Abstract
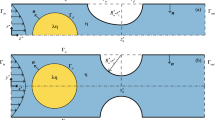
We investigate the flow resistance of a droplet trapped at a constriction in a microcavity located at a microchannel bifurcation as a function of system parameters including capillary number, drop confinement, and viscosity ratio. Using a combination of experiments and volume-of-fluid numerical simulations, we measure the hydrodynamic resistance of the trapped drop and connect it to drop deformation in the microcavity. For drop sizes smaller than the microcavity, we observe a bistable behavior in terms of the resistance of the trapped drop as a function of capillary number. For these underfilled drops, we find that the resistance is low at small capillary number (Ca < 10−3) and jumps to high resistance at a threshold capillary number. For drops equal to the microcavity size, we observe that the bistability vanishes and the drop resistance is of similar magnitude as that of underfilled drops at large capillary number. To explain these findings, we use confocal microscopy and simulations to obtain three-dimensional views of the drop deformation and continuous phase fluid in the microcavity. We observe that the low resistance is due to negligible drop deformation and unobstructed flow of continuous phase through the constriction. The high resistance is due to the drop interface protruding into the constriction restricting the flow of continuous phase through the gutters. Taken together, our results indicate that a trapped drop at a bifurcation can act as a nonlinear resistor and could be potentially used as a soft switch to control droplet trajectories in microfluidic devices.







Similar content being viewed by others
References
Abkarian M, Faivre M, Stone HA (2006) High-speed microfluidic differential manometer for cellular-scale hydrodynamics. Proc Natl Acad Sci USA 103:538–542. doi:10.1073/pnas.0507171102
Bithi SS, Vanapalli SA (2010) Behavior of a train of droplets in a fluidic network with hydrodynamic traps. Biomicrofluidics 4:044110. doi:10.1063/1.3523053
Bithi SS, Vanapalli SA (2015) Collective dynamics of non-coalescing and coalescing droplets in microfluidic parking networks. Soft Matter 11:5122–5132. doi:10.1039/c5sm01077b
Bithi SS, Vanapalli SA (2017) Microfluidic cell isolation technology for drug testing of single tumor cells and their clusters. Sci Rep 7:41707. doi:10.1038/srep41707
Bithi SS, Wang WS, Sun M, Blawzdziewicz J, Vanapalli SA (2014) Coalescing drops in microfluidic parking networks: A multifunctional platform for drop-based microfluidics. Biomicrofluidics 8:034118. doi:10.1063/1.4885079
Boukellal H, Selimovic S, Jia YW, Cristobal G, Fraden S (2009) Simple, robust storage of drops and fluids in a microfluidic device. Lab Chip 9:331–338. doi:10.1039/b808579j
Bruus H (2008) Theoretical microfludics. Oxford University Press, Oxford
Dangla R, Lee S, Baroud CN (2011) Trapping microfluidic drops in wells of surface energy. Phys Rev Lett 107:124501. doi:10.1103/PhysRevLett.107.124501
Du W, Li L, Nichols KP, Ismagilov RF (2009) SlipChip. Lab Chip 9:2286–2292. doi:10.1039/B908978K
Duffy DC, McDonald JC, Schueller OJ, Whitesides GM (1998) Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem 70:4974–4984. doi:10.1021/ac980656z
Edd JF, Humphry KJ, Irimia D, Weitz DA, Toner M (2009) Nucleation and solidification in static arrays of monodisperse drops. Lab Chip 9:1859–1865. doi:10.1039/B821785H
Fradet E, McDougall C, Abbyad P, Dangla R, McGloin D, Baroud CN (2011) Combining rails and anchors with laser forcing for selective manipulation within 2D droplet arrays. Lab Chip 11:4228–4234. doi:10.1039/C1LC20541B
Gerritsen MG, Durlofsky LJ (2005) Modeling fluid flow in oil reservoirs. Annu Rev Fluid Mech 37:211–238. doi:10.1146/annurev.fluid.37.061903.175748
Higgins JM, Eddington DT, Bhatia SN, Mahadevan L (2007) Sickle cell vasoocclusion and rescue in a microfluidic device. Proc Natl Acad Sci 104:20496–20500. doi:10.1073/pnas.0707122105
Hoang D, Portela L, Kleijn C, Kreutzer M, Van Steijn V (2013a) Dynamics of droplet breakup in a T-junction. J Fluid Mech 717:R4
Hoang DA, van Steijn V, Portela LM, Kreutzer MT, Kleijn CR (2013b) Benchmark numerical simulations of segmented two-phase flows in microchannels using the Volume of Fluid method. Comput Fluids 86:28–36
