Abstract
Aim
To examine the association between having a higher number of involved specialties, patient activation (PA), and health literacy (HL) in patients visiting a general hospital.
Subject and methods
Patients ≥ 18 years of age who had an appointment with the medical specialist or physician assistant were asked to participate in this study. Patients completed the Patient Activation Measure-13 (PAM) questionnaire and the European Health Literacy Survey Questionnaire (HLS-EU-Q). They were stratified into having < 3 or ≥ 3 involved medical specialties in the past 12 months. Two association models were built to examine the association.
Results
This study included 200 patients with 52% males (n = 104), a median age of 65 years, and low levels of education (67%). Patients with ≥ 3 involved medical specialties (58%) had lower total PAM scores (p = 0.03) and had lower HLS-EU-Q index scores (p = 0.23). The multivariable regression analysis showed that having ≥ 3 involved medical specialties was not associated with low PAM scores (OR = 1.59, p = 0.13) when adjusted for low education, low HLS-EU-Q scores, and higher age (> 65 years). In addition, having ≥ 3 involved medical specialties was not associated with low HLS-EU-Q scores (OR = 1.10, p = 0.76) when adjusted for low PAM scores and low education.
Conclusion
Patients with ≥ 3 involved medical specialties visiting the internal medicine department of a general (non-academic) hospital had variable levels of PA and HL. Moreover, having ≥ 3 involved medical specialties was not significantly associated with lower PA and HL. Importantly, the number of involved specialties may not be a proxy for recognizing low PA and HL. Organizations aiming to improve PA and HL could measure these constructs directly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medical advancements have resulted in an increase in life expectancy, which has in turn led to a growing number of patients with multiple chronic conditions (MCC), commonly known as multimorbidity (MacMahon and The Academy of Medical Sciences 2018). In the Netherlands, approximately 31% of the population had MCC in 2018 (5.3 million patients), and this number is expected to increase to 34% in 2040 (6.6 million patients) (Rijksinstituut voor Volksgezondheid en Milieu 2022). This increasing number of patients with MCC is putting pressure on current healthcare systems (Hiremath and Hiremath 2012, National Center for Chronic Disease Prevention and Health Promotion 2023).
Healthcare for patients with MCC is complex due to the organization of care (Tinetti et al. 2012; Sinnott et al. 2013). Current healthcare systems are not designed to adequately meet the care needs for patients with MCC (Tinetti et al. 2012). Outpatient hospital care is focused on managing single diseases, and care for patients with MCC is provided by multiple medical specialties simultaneously, yet individually (Vogeli et al. 2007). The resulting care fragmentation lowers the quality of care and leads to poor health(care) outcomes (Frandsen et al. 2015). Examples of poor outcomes include a higher number of diagnostic tests (Kern et al. 2017) and a higher risk of emergency department visits (Nyweide and Bynum 2017). In addition, patients may experience overtreatment and medication interactions due to conflicting or overlapping treatment regimes (Kearney et al. 2017). Even with these complex treatment regimes, patients are expected to self-manage and coordinate their own healthcare.
Fundamental components for self-management are patient activation (PA) and health literacy (HL). These components empower patients to take an active role in managing their health, have instrumental medical knowledge and adopt healthy behaviors (Paasche-Orlow and Wolf 2007; Hibbard 2014). Patient activation captures the patient’s necessary knowledge, skills and confidence in managing their health(care) and the likelihood to put skills into action (Hibbard 2014). It is essentially about how empowered a patient feels to take action and make informed decisions regarding their well-being. Health literacy refers to the capacity to find, understand, assess and apply health information (Santana et al. 2021). In other words, it is about having the tools to navigate and make sense of all health-related information patients encounter. Patients with lower PA are two to three times more likely to have unmet medical needs and to experience delayed medical care (Hibbard and Cunningham 2008). In addition, these patients are less likely to know about clinical practice guidelines for their condition and to be persistent in asking questions if they do not understand what the doctor told them (Fowles et al. 2009). Moreover, less activated patients may miss the skills and confidence to elicit the coordination of care that they need (Hibbard and Greene 2013). Low HL is associated with more hospitalizations, more use of emergency care, and higher mortality rates (Berkman et al. 2011).
