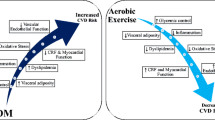
Summary
Type 2 diabetic patients have an increased level of systemic free radicals, which severely restrict the bioavailability of endothelium-derived nitric oxide (NO) and thus contribute to the development of an endothelial dysfunction. This review analyses the influence of physical training on molecular development mechanisms of the endothelial dysfunction and determines the significance of regular physical exercise for the endothelial function in type 2 diabetic patients. Systematic training reinforces the endogenic antioxidative capacity and results in a reduction in oxidative stress. Training – also combined with a change in diet – furthermore reduces hyperglycaemic blood sugar levels, thus curbing a major source of free radicals in diabetes. Moreover, physical exercise enhances vascular NO synthesis through an increased availability/activity of endothelial NO synthases (eNOS). Endurance, as well as resistance training with submaximal intensity or a combination of both forms of training is suitable to effectively improve the endothelial function in type 2 diabetic patients in the long term.
Zusammenfassung
Typ-2-Diabetiker weisen eine erhöhte Konzentration systemischer freier Radikale auf, welche die Bioverfügbarkeit von Stickstoffmonoxid (NO) im Endothel stark einschränken und damit zur Entstehung einer endothelialen Dysfunktion beitragen. Diese Übersichtsarbeit analysiert den Einfluss von körperlichem Training auf molekulare Entstehungsmechanismen der endothelialen Dysfunktion und eruiert die Bedeutung von regelmäßiger sportlicher Aktivität für die Endothelfunktion bei Typ-2-Diabetikern. Systematisches Training verstärkt die endogene antioxidative Kapazität und bewirkt eine Abnahme von oxidativem Stress. Sportliche Aktivität – auch in Kombination mit einer Ernährungsumstellung – verringert außerdem hyperglykämische Blutzuckerspiegel, wodurch eine Hauptquelle freier Radikale beim Diabetes eingedämmt wird. Zudem steigert körperliches Training die vaskuläre NO-Synthese durch eine erhöhte Verfügbarkeit/Aktivität der endothelialen NO-Synthasen (eNOS). Ein Ausdauer- als auch ein Krafttraining mit submaximalen Intensitäten oder eine Kombination beider Trainingsformen eignet sich langfristig, die Endothelfunktion bei Typ-2-Diabetikern wirksam zu verbessern.
Similar content being viewed by others
Literatur
Geiss LS, Herman WH, Smith PJ. Mortality in non-insulin-dependent diabetes. In: National Diabetes Data Group (Hrsg.) Diabetes in America. US Department of Health and Human Services, Public Health Service, National Institute of Health, Bethesda, MD, pp. 233–257, 1995
Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med, 340: 115–126, 1999
Rösen P. Endotheliale Dysfunktion: ein Synonym für funktionelle Atherosklerose. J Kardiol, 9: 556–652, 2002
Espinola-Klein C, Münzel T. Oxidativer Stress und Endothelfunktion: Welche Bedeutung hat das Geschlecht? Blickpunkt der Mann, 6: 29–31, 2004
Atalaya M, Laaksonen DE. Diabetes, oxidative stress and physical exercise. J Sports Sci Med, 1: 1–14, 2002
Münzel T, Daiber A, Ullrich V, Mulsch A. Vascular consequence of endothelial nitric oxide synthase uncoupling fort he activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Atherioscler Thromb Vasc Biol, 25: 1551–1557, 2005
Celermajer DS. Endothelial dysfunction: does it matter? Is it reversible? J Am Coll Cardiol, 30: 325–333, 1997
El Mabrouk M, Singh A, Touyz RM, Schiffrin EL. Antiproliferative effect of L-NAME on rat vascular smooth muscle cells. Life Sci, 67: 1613–1623, 2000
Gimbrone MA. Endothelial dysfunction and the pathogenesis of atherosclerosis. In: Fidge NH, Nestel PJ (eds) Atherosclerosis VII. Proceedings of the 7th Int. Symp. on Atherosclerosis. Excerpta Medica, Amsterdam, pp. 367–369, 1986
Karabag T, Kaya A, Yavuz S, et al. The relation of HOMA index with endothelial functions determined by flow mediated dilatation method among hyperglycemic patients. Indian Heart J, 59: 463–467, 2007
Steinberg HO, Chaker H, Leaming R, et al. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest, 97: 2601–2610, 1996
Steinberg HO, Paradisi G, Cronin J, et al. Type II diabetes abrogates sex differences in endothelial function in premenopausal women. Circulation, 101: 2040–2046, 2000
