Summary
Background
Rectovaginal fistulas represent 5% of all anorectal fistulas. For affected women, this pathology is associated with a reduction in quality of life (QoL) and self-esteem. Most commonly used methods of surgical closure have high recurrence rates or permanent perineal complaints, which in turn lead to negative effects on QoL and self-esteem. A fistula closure, using the “de-epithelialized Singapore flap” (SF), can be a good alternative therapy strategy.
Method
Our retrospective case series processes the long-term results of seven patients who were operated on for ano-/rectovaginal fistula using the SF. All patients underwent surgery at the University Hospital Graz, between May 2012 and July 2015. The data of the surgical follow-up examinations were collected and an additional telephone survey was carried out. The procedure is presented based on a structured description. All procedures were performed jointly by the Department of General Surgery and the Department of Plastic Surgery.
Results
The average age of the seven patients was 46.14 years (23–72 a). Five patients had a total of 12 previous operations with frustrating results. Of the seven patients treated, six had a permanent fistula closure (85.7%). The results of the telephone survey (n = 6) showed a high level of patient satisfaction (100%), and an improvement in QoL (83.3%), through our surgical method. In our cohort, neither urinary nor fecal incontinence occurred.
Conclusion
The treatment of an ano-/rectovaginal fistula using the “de-epithelialized pudendal thigh flap” (Singapore flap) is a promising treatment alternative. In particular, patients who have had previous proctological interventions show a benefit from this procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
While most studies, dealing with fistula repair, are focused on technical feasability and short term fistula closure rates our study is able to show long results and recurrence rates.
Descriptions of this technique are very rare in the literature.
Normally the technique is used for vaginal defects, and due to the de-epithelialization it can be used for fistula closure.
Introduction
Worldwide, the subentity of ano-/rectovaginal fistula accounts for approximately 5% of all rectal fistula formations [1]. For the affected patients, they represent an extremely stressful situation. Between 75 and 100% of affected women report significant psychological complaints with a reduction in self-esteem [2]. Regardless of the sociocultural or religious background of the patients, these fistula forms have dramatic negative effects on the social aspect, body feeling, sexual behavior, and, finally, on the mental health of the women concerned [3]. Especially in areas where access to high-end medicine is difficult or impossible and therefore a surgical solution seems less likely, the stigma perceived by those affected is even more pronounced [4]. This was well demonstrated by publications in BMC Women’s Health [3, 4].
In medically underdeveloped countries, the majority of anovaginal and rectovaginal fistulas in women are caused by obstetric trauma (95%; [5]). This is due to improper care of the injuries of the birth canal caused during the birth process. The origin of the fistula is related to communicating necrotizing recto/anal and vaginal tissue areas [4]. Because of the high frequency of recto-/anovaginal fistulas the sub-Saharan regions are also referred to as the “fistula belt” and the occurrence there is even considered endemic by some authors [6].
On the other hand, there is a significantly lower frequency of obstetric causes of such fistulas in Central Europe and North America. In 2016, Trovik et al. [7] were able to attribute a proportion of 12.87% of anal/rectal fistulas to obstetric trauma in a Norwegian patient collective. The resulting fistulas were all small and either healed spontaneously or could be sufficiently closed by a simple transvaginal procedure. This shows that both the frequency and the fistula expression vary greatly depending on the medical care and the healthcare system available. In developed countries, chronic inflammatory bowel disease (IBD) is the most common cause of ano-/rectovaginal fistula formation (45.6%; [8]). Other causes include surgical trauma (16.7%; [8]), radiation [9], very rarely cryptoglandular inflammation [10], and malignant diseases. The latter can rarely be successfully treated with surgical interventions.