Both patients with low PA and HL are frequent users of healthcare and may experience care fragmentation (Berkman et al. 2011; Maeng et al. 2012; Hibbard and Greene 2013; Hibbard et al. 2013). As a consequence, this fragmentation may negatively impact patients with low PA and HL (Hibbard and Greene 2013). Most studies examining the relationship between care coordination experiences (i.e., care fragmentation), HL, and PA rely on self-reported measures of care coordination experiences (O’Malley and Cunningham 2009; Hawley et al. 2011; Altin and Stock 2015; Rijken et al. 2022). Overall, these studies suggest that patients with low HL and PA are more likely to report problems with care coordination and this might be even more prominent in patients that visit multiple specialties. However, no literature is available on the relationship between the number of involved specialties, a measure that is readily available in electronic health records (EHR), PA, and HL. Therefore, the aim of this study was to investigate and explore the association between a higher number of involved specialties in the past 12 months, PA, and HL. Our hypothesis was that having a higher number of involved specialties is associated with low PA and HL.
Materials & methods
Study design, setting, and study population
A prospective cross-sectional cohort study was conducted between 2021 and 2023 (during the COVID-19 pandemic) at the internal medicine outpatient clinic of a medium-sized non-academic teaching hospital in the Netherlands. Patients were included who met the following inclusion criteria:
-
(1)
Patients were 18 years of age or older on the day they were approached for participation.
-
(2)
Patients consulted the medical specialist or physician assistant (under supervision of the medical specialist) at the outpatient clinic of the internal medicine department at the hospital during the inclusion period.
Patients were excluded if they had no or limited ability to speak Dutch, no capacity to provide informed consent, or if they had severe hearing or visual problems, hindering communication. In addition, patients were excluded if they provided implausible answers on the questionnaire (e.g., strongly (dis)agreeing with every statement or question).
Study procedure
Patients who had a scheduled outpatient clinic appointment were contacted by phone and asked to participate. After informed consent was received, patients were offered four options for filling in the questionnaire: by phone, by mail, online or physically at the outpatient clinic. To increase the response rate, patients who opted to fill in the questionnaire online were automatically reminded to complete the questionnaire after eight days.
In addition to the questionnaire, basic demographic and care-related variables were extracted from the EHR. Specific variables related to care complexity included number of medications (to determine polypharmacy, which was defined as using ≥ 5 medications), number of involved medical specialties in the past 12 months (dichotomized in having < 3 or ≥ 3 involved specialties), main internal medicine diagnosis (defined below), and comorbidities according to the medical history. The main internal medicine diagnosis refers to the medical reason for the patient’s visit to the outpatient clinic and was based on the records of the visits. Information from the Dutch Hospital Data – Clinical Classifications System (DHD-CCS) were used to categorize the main internal medicine diagnosis and to identify conditions as “chronic.” (Dutch Hospital Data 2023). The DHD-CCS classifies diagnoses according to the International Classification of Diseases 10th Revision Procedure Coding System (ICD-10-PCS), which was developed by the Agency for Healthcare Research and Quality (AHRQ). We considered oncologic conditions as chronic conditions when counting the number of chronic conditions. All data were coded, pseudonymized, and recorded with the Electronic Data Capture (EDC) system Castor. (Castor EDC 2021).
Study measures
Patient activation was measured using the shortened Dutch version of the Patient Activation Measure-13 (PAM-13) (Hibbard et al. 2005). This questionnaire includes 13 items that assess self-reported knowledge, skill, and confidence for health self-management of their chronic condition(s), and items can be scored with a 4-point Likert scale (1 = strongly disagree, 2 = disagree, 3 = agree, 4 = strongly agree, and 5 = not applicable). Examples items include: “Taking an active role in my own healthcare is the most important thing that affects my health” and “I am confident that I can maintain lifestyle changes, like diet and exercise, even during times of stress.” The total PAM-13 scores were calculated according to the description of the cooperation of General Practitioners (Dokterscoop 2014). In line with this description, patients were excluded if they answered all questions with “strongly disagree” or “strongly agree.” Total scores were categorized into four levels, based on known cut-off values: level 1: scores ≤ 35, level 2: scores 36–38, level 3: scores 39–42 and level 4: scores ≥ 43 (Magnezi et al. 2014), and subsequently dichotomized in lower PAM (level 1–2) and higher PAM scores (level 3–4).
