Abstract
Aim
Combination vaccines decrease the number of needles required, addressing a common concern of parents. However, some parents are hesitant about combination vaccines and/or want to opt out of certain vaccine components. This study assessed whether introduction of the combination MMRV vaccine influenced coverage levels for measles- and varicella-containing vaccines.
Study and methods
This was a population-based study of children born in Alberta, Canada between 2006 and 2012. We utilized administrative health data to evaluate coverage for the first dose of measles- and varicella-containing vaccines at the age of 24 months (i.e. between 2008 and 2014) before and after introduction of the combination MMRV vaccine in 2010. Among those who were vaccinated, we assessed whether any children continued to receive separate vaccines after the combination vaccine was introduced.
Results
Of 308,212 children, 272,345 (88.36%) were vaccinated with measles- and/or varicella-containing vaccines at the age of 24 months. Although coverage for measles-containing vaccines did not change overall between 2008 and 2014, coverage for varicella vaccine increased in the years following the introduction of MMRV. After the combination vaccine introduction, 96.55% of vaccinated children (n = 121,131) received MMRV vaccine.
Conclusion
Vaccine coverage for varicella increased after the introduction of the combination MMRV vaccine, and there was a narrowing in the gap between MMR and varicella coverage. Very few children received separate vaccines after the introduction of the combination MMRV vaccine. These findings suggest that combination vaccines are acceptable to most parents and increase coverage for varicella in our setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent outbreaks of vaccine-preventable diseases, such as measles, have highlighted the importance of achieving high levels of vaccination coverage (Centers for Disease Control and Prevention [CDC] 2019a; European Centre for Disease Prevention and Control [ECDC] 2018). One challenge facing current and future vaccination programs is the increasing number of vaccines scheduled for the infant and preschool years. Parents have concerns about the number of needles administered to their children at a single visit and in the vaccine schedule overall (Wallace et al. 2014). In most North American and European countries, children receive up to 12 separate injections between the ages of 2 and 18 months (CDC 2019b; ECDC 2019; Public Health Agency of Canada 2019). The large number of needles has been associated with deferral or refusal of vaccination (Bedford and Lansley 2007; Gidengil et al. 2012; Happe et al. 2007). One approach to reducing the number of injections is through combination vaccines that incorporate previously separate vaccines into a new single vaccine (Brown et al. 2010; Happe et al. 2007; Marshall et al. 2007). One example is the combination measles-mumps-rubella-varicella (MMRV) vaccine, which was developed to replace separate MMR and varicella vaccines. In 2010, the Canadian National Advisory Committee on Immunization (NACI) stated that MMRV may be used in place of individual MMR and varicella vaccines, but did not specify a preference for the combination MMRV vaccine versus separate MMR and varicella vaccines (NACI 2010). The decision of whether to adopt the combination MMRV vaccine varied among the 13 provinces and territories of Canada, and variability exists to this day.
The benefits of combination vaccines include (1) fewer numbers of needles, (2) less opportunity for parents to delay one vaccine component, and (3) more efficient vaccine administration process for providers (storage, consent, record-keeping). However, despite the apparent benefits of combination vaccines, some parents have expressed concerns about them, including fears that combining multiple vaccines in one injection will overload their child’s immune system or result in an increase in adverse events, despite lack of evidence to support these concerns (Gidengil et al. 2012; Glanz et al. 2018; Hulsey and Bland 2015; Marshall et al. 2007).
Given the potential pros and cons of combination vaccines, it is important to assess the impact of changing from separate to combination vaccines on vaccination coverage. The objectives of this study were to determine (a) whether the introduction of the combination MMRV vaccine changed coverage levels for the first dose of measles- and varicella-containing vaccines, and (b) whether children continued to receive separate vaccines after the combination vaccine was introduced to the routine vaccination schedule.