In the case of deep anterior rectal resections, the development of a rectovaginal fistula should be expected in 3% of cases. While in presence of IBD, a minimal fistula rate of 10% between the anastomotic region and the vagina can be awaited [11]. This underlines the major differences in pathomechanisms, healing processes, and therapy recommendations for ano-/rectovaginal fistulas with and without Crohn’s disease. Therefore, the S3 guidelines for the surgical treatment of rectovaginal fistulas are formulated accordingly (AMWF [Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften] compliant 2012, The treatment of recto-vaginal fistulas without Crohn’s disease [12]).
A common classification for rectovaginal fistulas is currently not available. As a decision-making aid in daily routine, it is advisable to apply a classification that is based on rectal altitude localization of the fistula mouth. From this, a therapeutic relevance can also be derived. Anovaginal fistulas are referred to as type I and rectovaginal fistulas as type II [13]. The type II fistulas are further divided into low-lying and high-lying fistulas (Table 1).
Most guidelines related to surgical therapy recommend a perineal or local procedure for type I and type IIa fistulas, whereas an abdominal access route is required for the treatment of higher type IIb fistulas [14,15,16].
For the surgical treatment of the former group (type I and type IIa), the following types of closure are described in the S3 guidelines [12]: endorectal occlusions, transvaginal occlusions, transperineal occlusions, local flap techniques, muscle flaps, or occlusion techniques with interposition of biomaterials. The technical possibilities can be divided into “direct closures” and closures by means of an interpolated tissue.
Table 2 provides an overview of the various methods with their risk of recurrence, the hazards described, and a review of the selected literature [17,18,19,20,21,22,23,24,25,26,27,28,29,30].
The techniques with direct closure are associated with a much higher rate of applicational limitations. Thus, they are reserved for simple fistula courses and primary interventions. For more complex situations and in the case of recurrent interventions, tissue interpositions offer a significantly better healing tendency [14].
However, the subgroup in which biomaterials were used for interposition has not shown the hoped-for effect on definitive fistula repair (34% recurrence rate in 10 months of follow-up; [29]). By contrast, this technique showed a high rate of local complications (Clavien–Dindo III 70%; [30]). On the other hand, flaps with a perfused tissue interposition yield better results, with fistula recurrence rates of 8% for primary procedures with Martius lobes [23] and 25% for “re-do” procedures with gracilis-plasties [24]. They are superior to direct closure methods in this aspect.
However, the muscle flaps often show functional defects at the removal site. The muscle-free lobes (fasciocutaneous flaps) frequently lead to perineal pain and dyspareunia [24]. Furthermore, unsightly and stressful genital scars can occur.
An alternative for a fasciocutaneous flap-plasty is a “deepithelialized pudendal-thigh flap” (Singapore flap). This technique was originally developed in 1989 for the treatment of vaginal wall lesions and congenital defects [31]. In 2006, the pudendal thigh flap was first used by Malaysian surgeons to occlude rectovaginal fistulas [32].
On the basis of the data of seven consecutive cases, in which we used the Singapore flap for the closure of deep-lying rectovaginal fistulas, we present the advantages and the technical aspects of this method.
Material and methods
We conducted a retrospective analysis of all patients who were surgically treated with a de-epithelialized Singapore flap between May 2012 and July 2015 due to a recurrent or complex rectovaginal fistula of type I or type IIa. All operations were performed at the University Hospital Graz. The procedures were carried out in cooperation between the Department of General Surgery and the Department of Plastic Surgery. During the observation period, seven patients underwent this procedure. The data were collected retrospectively from the reports of the surgical follow-up examinations. In addition, a recent telephone survey was conducted. Six out of seven patients could be reached. Four questions were asked, which could be answered with “yes” or “no.”: (“Is there urinary incontinence?”; “Is there a fecal incontinence?”; “Do you suffer from pain during sexual intercourse?”; “Would you decide to have the operation performed again with today’s knowledge?”). In addition, two questions with several answers were asked: “Has your quality of life improved?” with the answer options of “no change,” “improved,” and “deteriorated”; and “Are you satisfied with your sex life?” with the answering options “yes,” “no,” “improved,” and “deteriorated”.