Health literacy was assessed with the European Health Literacy Survey Questionnaire (HLS-EU-Q) (Sørensen et al. 2013). This multidimensional tool was developed to measure and compare HL among different populations in European countries. The questionnaire consists of six questions which can be scored on a 4-point Likert scale (0 = very easy, 1 = easy, 2 = difficult and 3 = very difficult). Example items include: “How easy or hard is it for you to estimate whether you should request help from another doctor?” and “How easy or hard is it for you to understand information from the media about how to become healthy?”. Index scores were obtained by dividing the total score by the number of questions (i.e., obtaining the mean score), as described in literature (Rouquette et al. 2018). Subsequently, these scores were categorized into inadequate HL (index score ≤ 2), problematic HL (index score 2 ≤ 3), and adequate HL (index score > 3) and thereafter dichotomized in inadequate/problematic HL and adequate HL.
Statistical analysis
Means and standard deviations (SD) were used for normally distributed continuous variables, and medians and interquartile ranges (IQR) for skewed continuous variables. Frequencies and percentages were used for categorical variables. To assess differences among patients with < 3 and ≥ 3 involved medical specialties in the past 12 months (dichotomized), the chi-square test, Mann–Whitey test, or the independent sample t-test were performed when appropriate. The relationship between the number of involved specialties in the past 12 months and total PAM scores or total HLS-EU-Q scores were visualized with boxplots and answers to PAM statements were explored if deemed relevant in the context of care coordination.
The associations between having a higher number of involved specialties, a lower PAM or HLS-EU-Q score (the associations of interest) were further explored using binary logistic regression analysis. Based on literature (Fowles et al. 2009; Berkman et al. 2011; Deen et al. 2011; Smith et al. 2013; Huang et al. 2021; Newland et al. 2021), we adjusted for the following: low educational levels, older age (> 65 years), low PAM scores (level 1 and 2), and problematic or inadequate HL. Confounders were identified and included in the model in a stepwise manner if they resulted in a coefficient change of more than 10% for the independent variable (Lee 2014; Twisk 2017). Missing variables were imputed if they had more than 5% missing. We considered p-values ≤ 0.05 as the cut-off for statistical significance.
Results
Baseline characteristics
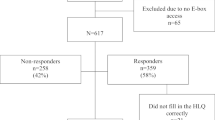
A total of 332 potential participating patients were approached, 200 patients were included and 132 patients could not be included. Of these 132 patients, 54 patients refused to participate, 77 patients did not respond or complete the questionnaire in time and one patient provided implausible answers to the PAM questionnaire (e.g., strongly agreeing with every statement).
Baseline characteristics are provided in Appendix A, Supplementary Table 1. The majority of patients were aged between 65 and 75 years (n = 79, 39.5%), male (n = 104, 52.0%), and had a partner (n = 157, 78.5%). Most patients had low education levels (n = 133, 66.5%) and were (pre)retired (n = 105, 52.5%). The most common disease group was “endocrine, nutritional, or metabolic disease” (n = 68, 34.0%), followed by malignancy (n = 52, 26.0%). The majority of patients (143, 71.5%) had MCC (≥ 2 chronic/oncologic conditions). The mean total PAM score (range 13–52) was 38.4 (SD = 4.8) with 99 patients having a low PAM score (49.5%). The median HLS-EU-Q index score (range 1–4) was 2.8 (IQR = 2.7–3.3, with 129 patients having problematic or inadequate HL (64.5%). For marital status, living situation, and working status 0.5% data was missing (n = 1), for HL scores 1.5% (n = 3).
The number of involved specialties, PA, and HL: descriptive statistics and exploration
Patients with ≥ 3 involved medical specialties in the past 12 months were significantly older (p = 0.01), more frequently not working (p = 0.04), had significantly more chronic/oncologic conditions (p = 0.01) and polypharmacy (p = 0.01) when compared to patients with 1–2 involved medical specialties (Table 1).
Furthermore, patients with ≥ 3 involved medical specialties had significantly lower mean total PAM scores (p = 0.03), and more often low dichotomized PAM scores (level 1 and 2, p = 0.04). The relationship between the number of involved specialties and total PAM scores is shown in Supplementary Fig. 1. The boxplots of 1 and 2 involved specialties show medians below and above the red dotted cut-off line for low PAM scores versus high PAM scores (dichotomized). In contrast, the boxplots of the number of specialties 3, 4, and ≥ 5 show medians on or below the red dotted cut-off line for low versus high PAM scores. Supplementary Fig. 2 shows the relationship between the number of involved specialties and total HLS-EU-Q scores. In this figure, the boxplots for all numbers of involved medical specialties show medians below or on the red dotted cut-off line for low problematic or inadequate HL versus adequate HL.