Methods
Setting
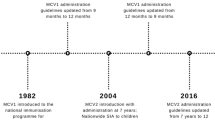
This study was conducted in the province of Alberta (population 4.2 million), Canada, which has a healthcare system that is publicly funded through the Alberta Health Care Insurance Plan (AHCIP). Each Alberta resident is issued a personal health number, which can be used to identify and link health service use. Children receive their routine vaccinations free of charge, according to the schedule recommended by the Alberta Ministry of Health (Alberta Health 2019). The first dose of separate measles and varicella-containing vaccines was scheduled at 12 months. In September 2010, Alberta switched from using separate MMR and varicella vaccines to the combination MMRV vaccine, still scheduled for the 12-month vaccination visit. After this date, MMRV was the vaccine routinely provided, but the separate vaccines continued to be available upon request by the parent.
Study design and population
This retrospective cohort study assessed coverage and type of vaccine delivered for the first dose of measles-containing vaccine (MMR or MMRV) and varicella-containing vaccine (varicella or MMRV) administered to children between the ages of 12 and 24 months. The cohort included children born in Alberta between January 1, 2006, and December 31, 2012, and assessed their vaccination status at the age of 24 months, i.e. between January 1, 2008, and December 31, 2014. Twenty-four months is a common age of coverage assessment for measles-containing vaccines in Canada and a number of other jurisdictions (National Centre for Immunisation Research and Surveillance [NCIRS] 2020; NHS 2020; Wilson et al. 2017). Data were obtained from three administrative data sets that were linked by personal health numbers: the Vital Statistics Registry, AHCIP, and the provincial immunization repository (known as ImmARI). Children born in Alberta in 2006–2012 were selected from the Vital Statistics Registry. Using AHCIP data, children were excluded if they had First Nations status (these Indigenous peoples of Canada may have received some vaccines on reserves, which are not included in ImmARI), had ever lived in the town of Lloydminster (where vaccines are provided by the neighbouring province of Saskatchewan), or had left Alberta or died before 24 months of age. The cohort was linked with ImmARI to capture immunization information.
Statistical analysis
We calculated vaccine coverage (defined as the percent of eligible children who received the relevant vaccine) for measles-containing and varicella-containing vaccines at the age of 24 months for each year from 2008 through 2014. For children who were vaccinated after the introduction of MMRV (2010), we assessed whether children received the combination MMRV vaccine or separate vaccines (i.e., MMR only, varicella only, MMR and varicella on the same day, or MMR and varicella on different days). Data management and statistical analyses were performed using SAS version 9.3 software (SAS Institute, Cary, NC).
Results
Change in coverage before and after introduction of MMRV
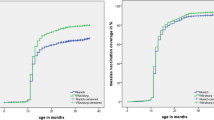
After 38,554 exclusions (23,956 First Nations children; 1810 lived in Lloydminster; 12,743 died or cancelled health insurance ≤24 months; and 45 missing postal code), 308,212 children born between January 2006 and December 2012 were included in the study. Of these, 272,345 (88.36%) were vaccinated with measles-containing and/or varicella-containing vaccines at the age of 24 months, while 35,867 children were not. As presented in Table 1 and Fig. 1, the coverage for measles-containing vaccines at 24 months of age was 89.0% in 2008, decreased slightly in 2009, stayed at 87.4–87.6% for four years, and then returned to 89.1% in 2014. For varicella-containing vaccines, the coverage was 86.7% in 2008 and 2009, decreased slightly to 85.6–85.8% from 2010 to 2011, and then gradually increased to 88.0% between 2012 and 2014.
Use of separate MMR and varicella vaccines after introduction of MMRV
Of the 272,345 vaccinated children in our cohort, 121,131 were born in or after 2010, the year when the combination MMRV vaccine was introduced (see Table 2). Among these, 116,955 children (96.55%) received MMRV; 1775 (1.47%) got separate MMR and varicella on the same day; 515 (0.42%) obtained separate MMR and varicella on different days; 1691 (1.40%) got MMR only; and 195 (0.16%) got only varicella vaccine. Of the children who did not receive varicella vaccine, 54.1% (n = 915) had a documented reason, including refusal/lack of consent (90.16%), history of disease (9.51%), and not recommended/previous adverse reaction (0.33%). Of the children who did not obtain MMR, only 25.6% (n = 50) had a documented reason, all of which were refusal/lack of consent.