Suzuki M, Takamisawa I, Yoshimasa Y, Harano Y. Association between insulin resistance and endothelial dysfunction in type 2 diabetes and the effects of pioglitazone. Diabetes Res Clin Pract, 76: 12–17, 2007
Williams SB, Cusco JA, Roddy MA, et al. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol, 27: 567–574, 1996
Victor VM, Rocha M, Sola E, et al. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des, 15: 2988–3002, 2009
Collier A, Rumley A, Rumley AG, et al. Free radical activity and hemostatic factors in NIDDM patients with and without microalbuminuria. Diabetes, 41: 909–913, 1992
Osuntokl AA, Fasanmade OA, Adekola AO, Amira CO. Lipid peroxidation and erythrocyte fragility in poorly controlled type 2 diabetes mellitus. Nig Q J Hosp Med, 17: 148–151, 2007
Pandey KB, Mishra N, Rizvi SI. Protein oxidation biomarkers in plasma of type 2 diabetic patients. Clin Biochem, 43: 508–511, 2010
Sato Y, Hotta N, Sakamoto N, et al. Lipid peroxide level in the plasma of diabetic patients. Biochem Med, 21: 104–107, 1979
Pandey KB, Mishra N, Rizvi SI. Myricetin may provide protection against oxidative stress in type 2 diabetic erythrocytes. Z Naturforsch C, 64: 626–630, 2009
Peuchant E, Delmas-Beauvieux MC, Couchouron A, et al. Short-term insulin therapy and normoglycemia. Effects on erythrocyte lipid peroxidation in NIDDM patients. Diabetes Care, 20: 202–207, 1997
Adaikalakoteswari A, Balasubramanyam M, Rema M, Mohan V. Differential gene expression of NADPH oxidase (p22phox) and hemoxygenase-1 in patients with Type 2 diabetes and microangiopathy. Diabet Med, 23: 666–674, 2006
Belia S, Santilli F, Beccaficio S, et al. Oxidative-induced membrane damage in diabetes lymphocytes: effects on intracellular Ca(2+) homeostasis. Free Radic Res, 43: 138–148, 2009
Scheede-Bergdahl C, Penkowa M, Hidalgo J, et al. Metallothionein-mediated antioxidant defense system and its response to exercise training are impaired in human type 2 diabetes. Diabetes, 54: 3089–3094, 2005
Sorrentino SA, Bahlmann FH, Besler C, et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation, 116: 163–173, 2007
Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm, 2010: 453892, 2010
Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med, 10: 339–352, 1991
Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature, 414: 813–820, 2001
Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature, 404: 787–790, 2000
Bravi MC, Pietrangeli P, Laurenti O, et al. Polyol pathway activation and glutathione redox status in non-insulin-dependent diabetic patients. Metabolism, 46: 1194–1198, 1997
Inoguchi T, Li P, Umeda F, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes, 49: 1939–1945, 2000
Anderssohn M, Schwedhelm E, Lüneburg N, et al. Asymmetric dimethylarginine as a mediator of vascular dysfunction and a marker of cardiovascular disease and mortality: an intriguing interaction with diabetes mellitus. Diab Vasc Dis Res, 7: 105–118, 2010
Newsholme P, Homem De Bittencourt PI, O'Hagan C, et al. Exercise and possible molecular mechanisms of protection from vascular disease and diabetes: the central role of ROS and nitric oxide. Clin Sci (Lond.), 118: 341–349, 2009
Maxwell SR, Thomason H, Sandler D, et al. Poor glycaemic control is associated with reduced serum free radical scavenging (antioxidant) activity in non-insulin-dependent diabetes mellitus. Ann Clin Biochem, 34: 638–644, 1997
Bhatia S, Shukla R, Venkata Madhu S, et al. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin Biochem, 36: 557–562, 2003
Kurtul N, Bakan E, Aksoy H, Baykal O. Leukocyte lipid peroxidation, superoxide dismutase and catalase activities of type 2 diabetic patients with retinopathy. Acta Medica (Hradec Kralove), 48: 35–38, 2005
Memisogullari R, Taysi S, Bakan E, Capoglu I. Antioxidant status and lipid peroxidation in type II diabetes mellitus. Cell Biochem Funct, 21: 291–296, 2003
Sundaram RK, Bhaskar A, Vijayalingam S, et al. Antioxidant status and lipid peroxidation in type II diabetes mellitus with and without complications. Clin Sci (Lond.), 90: 255–260, 1996
Vijayalingam S, Parthiban A, Shanmugasundaram KR, Mohan V. Abnormal antioxidant status in impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Diabet Med, 13: 715–719, 1996