Table 2 shows no significant differences in answers to relevant PAM statements among patients with < 3 or ≥ 3 involved medical specialties. The group of patients with 1–2 involved specialties agreed more often with the following statement ((84.7% versus 74.8% in patients with ≥ 3 involved medical specialties, p = 0.11): “I know which treatments are available for my health problems” (Table 2).
The number of involved specialties, PA, and HL: multivariable regression analysis
In the crude model of the regression analysis, a significant association between a higher number of involved specialties in the past 12 months and a low PAM score (dichotomized) was found (Table 3, odds ratio (OR) = 1.79, 95% confidence interval (CI) = 1.02–3.16, p = 0.04). In the adjusted regression model, when adjusted for low education level, problematic or inadequate HL (dichotomized) and older age (> 65 years), the association was still present but no longer significant (Table 3, OR = 1.59, 95% CI = 0.87–2.90 and p = 0.13). In this adjusted model, the confounders low education level (OR = 2.01, 95% CI = 1.07–3.76 and p = 0.03) and problematic or inadequate HL (OR = 1.92, 95% CI = 1.0–3.55 and p = 0.04) were significantly associated with a low PAM score.
The association between a higher number of involved specialties in the past 12 months and problematic or inadequate HL was not significant in the crude model (Table 4, OR = 1.24, 95% CI = 0.69–2.25, p = 0.48). In the adjusted model, this association was still not significant (OR = 1.10, 95% CI = 0.60–2.03, p = 0.76) when adjusted for the significant confounders low PAM score (OR = 1.92, 95% CI = 1.04–3.54, p = 0.04) and low education level (OR = 1.24, 95% CI = 0.66–2.35, p = 0.51).
Discussion
The aim of this study was to examine the relationship between having a higher number of involved specialties, PA, and HL in patients visiting the outpatient clinic of the internal medicine department of a Dutch general hospital. We hypothesized that a higher number of involved specialties would be associated with low PAM and HLS-EU-Q scores. Using descriptive statistics, patients with ≥ 3 involved specialties showed significantly lower PAM scores and, although not significant, lower HLS-EU-Q scores. The significant association between a higher number of involved specialties and low PAM score appeared to be mediated by low HLS-EU-Q scores and low education level in the adjusted regression model and was consequently no longer significant in the adjusted regression model. Further, a high number of involved specialties was not associated with low HLS-EU-Q scores in the adjusted regression model. The identified association between low HLS-EU-Q scores or low education level and low PAM scores is in line with existing literature (Fowles et al. 2009; Smith et al. 2013).
Patients with low PA and HL more frequently have MCC and it is therefore assumable that these patients have a higher number of involved specialties (Pedersen et al. 2023; Paukkonen et al. 2022). However, our findings suggest that patients with a higher number of involved specialties are not a distinct subgroup with either low PA and HL. This is substantiated by the wide spread in all boxplots (1–5 number of involved specialties) in both Supplementary figures. We explored answers to PAM statements we deemed relevant for the research question and expected to find (significant) differences. However, even in this exploration, no significant differences were found. Thus, the hypothesis that patients with multiple involved specialties tend to have low PA or HL was not supported by our research.
Previous studies focused on identifying determinants for low PA and HL. One study identified low education as a determinant for low PA and HL among multimorbid patients with chronic pulmonary obstructive disease. Goran et al. (2018) showed that a high treatment burden, being divorced, having mobility problems, and having anxiety/depression problems were associated with low HL in patients with multimorbidity in primary care. We conclude that having a higher number of involved specialties may not be a suitable proxy for identifying patients with low PA and HL. Hence, it cannot be assumed that patients with more involved specialties have low PA and HL when setting up interventions aimed at improving self-management. Organizations setting up these interventions could use other determinants (such as low education level) for low PA and HL or measure these constructs directly in patients. Finding optimal ways to identify patients with low PA and HL is important since patients with low PA and HL specifically are most likely to benefit from these interventions (Hosseinzadeh et al. 2022).