Discussion
Change in vaccine coverage
Vaccine coverage for MMR decreased after 2008 and did not increase back to 2008 levels until 2014. In contrast, vaccine coverage for varicella decreased after 2008 and then began to increase after 2010, following the introduction of the combination MMRV vaccine. It is unclear why coverage dropped and then rebounded for both vaccines. It is possible that other factors may have changed during the same period, such as a change in attitudes about vaccines driven by social media reports or disease outbreaks that changed perceptions of disease susceptibility.
It is significant that there was a narrowing in the gap between MMR and varicella coverage after introduction of the MMRV vaccine. Our study mirrors findings of studies in Australia (Macartney et al. 2017) and Germany (Streng and Liese 2014), showing that introduction of the MMRV vaccine resulted in an increase in varicella vaccine coverage. In 2013, Australia changed from MMR at 12 months, varicella at 18 months, and a second dose of MMR at 4 years, to the current schedule of MMR at 12 months and MMRV at 18 months. In the study by Macartney et al. (2017), they found that varicella coverage increased by 4% after the introduction of MMRV at 18 months, a result slightly higher than our study finding (which revealed a 2.4% change between the introduction of MMRV in 2010 and the end of our study period in 2014). The difference between Macartney’s study and our study may be associated with the fact that the switch to MMRV vaccine in Australia was also accompanied by a change in the MMR administration schedule from 4 years to 18 months, whereas our study was conducted in the context of a consistent schedule. A German study by Streng and Liese (2014), following the 2011 switch from MMRV back to separate MMR and varicella vaccines (based on concerns about febrile seizures related to MMRV) (Klein et al. 2010; MacDonald et al. 2014; Schink et al. 2014), resulted in a corresponding 4–12% decrease in varicella coverage (depending on region) and no change in MMR coverage.
The concern about febrile seizures related to MMRV resulted in a change in the national recommendation in Canada in 2016 (NACI 2016). The revised recommendation stated that the first dose can be given either as MMRV or as separate MMR and varicella vaccines, with the following considerations: parental acceptability of the increased risk of febrile seizure, potential impact on the perception of safety and vaccination coverage, and the need for an additional injection. The decision of whether to adopt the recommendation is jurisdiction-specific, with many provinces, including Alberta, continuing to provide the combination MMRV vaccine for the first dose.
Use of separate versus combination vaccines
Of the children in our study vaccinated with MMR and/or varicella after 2010, the vast majority (>96%) received the combination MMRV vaccine, with only a small proportion receiving separate vaccines. Our study could not determine whether the use of separate vaccines was due to contraindications or supply shortages, as opposed to parental choice (Happe et al. 2007). However, we hypothesize that the use of separate vaccines on the same day (1.47% of children) might be equally due to supply issues or parental choice, i.e. we cannot distinguish the reason. In contrast, the receipt of separate vaccines on different days (0.43% of children) or receipt of only MMR (1.40% of children) or varicella vaccine (0.16% of children) suggests parental choice might be the reason, because there is no reason related to supply to explain why a separate vaccine would be omitted or delayed.
The role of combination vaccines in achieving national coverage targets
Combination vaccines have been shown, in our study and others (Kalies et al. 2006; Happe et al. 2007; Macartney et al. 2017; Streng and Liese 2014), to increase vaccine coverage for individual antigens. It has been argued that as more and more new vaccines are added to the childhood vaccine schedule, coverage rates will begin to erode. Combination vaccines are a potential solution to this problem (Marshall et al. 2007), and some national immunization advisory bodies are recommending their use whenever possible (Skibinski et al. 2011). In fact, it has been proposed that increased use of combination vaccines could be key to achieving regional and national public health targets for vaccine coverage (Maman et al. 2015; Skibinski et al. 2011).
Strengths and limitations
A strength of this study is the use of a comprehensive population-based database in a publicly funded vaccination program, which reduces potential bias arising from non-representative sampling. However, one of the limitations of administrative data is that it only shows us vaccine uptake behaviour, and not the reasons or mechanisms behind that behaviour. Certainly, there is a need for future studies to explore reasons for refusal or deferral of combination vaccines (Happe et al. 2007). One cannot discount the possibility that unidentified historical effects are responsible for the trends observed in MMR and varicella coverage, but it is unlikely that these would be responsible for the narrowing in the coverage gap between the two vaccines, suggesting that an increase in varicella coverage has resulted from the introduction of the combination vaccine.