Huerre A, Theodoly O, Leshansky AM, Valignat M-P, Cantat I, Jullien M-C (2015) Droplets in microchannels: dynamical properties of the lubrication film. Phys Rev Lett 115:064501
Jeong H-H, Jin SH, Lee BJ, Kim T, Lee C-S (2015) Microfluidic static droplet array for analyzing microbial communication on a population gradient. Lab Chip 15:889–899. doi:10.1039/C4LC01097C
Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WEF, Goldbrunner R, Herms J, Winkler F (2010) Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 16:116–122 doi:http://www.nature.com/nm/journal/v16/n1/suppinfo/nm.2072_S1.html
Korczyk PM, Cybulski O, Makulska S, Garstecki P (2011) Effects of unsteadiness of the rates of flow on the dynamics of formation of droplets in microfluidic systems. Lab Chip 11:173–175. doi:10.1039/C0LC00088D
Korczyk PM, Derzsi L, Jakiela S, Garstecki P (2013) Microfluidic traps for hard-wired operations on droplets. Lab Chip 13:4096–4102. doi:10.1039/C3LC50347J
Lau BTC, Baitz CA, Dong XP, Hansen CL (2007) A Complete Microfluidic Screening Platform for Rational Protein Crystallization. J Am Chem Soc 129:454–455. doi:10.1021/ja065855b
Li Z, Mak SY, Sauret A, Shum HC (2014) Syringe-pump-induced fluctuation in all-aqueous microfluidic system implications for flow rate accuracy. Lab Chip 14:744–749. doi:10.1039/C3LC51176F
Ling Y, Fullana J-M, Popinet S, Josserand C (2016) Droplet migration in a Hele–Shaw cell: effect of the lubrication film on the droplet dynamics. Phys Fluids 28:062001. doi:10.1063/1.4952398
Nekouei M, Vanapalli SA (2017) Volume-of-fluid simulations in microfluidic T-junction devices: influence of viscosity ratio on droplet size. Phys Fluids 29:032007
Nieves-Remacha MJ, Yang L, Jensen KF (2015) OpenFOAM computational fluid dynamic simulations of two-phase flow and mass transfer in an advanced-flow reactor. Ind Eng Chem Res 54:6649–6659
Olbricht WL (1996) Pore-scale prototypes of multiphase flow in porous media. Annu Rev Fluid Mech 28:187–213. doi:10.1146/annurev.fl.28.010196.001155
OpenCFD O (2009) The Open Source CFD Toolbox User Guide. OpenCFD Ltd
Pompano RR, Liu WS, Du WB, Ismagilov RF (2011) Microfluidics using spatially defined arrays of droplets in one, two, and three dimensions. Annu Rev Anal Chem 4:59–81
Seemann R, Brinkmann M, Pfohl T, Herminghaus S (2011) Droplet based microfluidics. Rep Prog Phys 75:016601
Shemesh J et al (2014) Stationary nanoliter droplet array with a substrate of choice for single adherent/nonadherent cell incubation and analysis. Proc Natl Acad Sci 111:11293–11298. doi:10.1073/pnas.1404472111
Shi WW, Qin JH, Ye NN, Lin BC (2008) Droplet-based microfluidic system for individual Caenorhabditis elegans assay. Lab Chip 8:1432–1435. doi:10.1039/b808753a
Sun M, Bithi SS, Vanapalli SA (2011) Microfluidic static droplet arrays with tuneable gradients in material composition. Lab Chip 11:3949–3952. doi:10.1039/C1LC20709A
Vanapalli SA, Dvd Ende, Duits MHG, Mugele F (2007) Scaling of interface displacement in a microfluidic comparator. Appl Phys Lett 90:114109. doi:10.1063/1.2713800
Vanapalli SA, Banpurkar AG, van den Ende D, Duits MHG, Mugele F (2009) Hydrodynamic resistance of single confined moving drops in rectangular microchannels. Lab Chip 9:982–990. doi:10.1039/B815002H
Zagnoni M, Cooper JM (2010) A microdroplet-based shift register. Lab Chip 10:3069–3073. doi:10.1039/C0LC00219D
Zhang C, Xing D (2010) Single-molecule DNA amplification and analysis using microfluidics. Chem Rev 110:4910–4947
Zhu L, Gallaire F (2016) A pancake droplet translating in a Hele–Shaw cell: lubrication film and flow field. J Fluid Mech 798:955–969. doi:10.1017/jfm.2016.357
Acknowledgments
We gratefully acknowledge National Science Foundation (NSF) for supporting this work through a CAREER Grant No. 1150836. The SEM characterization was conducted using Hitachi S-4300 acquired through NSF Major Research Instrumentation Program Award #0421032.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bithi, S.S., Nekouei, M. & Vanapalli, S.A. Bistability in the hydrodynamic resistance of a drop trapped at a microcavity junction. Microfluid Nanofluid 21, 164 (2017). https://doi.org/10.1007/s10404-017-2006-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-017-2006-4