Other literature focused on the relationship between self-reported care coordination and the number of involved specialties, and between self-reported care coordination, PA and HL. Rijken et al. (2022) conducted research in Dutch general practices (n = 1098) and concluded that patients with more involved medical specialties experienced higher levels of self-reported care coordination. In addition, literature showed that lower PA and HL was associated with less self-reported care coordination (O’Malley and Cunningham 2009; Maeng et al. 2012; Altin and Stock 2015; Rijken et al. 2022). Therefore, we propose that patients with low PA or HL are a distinct risk group in regard to experiencing low care coordination. When enhancing care coordination, PA and HL must be taken into account as separate concepts and the number of involved medical specialties is not a suitably proxy for these concepts.
This study had several strengths. To our knowledge, this was the first study to examine the relationship between the number of involved specialties, PA, and HL. Second, the sample was representative for the Dutch population at the internal medicine outpatient clinic and related research populations on this subject, as it included a wide range of age groups, education levels and working status (Rijken et al. 2022; van der Mooren and de Vries 2022).
There are some limitations that need to be considered. First, this study might be subject to selection bias since patients with lower PA and HL may have refused to participate in this study. To illustrate, patients with lower HL may lack confidence to participate in a questionnaire-based study because of lower skills to fill in questionnaires, even though we offered different options to fill out the questionnaire. In addition, inactivated patients may experience known barriers to participate in research (Sheridan et al. 2020). Therefore, the actual PA and HL scores might be lower than depicted in this study. Second, only patients from the internal medicine department were asked to participate. Therefore, the results of this study may not be generalizable to patients with other types of disease that do not visit the internal medicine department. Third, it is possible that other covariates than those included in this study could have influenced the examined association, such as ethnical background and support in care coordination that was already applied. However, the aim of this study was not to explain variance, but rather to use prospective collected data to test the association between having more involved specialties, low PA, and HL in a general population, without delving too deep into specific subgroups or covariates.
Conclusion
Patients visiting the internal medicine department of a Dutch general hospital had variable levels of PA and HL, and having ≥ 3 involved medical specialties was not significantly associated with lower PA and HL in the final multivariable regression analysis. The number of involved medical specialties may therefore not be a proxy for recognizing low PA and HL in patients.
Implications for public health & practice
This study’s results are important for organizations setting up interventions aimed at enhancing care coordination since the number of involved specialties is not a significant proxy for identifying patients with low PA and HL. Alternatively, these organizations could measure PA and HL directly or use other determinants. In addition, due to the cross-sectional design of this study, it was difficult to understand the direction of causality in the examined relationship between PA, HL, and the number of involved specialties. Future research should focus on identifying the underlying causal pathways by linking PA and HL with care coordination.
Data availability
Data might be available upon reasonable request.
Code availability
Not applicable.
References
Altin SV, Stock S (2015) Impact of health literacy, accessibility and coordination of care on patient’s satisfaction with primary care in Germany. BMC Fam Pract BMC Family Practice 16(1):1–7
Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K (2011) Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 155(2):97–107
Castor EDC (2021) Castor electronic data capture. https://www.castoredc.com. Accessed 18 Jun 2023
Deen D, Lu WH, Rothstein D, Santana L, Gold MR (2011) Asking questions: the effect of a brief intervention in community health centers on patient activation. Patient Educ Couns Elsevier Ireland Ltd 84(2):257–260