The surgical follow-up took place 6 weeks postoperatively and then after 3, 9, 15, and 27 months. Between month 27 and month 31, the follow-up care was terminated for all patients. Two patients were lost to follow-up. One of these patients could be reached as part of the telephone survey.
Description of the surgical procedure
All procedures were performed jointly by an experienced proctological surgeon and an experienced plastic surgeon (Table 3). The patients were placed in the lithotripsy position and a Foley catheter was inserted (Fig. 1).
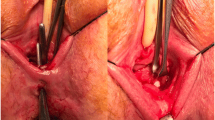
The perineal preparation is the second step. Here, after transverse perineal skin incision, the posterior vaginal wall is prepared. The preparation must be continued, at minimum 1.5–2 cm cranially of the vaginal fistula mouth. The space which is later needed for an optimal flap positioning is already created yet (Fig. 1).
After that, the vaginal fistula mouth can be closed. For this we use absorbable, poly-fil suture material, with a thickness of 2‑0 (Fig. 1).
Step four is the preparation of the rectal fistula mouth and the excision of the fistula duct. Usually this succeeds very well because the perineal and rectovaginal space is already opened and easily accessible at this time. The peri-fistular soft tissue cuff can be created generously, as sufficient inflammation-free tissue will be available through the later flap. The flap closure is again performed with poly-fil, absorbable suture material, with a thickness of 2‑0 (Fig. 1).
Now the flap is lifted. After lanceolate skin incision in the groin (clearly lateral of the equilateral labium majus), further preparation in the cranial wound angle has to be made down to the fascial layer. Once this landmark is reached, the area is prepared in a caudo-dorsal direction. Thus, the final entifications of the vascular/nerval bundle of the internal pudendal artery lies securely in the transposition flap (Fig. 2).
The sixth step is the de-epithelialization of the flap. This is carried out without the use of cauterization. Remaining bleedings are then sealed selectively (Fig. 2).
Subsequently, a short tunnel from the flaps sampling point into the preformed perineal cavity is created and then the flap is affixed with sutures in the ideal position. We also introduce fibrin glue because we believe that this prevents seroma formations and bleedings. However, the use of glue certainly depends on the respective routine and clinical philosophy regarding this procedure (Fig. 3).
As the eighth and final surgical step, the procedure is finished with a single-layer perineal skin closure and a two-layer skin closure at the removal site (Fig. 3).
All patients received preoperative emptying with mini-enema and perioperative antibiotics with piperacillin/tazobactam. A preoperative stoma system is not required but can off course be discussed for specific cases.
Results
During a period of 3 years (2012–2015), a total of seven patients were treated with a “de-epithelialized pudendal thigh flap” (Singapore flap). All patients had a rectovaginal fistula of type I or type IIa. Surgical care was conducted by a joint team consisting of a visceral surgeon and a plastic surgeon. Only one patient had no previous operations in the anamnesis. The mean age of the patients was 46.14 years (range 23–72). Five patients had already had a total of 12 previous proctological procedures, with the aim of local fistula remediation. Another patient had not yet had any previous fistula operation, but at the time of the operation there was stool deviation by means of stoma.
We achieved complete fistula healing in six cases (85.7%). In the area of the perineal wound as well as at the removal site of the flap, there were no surgical site infections. Overall, there were no postoperative complications in these six patients, whose condition was graded higher than Type I according to the Clavien–Dindo classification. The first planned follow-up took place after 6 weeks. The next follow-up visits were carried out in the 4th, 7th, 15th, and 27th month postoperatively. The follow-up ended after 31 months. During this period, none of the six patients experienced a fistula recurrence. Furthermore, no new proctological operations were performed on any of these patients during the observation period.