Conclusions
Introduction of the combination MMRV vaccine in our study setting led to a narrowing in the gap of coverage between measles and varicella, with most vaccinated children receiving MMRV. This study provides some evidence that a large determinant of vaccine coverage patterns is the availability of vaccine products. In our study, few children continued to receive available separate vaccines after introduction of the combination MMRV vaccine. With the development of new vaccines to protect children from current and emerging infectious disease, it is likely that more combination vaccines will be developed to minimize the number of injections required. The potential for combination vaccines to increase vaccine coverage, and thus to improve population protection against disease, should be reassuring to parents, clinicians, and policymakers.
References
Alberta Health (2019) Interactive Health Data Application. http://www.ahw.gov.ab.ca/IHDA_Retrieval/. Accessed 5 December 2019
Bedford H, Lansley M (2007) More vaccines for children? Parents’ views. Vaccine 25(45):7818–7823. https://doi.org/10.1016/j.vaccine.2007.08.057
Brown KF, Kroll JS, Hudson MJ, Ramsay M, Green J, Long SJ et al (2010) Factors underlying parental decisions about combination childhood vaccinations including MMR: a systematic review. Vaccine 28(26):4235–4248. https://doi.org/10.1016/j.vaccine.2010.04.052
Centers for Disease Control & Prevention [CDC] (2019a) Measles cases and outbreaks. https://www.cdc.gov/measles/cases-outbreaks.html. Accessed 5 Dec 2019
Centers for Disease Control and Prevention [CDC] (2019b) Recommended child and adolescent immunization schedule for ages 18 years or younger, United States. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed 5 Dec 2019
European Centre for Disease Prevention and Control [ECDC] (2018) Monthly measles and rubella monitoring report. https://www.ecdc.europa.eu/en/publications-data/monthly-measles-and-rubella-monitoring-report-december-2018. Accessed 15 November 2019
European Centre for Disease Prevention and Control [ECDC] (2019) Vaccination schedules for individual European countries and specific age groups. https://www.ecdc.europa.eu/en/immunisation-vaccines/EU-vaccination-schedules. Accessed 5 December 2019
Gidengil C, Lieu TA, Payne K, Rusinak D, Messonnier M, Prosser LA (2012) Parental and societal values for the risks and benefits of childhood combination vaccines. Vaccine 30(23):3445–3452. https://doi.org/10.1016/j.vaccine.2012.03.022
Glanz JM, Newcomer SR, Daley MF, DeStefano F, Groom HC et al (2018) Association between estimated cumulative vaccine antigen exposure through the first 23 months of life and non-vaccine-targeted infections from 24 through 47 months of age. JAMA 319(9):906–913
Happe LE, Lunacsek OE, Marshall GS, Lewis T, Spencer S (2007) Combination vaccine use and vaccination quality in a managed care population. Am J Manag Care 13(9):506–512
Hulsey E, Bland T (2015) Immune overload: parental attitudes toward combination and single antigen vaccines. Vaccine 33(22):2546–2550. https://doi.org/10.1016/j.vaccine.2015.04.020
Kalies H, Grote V, Verstraeten T, Hessel L, Schmitt HJ, von Kries R (2006) The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J 25(6):507–512. https://doi.org/10.1097/01.inf.0000222413.47344.23
Klein NP, Fireman B, Yih WK, Lewis E, Kulldorff M, Ray P et al (2010) Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics 126(1):1. https://doi.org/10.1542/peds.2010-0665
Macartney K, Gidding HF, Trinh L et al (2017) Evaluation of combination measles-mumps-rubella-varicella vaccine introduction in Australia. JAMA Pediatr 171(10):992–998. https://doi.org/10.1001/jamapediatrics.2017.1965
MacDonald SE, Dover DC, Simmonds KA, Svenson LW (2014) Risk of febrile seizures after first dose of measles-mumps-rubella-varicella vaccine: a population-based cohort study. Can Med Assoc J 186(11):824–829. https://doi.org/10.1503/cmaj.140078
Maman K, Zöllner Y, Greco D, Duru G, Sendyona S, Remy V (2015) The value of childhood combination vaccines: from beliefs to evidence. Hum Vaccin & Immun 11(9):2132–2141. https://doi.org/10.1080/21645515.2015.1044180