Dokterscoop (2014) PAM introductie Voor Zorgverlener. https://docplayer.nl/20189667-Introductie-voor-zorgverlener.html#google_vignette. Accessed 18 Jun 2023
Dutch Hospital Data (2023) CCS code adviezen. Accessed on 8 July 2023 at https://www.dhd.nl/assets/uploads/Codeadviezen-Expertgroep-ICD-10-01-10-2023.pdf
Fowles JB, Terry P, Xi M, Hibbard J, Bloom CT, Harvey L (2009) Measuring self-management of patients’ and employees’ health: further validation of the Patient Activation Measure (PAM) based on its relation to employee characteristics. Patient Educ Couns 77(1):116–122
Frandsen B, Joynt Maddox K, Rebitzer J, Jha A (2015) Care fragmentation, quality, and costs among chronically Ill patients. Am J Manag Care 21:355–362
Goran AAN, Pasquier J, Deruaz-luyet A, Burnand B, Haller DM, Neuner-jehle S et al (2018) Factors associated with health literacy in multimorbid patients in primary care: a cross-sectional study in Switzerland. 1–9. https://doi.org/10.1136/bmjopen-2017-018281
Hawley ST, Janz NK, Lillie SE, Friese CR, Jennifer J, Graff JJ, Hamilton AS, Jain S, Katz SJ (2011) Perceptions of care coordination in a population-based sample of diverse breast cancer patients. Patient Educ Couns (1):1–16
Hibbard J (2014) Supporting people to manage their health. An introduction to patient activation. https://assets.kingsfund.org.uk/f/256914/x/d5fbab2178/supporting_people_manage_their_health_2014.pdf. Accessed 18 Jun 2023
Hibbard JH, Cunningham PJ (2008) How engaged are consumers in their health and health care, and why does it matter? Res Brief 8:1–9
Hibbard JH, Greene J (2013) What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff 32(2):207–214
Hibbard JH, Greene J, Overton V (2013) Patients with lower activation associated with higher costs; delivery systems should know their patients’ “scores”. Health Aff 32(2):216–222
Hibbard JH, Mahoney ER, Stockard J, Tusler M (2005) Development and testing of a short form of the patient activation measure. Health Serv Res 40(6 I):1918–30
Hiremath L, Hiremath D (2012) Noncommunicable diseases. In: Essentials of community medicine: a practical approach. Jaypee Brothers Medical Publishers (P) Ltd, pp 76–76. https://doi.org/10.5005/jp/books/11660_5
Hosseinzadeh H, Downie S, Shnaigat M (2022) Effectiveness of health literacy- and patient activation-targeted interventions on chronic disease self-management outcomes in outpatient settings: a systematic review. Aust J Prim Health 28(2):83–96
Huang LY, Lin YP, Glass GF, Chan EY (2021) Health literacy and patient activation among adults with chronic diseases in Singapore: a cross-sectional study. Nurs Open 8(5):2857–2865
Kearney M, Treadwell J, Marshall M (2017) Overtreatment and undertreatment: time to challenge our thinking. Br J Gen Pract 67(663):442–443
Kern LM, Seirup JK, Casalino LP, Safford MM (2017) Healthcare fragmentation and the frequency of radiology and other diagnostic tests: a cross-sectional study. J Gen Intern Med 32(2):175–181
Lee PH (2014) Is a cutoff of 10% appropriate for the change-in-estimate criterion of confounder identification? J Epidemiol 24(2):161–167
MacMahon S (2018) The academy of medical sciences. Multimorbidity: a priority for global health research. https://acmedsci.ac.uk/file-download/82222577. Accessed 6 Jun 2023
Maeng DD, Martsolf GR, Scanlon DP, Christianson JB (2012) Care coordination for the chronically ill: understanding the patient’s perspective. Health Serv Res 47(5):1960–1979
Magnezi R, Glasser S, Shalev H, Sheiber A, Reuveni H (2014) Patient activation, depression and quality of life. Patient Educ Couns Elsevier Ireland Ltd 94(3):432–437
National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP) (2023) Health and economic costs of chronic diseases. https://www.cdc.gov/chronicdisease/index.html. Accessed 18 Jun 2023
Newland P, Lorenz R, Oliver BJ (2021) Patient activation in adults with chronic conditions: a systematic review. J Health Psychol 26(1):103–114
Nyweide D, Bynum J (2017) Relationship between continuity of ambulatory care and risk of emergency department episodes among older adults. Ann Emerg Med 69(4):407–415
O’Malley AS, Cunningham PJ (2009) Patient experiences with coordination of care: the benefit of continuity and primary care physician as referral source. J Gen Intern Med 24(2):170–177
Paasche-Orlow MK, Wolf MS (2007) The causal pathways linking health literacy to health outcomes. Am J Health Behav 31(SUPPL. 1):S19–S26
Paukkonen L, Oikarinen A, Kähkönen O, Kaakinen P (2022) Patient activation for self-management among adult patients with multimorbidity in primary healthcare settings. Heal Sci Reports 5(4):1–17