In one patient, the surgical method was not successful. This resulted in an early fistula recurrence within 3 weeks. The reason for this is an initially poor suture fixation of the lobe. In this patient, a stoma system was performed in an acute setting and then the flap was affixed again. The subsequent course was also unpleasant in this patient. Thus, two further local revisional interventions were necessary. In addition, the relocation of the stoma led to anastomotic insufficiency and the patient subsequently had to undergo further laparotomies (three times). In further examinations it was discovered that this patient had Crohn’s disease, which had gone unnoticed in the preoperative examinations.
No postoperative stool incontinence symptoms occurred in the remaining patients. One patient reported discomfort in regard to urinary incontinence, which had already been present preoperatively to the same extent. Two patients described their sex life as being restricted, but five out of six patients described an improvement in the same domain compared to the preoperative condition (83.3%). No dyspareunia was detected in the follow-up investigations and in the subsequent telephone survey (Table 4).
Referring to the current condition, all six patients who were reached by telephone stated they would decide to undergo the procedure again (Table 4).
The patient who already had a stoma treatment preoperatively, underwent successful colostomy reversal 6 months after the initial fistula repair.
One patient could not be reached for the telephone survey.
Discussion
The results of our work show very nicely that the pudendal thigh flap is a suitable method to treat deep-lying rectovaginal fistulas surgically. In our case series, we were able to achieve an optimal result in six out of seven patients. Only in one patient did the surgical technique prove to be inadequate. This patient suffered from a preoperatively unrecognized Crohn’s disease. The remaining patients all showed benefit from the treatment, although these were “re-do procedures” after multiple frustrating previous operations. Fistula operations after previous proctological procedures per se show an increased rate of fistula recurrences. In a large-scale meta-analysis in 2019, Mei et al. were able to identify previous proctological interventions as an independent risk factor for the development of a postoperative fistula recurrence (RR 1.52; [33]). This was also incorporated into an expert consensus paper and published in the International Journal of Surgery in 2021 [34]. With our method, we were able to achieve a permanent occlusion rate of 85.7% for this complex patient collective.
In addition to the low recurrence rate, further advantages are the minor soft tissue trauma and the absence of a functional deficit at the harvesting area of the flap. We also consider that the absence of a scar in the genital region is a benefit for the affected patients. From a surgical point of view, a tissue-strong flap-plasty between the anterior rectal wall and the vaginas posterior wall is often thought to be the reason for perineal discomfort and dyspareunia. In our patient population, a dyspareunia rate of 16.7% was found after using the slim and well-supplied Singapore flap. By comparison, dyspareunia is to be expected in 31% of cases with a flap supply using the Martius flap (McNevin et al.; [35]). In 2021 Hauch et al. (Annals of Plastic Surgery) described a dyspareunia rate of 36% [36] after plastic surgery with the aim of closing a rectovaginal fistula.
The rate of local surgical site infections was low. The six non-Crohn patients showed no wound healing disorders at all. Overall, we found a rate of surgical site infections of only 14.28% with our technique. This is even more remarkable since no patient had a stool-deviation via stoma prior to the fistula closure. Only one patient was a stoma carrier at the time of surgery. However, this had already been created before the first contact with our clinic. Garg et al. (World Journal of Clinical Cases) in 2021 reported a stoma rate of 63% with conventional occlusion techniques of anal fistulas [37]. However, this work was based on a study that included patients with and without Crohn’s disease.
All the patients we were able to reach by telephone would undergo this operation again for the aim of a fistula closure. Of course, this certainly reflects, in some way, the unpleasant and stressful preoperative situation. Nevertheless, this shows a prominent level of patient satisfaction with the method itself. None of the six patients surveyed reported a decrease in their quality of life. While one patient reported “no change” (16.7%), five patients reported an improvement (83.3%). The result is distorted by the fact that the patient who experienced the most complicated course could not be reached by telephone during the survey.
Encouragingly, the telephone survey has shown that no patient experienced fecal incontinence. Likewise, no urinary incontinence of new onset was detected. In one patient only, who had previously suffered from urinary incontinence, this was also found postoperatively.