Marshall GS, Happe LE, Lunacsek OE, Szymanski MD, Woods CR, Zahn M, Russell A (2007) Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J 26(6):496–500. https://doi.org/10.1097/INF.0b013e31805d7f17
National Advisory Committee on Immunization (NACI) (2010) An advisory committee statement of NACI: Statement on measles-mumps-rubella-varicella vaccine. Canada Communicable Disease Report (CCDR), Public Health Agency of Canada (PHAC). https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2010-36/canada-communicable-disease-report-14.html. Accessed June 12 2020
National Advisory Committee on Immunization (NACI) (2016) An advisory committee statement of NACI: Update on measles-mumps-rubella-varicella vaccine and febrile seizures. Public Health Agency of Canada (PHAC). 2016. https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/healthy-living/update-measles-mumps-rubella-varicella-vaccine-febrile-seizures/pub1-eng.pdf. Accessed June 12 2020
National Centre for Immunisation Research and Surveillance (NCIRS). Vaccine coverage, Australia (2020). http://ncirs.org.au/our-work/vaccine-coverage. Accessed 12 June 2020
National Health Service (NHS). Childhood Vaccination Coverage Statistics – England 2018–19 (2020). https://digital.nhs.uk/data-and-information/publications/statistical/nhs-immunisation-statistics. Accessed 12 June 2020
Public Health Agency of Canada (PHAC) (2019) Canada’s provincial and territorial routine (and catch-up) vaccination programs for infants and children. https://www.canada.ca/en/public-health/services/provincial-territorial-immunization-information/provincial-territorial-routine-vaccination-programs-infants-children.html. Accessed 15 November 2019
Schink T, Holstiege J, Kowalzik F, Zepp F, Garbe E (2014) Risk of febrile convulsions after MMRV vaccination in comparison to MMR or MMR+V vaccination. Vaccine 32(6):645–650. https://doi.org/10.1016/j.vaccine.2013.12.011
Skibinski DAG, Baudner BC, Singh M, O’Hagan DT (2011) Combination vaccines. J Global Infect Dis 3(1):63–72
Streng A, Liese JG (2014) Decline of varicella vaccination in German surveillance regions after recommendation of separate first-dose vaccination for varicella and measles–mumps–rubella. Vaccine 32(8):897–900. https://doi.org/10.1016/j.vaccine.2013.12.065
Wallace AS, Mantel C, Mayers G, Mansoor O, Gindler JS, Hyde TB (2014) Experiences with provider and parental attitudes and practices regarding the administration of multiple injections during infant vaccination visits: lessons for vaccine introduction. Vaccine 32(41):5301–5310. https://doi.org/10.1016/j.vaccine.2014.07.076
Wilson SE, Quach S, MacDonald SE, Naus M, Deeks SL, Crowcroft NS et al (2017) Methods used for immunization coverage assessment in Canada: a Canadian immunization research network (CIRN) study. Hum Vaccin & Immun 13(8):1928–1936. https://doi.org/10.1080/21645515.2017.1319022
Acknowledgements
This work would not have been possible without the support of Larry Svenson and his team in the Analytics and Performance Reporting Branch of the Alberta Ministry of Health.
Funding
No direct funding was received for this study. At the time this study was conducted, Shannon MacDonald was supported by Post-Doctoral Fellowships from the Canadian Institutes of Health Research and Alberta Innovates Health Solutions.
Author information
Authors and Affiliations
Contributions
SM, ST, and JK conceptualized the study; SM and XG designed the study; XG analysed the data; all authors interpreted the findings; SM drafted the MS; all authors reviewed the manuscript critically and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest with regard to the organization that sponsored the research.
Ethics approval
This study was approved by the University of Calgary Conjoint Health Research Ethics Board.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
MacDonald, S.E., Tough, S., Guo, X. et al. Impact of combination MMRV vaccine on first-dose coverage for measles and varicella: a population-based study. J Public Health (Berl.) 30, 1063–1068 (2022). https://doi.org/10.1007/s10389-020-01379-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10389-020-01379-9