Landmesser U, Merten R, Spiekermann S, et al. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation, 16: 2264–2270, 2000
Ashok Kumar P, Rajagopal G. Lipid peroxidation in erythrocytes of patients with type 2 diabetes mellitus. Indian J Clinical Biochem, 18: 71–74, 2003
Akkus I, Kalak S, Vural H, et al. Leukocyte lipid peroxidation, superoxide dismutase, glutathione peroxidase and serum and leukocyte vitamin C levels of patients with type II diabetes mellitus. Clin Chim Acta, 244: 221–227, 1996
Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem, 278: 22546–22554, 2003
Schulze E, Jansen T, Wenzel P, et al. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal, 10: 1115–1126, 2008
Weber M, Lauer N, Mülsch A, Kojda G. The effect of peroxynitrite on the catalytic activity of soluble guanylyl cyclase. Free Radic Biol Med, 31: 1360–1367, 2001
Zou M, Martin C, Ullrich V. Tyrosine nitration as a mechanism of selective inactivation of prostacyclin synthase by peroxynitrite. Biol Chem, 378: 707–713, 1997
Steinberg HO, Baron AD. Vascular function, insulin resistance and fatty acids. Diabetologica, 45: 623–634, 2002
Kearney MT, Duncan ER, Kahn M, Wheatcroft SB. Insulin resistance and endothelial cell dysfunction: studies in mammalian models. Exp Physiol, 93: 158–163, 2008
Wei Y, Chen K, Whaley-Connell AT, et al. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol, 294: R673–R680, 2008
Du XL, Edelstein D, Dimmeler S, et al. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest, 108: 1341–1348, 2001
Itabe H. Oxidative modification of LDL: its pathological role in atherosclerosis. Clin Rev Allergy Immunol, 37: 4–11, 2009
Yoshida H, Sasaki K, Namiki Y, et al. Edaravone, a novel radical scavenger, inhibits oxidative modification of low-density lipoprotein (LDL) and reverses oxidized LDL-mediated reduction in the expression of endothelial nitric oxide synthase. Atherosclerosis, 179: 97–102, 2005
Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-kappaB (Review). Int J Mol Med, 4: 223–230, 1999
Balletshofer BM, Häring HU. Typ-2 Diabetes, Insulinresistenz und endotheliale Dysfunktion. Hämostaseologie, 4: 159–166, 2001
Davda RK, Stepniakowski KT, Lu G, et al. Oleic acid inhibits endothelial nitric oxide synthase by a protein kinase C-independent mechanism. Hypertension, 26: 764–770, 1995
Pieper GM, Dondlinger LA. Plasma and vascular tissue arginine are decreased in diabetes: acute arginine supplementation restores endothelium-dependent relaxation by augmenting cGMP production. J Pharmacol Exp Ther, 283: 684–691, 1997
Ashton T, Rowlands CC, Jones E, et al. Electron spin resonance spectrometric detection of oxygen-centred radicals in human serum following exhaustive exercise. Eur J Appl Physiol, 77: 498–502, 1998
Bloch W, Schmidt A. Sport und freie Radikale. Blickpunkt der Mann, 3: 13–18, 2004
Niess AM, Fehrenbach E, Northoff H, Dickhuth HH. Freie Radikale und oxidativer Stress bei körperlicher Belastung und Trainingsanpassung – Eine aktuelle Übersicht. Dt Z Sportmed, 12: 345–353, 2002
Reid MB. Redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol, 90: 724–731, 2001
Hollander J, Fiebig R, Gore M, et al. Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Pflügers Arch, 442: 426–434, 2001
Mann GE, Niehueser-Saran J, Watson A, et al. Nrf2/ARE regulated antioxidant gene expression in endothelial and smooth muscle cells in oxidative stress: implications for atherosclerosis and preeclampsia. Acta Physiologica Sinica, 59: 117–127, 2007
Miyazaki H, Oh-ishi S, Ookawara T, et al. Strenuous endurance training in humans reduces oxidative stress following exhaustive exercise. Eur J Appl Physiol, 84: 1–6, 2001
Elosua R, Molina L, Fito M, et al. Response of oxidative stress biomarkers to a 16-week aerobic physical activity program, and to acute physical activity, in healthy young men and women. Atherosclerosis, 167: 327–334, 2003
Ennezat PV, Malendowicz SL, Testa M, et al. Physical training in patients with chronic heart failure enhances the expression of genes encoding antioxidative enzymes. J Am Coll Cardiol, 38: 194–198, 2001
Linke A, Adams V, Schulze PC, et al. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation, 111: 1763–1770, 2005