Pedersen SE, Aaby A, Friis K, Maindal HT (2023) Multimorbidity and health literacy: a population-based survey among 28,627 Danish adults. Scand J Public Health 51(2):165–172
Rijken M, Close J, Menting J, Lette M, Stoop A, Zonneveld N, de Bruin SR, Lloyd H, Heijmans M (2022) Assessing the experience of person-centred coordinated care of people with chronic conditions in the Netherlands: validation of the Dutch P3CEQ. Heal Expect 25(3):1069–1080
Rijksinstituut voor Volksgezondheid en Milieu (2022) Trendscenario | Ziekten en aandoeningen. https://www.volksgezondheidtoekomstverkenning.nl/c-vtv/trendscenario-update-2020/ziekten-aandoeningen. Accessed 18 Jun 2023
Rouquette A, Nadot T, Labitrie P, Van den Broucke S, Mancini J, Rigal L, Ringa V (2018) Validity and measurement invariance across sex, age, and education level of the French short versions of the European health literacy survey questionnaire. PLoS ONE 13(12):1–15
Santana S, Brach C, Harris L, Ochiai E, Blakey C, Bevington F, Kleinman D, Pronk N (2021) Updating health literacy for healthy people 2030: defining its importance for a new decade in public health. J Public Heal Manag Pract 27(12):S258–S264
Sheridan R, Martin-Kerry J, Hudson J, Parker A, Bower P, Knapp P (2020) Why do patients take part in research? An overview of systematic reviews of psychosocial barriers and facilitators. Trials 21(1):259
Sinnott C, McHugh S, Browne J, Bradley C (2013) GPs’ perspectives on the management of patients with multimorbidity: systematic review and synthesis of qualitative research. BMJ Open 3(9):e003610
Smith SG, Curtis LM, Wardle J, von Wagner C, Wolf MS (2013) Skill set or mind set? Associations between health literacy, Patient activation and health. Plos One 8(9):1–7
Sørensen K, Van Den Broucke S, Pelikan JM, Fullam J, Doyle G, Slonska Z, Kondilis B, Stoffels V, Osborne RH, Brand H (2013) Measuring health literacy in populations: illuminating the design and development process of the European Health Literacy Survey Questionnaire (HLS-EU-Q). BMC Public Health 13–948
Tinetti ME, Fried TR, Boyd CM (2012) Designing health care for the most common chronic condition - Multimorbidity. JAMA 307(23):2493–2494
Twisk JWR (2017) Inleiding in de toegepaste biostatistiek, 4th edn. Bohn Stafleu en van Loghum
van der Mooren F, de Vries R (2022) Steeds meer hoogopgeleiden in Nederland: wat voor beroep hebben ze? https://www.cbs.nl/nl-nl/longread/statistische-trends/2022/steeds-meer-hoogopgeleiden-in-nederland-wat-voor-beroep-hebben-ze-. Accessed 7 Jun 2023
Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB, Blumenthal D (2007) Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med 22(SUPPL. 3):391–395
Acknowledgements
The authors thank Annerie Treffers and Lotte Doude van Troostwijk for their valuable contribution to the data collection of this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: Hidde Dijkstra, Liann I. Weil, Y. Vermeeren, M. Verhoeff, Barbara C. van Munster; Methodology: Hidde Dijkstra, Liann I. Weil, M. Verhoeff, Barbara C. van Munster; Formal analysis and investigation: Hidde Dijkstra, M. Verhoeff; Writing—original draft preparation: Hidde Dijkstra, Liann I. Weil; Writing—review and editing: Hidde Dijkstra, Liann I. Weil, Y. Vermeeren, M. Verhoeff, Barbara C. van Munster; Resources: Y. Vermeeren, Barbara C. van Munster; Supervision: Liann I. Weil, Y. Vermeeren, M. Verhoeff, Barbara C. van Munster.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Local Ethics Committee of Gelre hospitals (Reference number LTC number 2021_22).
Consent to participate
Patients provided written or digital informed consent to participate in the study, which included permission to use information from their Electronic Health Record for research.
Consent for publication
Not applicable.
Conflict of interest
Each author certifies that he or she has no commercial associations that might pose a conflict of interest in connection with the submitted article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This research was performed at University Medical Center of Groningen, the Netherlands.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dijkstra, H., Weil, L.I., Vermeeren, Y. et al. Patient activation and health literacy in Dutch patients with multiple involved specialties. J Public Health (Berl.) (2024). https://doi.org/10.1007/s10389-024-02248-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10389-024-02248-5