Even though our results on the closure of rectovaginal fistulas by means of a de-epithelialized Singapore flap are very promising, the importance of the correct patient selection must be underlined. The height and the course of the fistula duct must be determined precisely, both radiologically with magnetic resonance imaging and endoscopically. This has to be done because, on the one hand, fistula courses, in which the rectal fistula mouth is located orally to the lower transverse fold (Houston’s valve), are not sufficiently treatable with this method. And on the other hand, to be aware of a complex and communicating fistula course preoperatively. Although van Buren et al. (Radiographics) were able to show the superiority of magnetic resonance imaging diagnostics in relation to rectovaginal fistulas [38], endoanal and endorectal ultrasound is of course also of immense importance. Ultrasound allows the examiner to gain a variety of information about the surgical area. However, this investigation requires appropriate expertise.
Furthermore, all efforts must be on ensuring that Crohn’s disease can be excluded preoperatively with a high degree of certainty. We experienced the hard way how important this is and how difficult it can be to exclude Crohn’s disease.
Limitations
Of course, our study has limitations. The patient population comprising seven patients is small. It is also a retrospective analysis, which has disadvantages per se. However, we were able to show the long-term effects and the sustainable success of our surgical method very well through a long follow-up period (31 months) and an additional telephone survey.
Conclusion
Altogether, our work shows that a de-epithelialized pudendal thigh flap is a good method to surgically treat patients with a type I or type IIa rectovaginal fistula without Crohn’s disease. Especially after previous fistula surgery, this method is a promising alternative that should be offered to affected patients. To perform this technique optimally, a proctological surgical unit and a plastic surgical unit with proven expertise in “flap surgery” must be available at the performing center.
References
Tsang CB, Rothenberger DA. Rectovaginal fistulas. Therapeutic options. Surg Clin North Am. 1997;77(1):95–114. https://doi.org/10.1016/s0039-6109(05)70535-1.
Leroy A, Azaïs H, Giraudet G, et al. Quality of life and symptoms before and after surgical treatment of rectovaginal fistula. Prog Urol. 2017;27(4):229–37. https://doi.org/10.1016/j.purol.2016.12.001.
Touhidi Nezhad F, Jalali R, Karimi F, et al. Women’s experiences of rectovaginal fistula: an ethno-religious experience. BMC Womens Health. 2020;20(1):130. https://doi.org/10.1186/s12905-020-00992-w.
Roush KM. Social implications of obstetric fistula: an integrative review. J Midwifery Womens Health. 2009;54(2):e21–e33. https://doi.org/10.1016/j.jmwh.2008.09.005.
Kpatcha TM, Wangala P, Botcho G, et al. Epidemiologic, anatomoclinic and therapeutic profil of urogenital and rectovaginal fistula in Togo. Prog Urol. 2020;30(11):597–603. https://doi.org/10.1016/j.purol.2020.06.008.
Rundasa DN, Wolde TF, Ayana KB, Worke AF. Awareness of obstetric fistula and associated factors among women in reproductive age group attending public hospitals in southwest Ethiopia, 2021. Reprod Health. 2021;18(1):183. https://doi.org/10.1186/s12978-021-01228-2.
Trovik J, Thornhill HF, Kiserud T. Incidence of obstetric fistula in Norway: a population-based prospective cohort study. Acta Obstet Gynecol Scand. 2016;95(4):405–10. https://doi.org/10.1111/aogs.12845.
Zoulek E, Karp DR, Davila GW. Rectovaginal fistula as a complication to a Bartholin gland excision. Obstet Gynecol. 2011;118(2 Pt 2):489–91. https://doi.org/10.1097/AOG.0b013e3182235548.
Narayanan P, Nobbenhuis M, Reynolds KM, et al. Fistulas in malignant gynecologic disease: etiology, imaging, and management. Radiographics. 2009;29(4):1073–83. https://doi.org/10.1148/rg.294085223.
