA) assay for protein quantitation. 11–15. https://doi.org/10.1007/978-1-59745-198-7_3
Wallin P, Zandén C, Carlberg B et al (2012) A method to integrate patterned electrospun fibers with microfluidic systems to generate complex microenvironments for cell culture applications. Biomicrofluidics 6. https://doi.org/10.1063/1.4729747
Xu H, Zhang F, Wang M et al (2022) Electrospun hierarchical structural films for effective wound healing. Biomater Adv 136:212795. https://doi.org/10.1016/j.bioadv.2022.212795
Xu C, Bonfante G, Park J et al (2023) Fabrication of an electrospun polycaprolactone substrate for colorimetric bioassays. Biomed Microdevices 25. https://doi.org/10.1007/s10544-023-00673-z
Yan J, Qiang L, Gao Y et al (2012) Effect of fiber alignment in electrospun scaffolds on keratocytes and corneal epithelial cells behavior. J Biomed Mater Res - Part A 100 A:527–535. https://doi.org/10.1002/jbm.a.33301
Yang Y, Liu S, Chen C et al (2020) Microfluidic-enabled self-organized tumor model for in vitro cytotoxicity assessment of doxorubicin. Biomed Microdevices 22:1–11. https://doi.org/10.1007/s10544-020-00523-2
Zhang B, Korolj A, Lai BFL, Radisic M (2018) Advances in organ-on-a-chip engineering. Nat Rev Mater 3:257–278. https://doi.org/10.1038/s41578-018-0034-7
Zhong S, Teo WE, Zhu X et al (2006) An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J Biomed Mater Res - Part A 79:456–463. https://doi.org/10.1002/jbm.a.30870
Zhu B, Lu Q, Yin J et al (2005) Alignment of osteoblast-like cells and cell-produced collagen matrix induced by nanogrooves. Tissue Eng 11:825–834. https://doi.org/10.1089/ten.2005.11.825
Acknowledgements
The authors acknowledge İzmir Institute of Technology Biotechnology and Bioengineering Research and Application Center and İzmir Institute of Technology Materials Research Center for the instrumental facilities provided to accomplish this study.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Özüm Yıldırım-Semerci: Investigation, formal analysis, and writing – original draft. Ahu Arslan-Yildiz: Conceptualization, supervision, and writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yildirim-Semerci, Ö., Arslan-Yildiz, A. Engineering free-standing electrospun PLLCL fibers on microfluidic platform for cell alignment. Microfluid Nanofluid 28, 42 (2024). https://doi.org/10.1007/s10404-024-02736-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-024-02736-w