Ohno H, Yahata T, Sato Y, et al. Physical training and fasting erythrocyte activities of free radical scavenging enzyme systems in sedentary men. Eur J Appl Physiol Occup Physiol, 57: 173–176, 1988
Parise G, Phillips SM, Kaczor JJ, Tarnopolsky MA. Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults. Free Radic Biol Med, 39: 289–295, 2005
Shin YA, Lee JH, Song W, Jun TW. Exercise training improves the antioxidant enzyme activity with no changes of telomere length. Mech Ageing Dev, 129: 254–260, 2008
Evelo CT, Palmen NG, Artur Y, Janssen GM. Changes in blood glutathione concentrations, and in erythrocyte glutathione reductase and glutathione S-transferase activity after running training and after participation in contests. Eur J Appl Physiol Occup Physiol, 64: 354–358, 1992
Adams V, Linke A, Kränkel N, et al. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation, 111: 555–562, 2005
Iborra RT, Ribeiro IC, Neves MQ, et al. Aerobic exercise training improves the role of high-density lipoprotein antioxidant and reduces plasma lipid peroxidation in type 2 diabetes mellitus. Scand J Med Sci Sports, 18: 742–750, 2008
Lazarevic G, Antic S, Cvetkovic T, et al. Effects of regular exercise on cardiovascular risk factors profile and oxidative stress in obese type 2 diabetic patients in regard to SCORE risk. Acta Cardiol, 63: 485–491, 2008
Nojima H, Watanabe H, Yamane K, et al. Effect of aerobic exercise training on oxidative stress in patients with type 2 diabetes mellitus. Metabolism, 57: 170–176, 2008
Roberts CK, Won D, Pruthi S, et al. Effect of a diet and exercise intervention on oxidative stress, inflammation and monocyte adhesion in diabetic men. Diabetes Res Clin Pract, 73: 249–259, 2006
Wycherley TP, Brinkworth GD, Noakes M, et al. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes Metab, 10: 1062–1073, 2008
Kadoglou NP, Iliadis F, Angelopoulou N, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil, 14: 837–843, 2007
Santos JM, Ribeiro SB, Gaya AR, et al. Skeletal muscle pathways of contraction-enhanced glucose uptake. Int J Sports Med, 29: 785–794, 2008
Walther C, Gielen S, Hambrecht R. The effect of exercise training on endothelial function in cardiovascular disease in humans. Exerc Sport Sci Rev, 32: 129–134, 2004
Resnick N, Gimbrone MA. Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J, 9: 874–882, 1995
Boo YC, Sorescu G, Boyd N, et al. Shear stress at stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms – role of protein kinase A. J Biol Chem, 277: 3388–3396, 2002
Corson MA, James NL, Latta SE, et al. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ Res, 79: 984–991, 1996
Dimmler S, Fleming I, Fisslthaler B, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature, 399: 601–605, 1999
Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol, 561: 1–25, 2004
Hambrecht R, Adams V, Erbs S, et al. Regular physical activity improves endothelial funtion in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation, 107: 3152–3158, 2003
Maiorana A, O'Driscoll G, Cheetham C, et al. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol, 38: 860–866, 2001
Okada S, Hiuge A, Makino H, et al. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J Atheroscler Thromb, 13: 2–7, 2010
Lavrencic A, Salobir BG, Keber I. Physical training improves flow-mediated dilation in patients with the polymetabolic syndrome. Arterioscler Thromb Vasc Biol, 20: 551–555, 2000
Cohen ND, Dunstan DW, Robinson C, et al. Improved endothelial function following a 14-month resistance exercise training program in adults with type 2 diabetes. Diabetes Res Clin Pract, 79: 405–411, 2008
Author information
Authors and Affiliations
Corresponding author
Additional information
Ein Erratum zu diesem Beitrag ist unter http://dx.doi.org/10.1007/s10354-011-0036-1 zu finden.
Rights and permissions
About this article
Cite this article
Brinkmann, C., Schwinger, R. & Brixius, K. Körperliche Aktivität und endotheliale Dysfunktion bei Typ-2-Diabetikern: über die Rolle von Stickstoffmonoxid und oxidativem Stress. Wien Med Wochenschr 161, 305–314 (2011). https://doi.org/10.1007/s10354-011-0868-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-011-0868-8