Ommer A, Herold A, Berg E, et al. Cryptoglandular anal fistulas. Dtsch Arztebl Int. 2011;108(42):707–13. https://doi.org/10.3238/arztebl.2011.0707.
Schloericke E, Zimmermann M, Benecke C, et al. Surgical management of complicated rectovaginal fistulas and the role of omentoplasty. Tech Coloproctol. 2017;21(12):945–52. https://doi.org/10.1007/s10151-017-1657-1.
Ommer A, Herold A, Berg E, et al. S3-Leitlinie: Rektovaginale Fisteln (ohne M. Crohn). AWMF-Registriernummer: 088/004S3. Coloproctology. 2012;34:211–46.
Abendstein BJ. Rektovaginale Fisteln und ihre Behandlungsmöglichkeiten. J Urol Urogynäkol. 2009;16(4):5–9.
Studniarek A, Abcarian A, Pan J, et al. What is the best method of rectovaginal fistula repair? A 25-year single-center experience. Tech Coloproctol. 2021;25(9):1037–44. https://doi.org/10.1007/s10151-021-02475-y.
Raju R, Linder BJ, Bews KA, et al. Perioperative outcomes of rectovaginal fistula repair based on surgical approach: a national contemporary analysis. Female Pelvic Med Reconstr Surg. 2021;27(2):e342–e7. https://doi.org/10.1097/SPV.0000000000000924.
Hauch A, Ramamoorthy S, Zelhart M, et al. Refining approaches to surgical repair of rectovaginal fistulas. Ann Plast Surg. 2020;84(5S Suppl 4):S250–S6. https://doi.org/10.1097/SAP.0000000000002207.
Baig MK, Zhao RH, Yuen CH, et al. Simple rectovaginal fistulas. Int J Colorectal Dis. 2000;15(5–6):323–7. https://doi.org/10.1007/s003840000253.
Lowry AC, Thorson AG, Rothenberger DA, et al. Repair of simple rectovaginal fistulas. Influence of previous repairs. Dis Colon Rectum. 1988;31(9):676–8. https://doi.org/10.1007/BF02552581.
Yuan X, Chen H, Chen C, et al. Minimally invasive treatment of mid-low rectovaginal fistula: a transanal endoscopic surgery study. Surg Endosc. 2020;34(9):3971–7. https://doi.org/10.1007/s00464-019-07174-2.
Thayalan K, Krause H, Goh J. A retrospective case series on transvaginal repair of rectovaginal fistula performed by a urogynaecology operative team in Australia. Aust N Z J Obstet Gynaecol. 2021; https://doi.org/10.1111/ajo.13444.
Chong W, Liu T, Bui A. Incidence and risk factors for postoperative complications of rectovaginal fistula repairs, based on different surgical routes. Female Pelvic Med Reconstr Surg. 2021;27(1):e82–e90. https://doi.org/10.1097/SPV.0000000000000820.
Tsang CB, Madoff RD, Wong WD, Rothenberger DA, et al. Anal sphincter integrity and function influences outcome in rectovaginal fistula repair. Dis Colon Rectum. 1998;41(9):1141–6. https://doi.org/10.1007/BF02239436.
Gosselink MP, Oom DM, Zimmerman DD, et al. Martius flap: an adjunct for repair of complex, low rectovaginal fistula. Am J Surg. 2009;197(6):833–4. https://doi.org/10.1016/j.amjsurg.2007.07.023.
Cui L, Chen D, Chen W, et al. Interposition of vital bulbocavernosus graft in the treatment of both simple and recurrent rectovaginal fistulas. Int J Colorectal Dis. 2009;24(11):1255–9. https://doi.org/10.1007/s00384-009-0720-4.
Ruiz D, Bashankaev B, Speranza J, et al. Graciloplasty for rectourethral, rectovaginal and rectovesical fistulas: technique overview, pitfalls and complications. Tech Coloproctol. 2008;12(3):277–81. https://doi.org/10.1007/s10151-008-0433-7. discussion 281–282.
Picciariello A, Papagni V, De Fazio M, et al. Functional outcome and quality of life evaluation of graciloplasty for the treatment of complex recto-vaginal and recto-urethral fistulas. Updates Surg. 2020;72(1):205–11. https://doi.org/10.1007/s13304-020-00704-x.
Frontali A, Rottoli M, Chierici A, et al. Rectovaginal fistula: risk factors for failure after graciloplasty—a bicentric retrospective European study of 61 patients. Colorectal Dis. 2021;23(8):2113–8. https://doi.org/10.1111/codi.15673.
Göttgens KWA, Heemskerk J, van Gemert W, Smeets R, et al. Rectovaginal fistula: a new technique and preliminary results using collagen matrix biomesh. Tech Coloproctol. 2014;18(9):817–23. https://doi.org/10.1007/s10151-014-1145-9.
Neal Ellis C. Outcomes after repair of rectovaginal fistulas using bioprosthetics. Dis Colon Rectum. 2008;51(7):1084–8. https://doi.org/10.1007/s10350-008-9339-8.
Mege D, Frasson M, Maggiori L, et al. Is biological mesh interposition a valid option for complex or recurrent rectovaginal fistula? Colorectal Dis. 2016;18(2):O61–O5. https://doi.org/10.1111/codi.13242.
Wee JT, Joseph VT. A new technique of vaginal reconstruction using neurovascular pudendal-thigh flaps: a preliminary report. Plast Reconstr Surg. 1989;83(4):701–9. https://doi.org/10.1097/00006534-198904000-00018.
Sathappan S, Rica MAI. Pudendal thigh flap for repair of rectovaginal fistula. Med J Malaysia. 2006;61(3):355–7.
Mei Z, Wang Q, Zhang Y, et al. Risk factors for recurrence after anal fistula surgery: a meta-analysis. Int J Surg. 2019;69:153–64. https://doi.org/10.1016/j.ijsu.2019.08.003.
Mei Z, Li Y, Wang Q, et al. Risk factors for postoperative recurrence of anal fistula identified by an international, evidence-based Delphi consultation survey of surgical specialists. Int J Surg. 2021;92:106038. https://doi.org/10.1016/j.ijsu.2021.106038.
McNevin MS, Lee PYH, Bax TW. Martius flap: an adjunct for repair of complex, low rectovaginal fistula. Am J Surg. 2007;193(5):597–9. https://doi.org/10.1016/j.amjsurg.2007.01.009.
Hauch A, McKee RM, Li W‑Y, et al. Rectovaginal fistula repair 1 year later: lessons learned. Ann Plast Surg. 2021;87(2):187–93. https://doi.org/10.1097/SAP.0000000000002626.
Garg P, Yagnik VD, Dawka S. Fecal diversion in complex anal fistulas: Is there a way to avoid it? World J Clin Cases. 2021;9(25):7306–10. https://doi.org/10.12998/wjcc.v9.i25.7306.
Van Buren WM, Lightner AL, Kim ST, et al. Imaging and surgical management of anorectal vaginal fistulas. Radiographics. 2018;38(5):1385–401. https://doi.org/10.1148/rg.2018170167.
Funding
Open access funding provided by Medical University of Graz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Sauseng, J. Kresic, M. Mayerhofer, M.A. Ribeiro Skreinig, L.-P. Kamolz, S. Spendel, M. Schintler, A. Imamovic and J. Pfeifer declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sauseng, S., Kresic, J., Mayerhofer, M. et al. Surgical treatment of deep-lying ano-/rectovaginal fistulas using a de-epithelialized “Singapore flap” (pudendal thigh flap). Eur Surg 54, 136–143 (2022). https://doi.org/10.1007/s10353-022-00759-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-022-00759-7