Summary
Background
Schwannoma is a benign tumor arising from Schwann cells of the peripheral nerves. It is often asymptomatic and can develop in the retroperitoneum, mediastinum, head and neck region, and upper and lower extremities. Schwannoma of the abdominal wall is extremely rare, but differential diagnosis with malignant neoplasms is important to reduce the risk of undertreatment.
Methods
A narrative review of abdominal wall schwannoma was performed using PubMed, EMBASE, and Web of Science database and the search terms “schwannoma”, “neurinoma”, “neurilemmoma”, “soft tissue tumors”, “neurogenic tumor”, “rectus abdominis mass”, “abdominal wall”. In addition, the hospital charts were reviewed to report the personal experience.
Results
Only 9 single case-reports of benign schwannoma of the abdominal wall were found in the English medical literature over the past decade. None of the patients received preoperative biopsy and all were resected with clear margins. In addition to the literature review, we report the case of a 58-year-old man referred for a palpable mass in the left upper abdominal quadrant. Ultrasonography and magnetic resonance imaging revealed a solid and well-encapsulated mass inside the left rectus abdominis muscle. A core biopsy of the lesion provided the diagnosis of cellular schwannoma and this was confirmed by histopathologic examination of the surgical specimen.
Conclusions
Benign schwannoma of the abdominal wall is extremely rare. Percutaneous core needle biopsy is important for the differential diagnosis with more common and biologically more aggressive malignancies, such as desmoid tumors and sarcomas, and may be relevant for planning the most appropriate management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
-
Differential diagnosis of an abdominal wall mass should include malignant tumors (desmoid, soft tissue sarcoma, and dermatofibrosarcoma protuberans), benign tumors (schwannoma, lipoma, and fibromatosis), and pseudo-tumors (hematoma, myositis, and nodular fasciitis).
-
Preoperative histological diagnosis can easily be obtained by percutaneous core biopsy and is essential to define the appropriate surgical approach and to reduce the risk of undertreatment and local recurrence.
Introduction
Among tumors of the abdominal wall, desmoid tumors and sarcomas are the most represented [1, 2]. These are a heterogeneous group of malignant neoplasms with different biological behavior, metastatic potential, and rates of local recurrence after surgery. In contrast, schwannoma is a benign neurogenic tumor, also known as neurinoma or neurilemmoma, and represents approximately 5% of all benign soft-tissue neoplasms [3]. It is a slow-growing neoplasm, composed of Schwann cells, i.e., the glial cells forming the myelin sheath which is necessary for rapid propagation of the action potentials in nerve fibers [4]. Schwannoma is often asymptomatic, incidentally discovered during computed tomography (CT), and is usually located in the retroperitoneum, mediastinum, head and neck region, and upper and lower extremities. Schwannomas of the abdominal wall have rarely been reported. CT and magnetic resonance imaging (MRI) have emerged as the diagnostic modality of choice but the sensitivity remains low. A preoperative histological diagnosis obtained by percutaneous core needle biopsy has been considered essential to plan the correct surgical approach and reduce the risk of recurrence of soft tissue tumors [5]. Aim of this study was to analyze the literature and to discuss a new case of benign, cellular schwannoma of the abdominal wall.
Materials and methods
A literature review was performed to identify all English-written published articles on schwannoma of the abdominal wall in PubMed, EMBASE, and Web of Science database, using the keywords “schwannoma”, “neurinoma”, “neurilemmoma”, “soft tissue tumors”, “neurogenic tumor”, “rectus abdominis mass”, “abdominal wall”, with “AND” and “OR”. Based on the 2020 World Health Organization (WHO) classification of soft tissue tumors, the cases of schwannoma with malignant characteristics or incorporated in new recognized entities and subtypes, were excluded [6, 7].
We also consulted the hospital electronic database to find the records of patients admitted for treatment of abdominal wall tumors. Demographic and clinical patient data were extracted from the hospital charts. Written informed consent was obtained from patients.
Results
Our literature search yielded 12 single case reports published between 2001 and March 2021, and 9 of these were included in the review [8,9,10,11,12,13,14,15,16]. According to the WHO classification of soft tissue tumors, we excluded 3 case reports (1 schwannoma with melanocytic differentiation, 1 malignant, 1 with low malignant potential) [17,18,19].
The main demographic and clinical characteristics of the patients included in the review are summarized in Table 1. The median age was 52 (range 22–70), and the sex distribution was similar. The tumor was more frequently located in the left anterolateral upper abdominal quadrant (44.5%), and pain was reported by 6 (66.6%) patients. No preoperative biopsy was performed. Histology of the surgical specimen showed an “ancient” morphologic variant in 4 patients (44.4%). Follow-up was reported in 4 patients and ranged from 1 to 12 months. No local recurrences were reported.
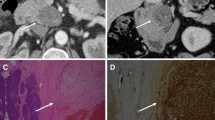
In addition to the narrative review, we analyzed the clinical characteristic of a 58-year-old man referred for surgical evaluation of a palpable mass in the proximal portion of the left rectus abdominis muscle, just below the costal margin. The patient noted a progressive enlargement of the mass, not associated with pain, paresthesia or other symptoms. Medical history included hypertension, type 2 diabetes, and coronary artery disease with previous angioplasty and stent placement. On physical examination, a large oval-shaped solid mass with regular borders was palpable. Ultrasonography demonstrated a hypoechoic area inside the left rectus abdominis muscle, well-encapsulated and with regular borders and vascularization. MRI with gadolinium confirmed the presence of 64 × 42 × 30 mm mass, with low intensity signal on T1-weighted images, heterogeneous increased signal intensity on T2-weighted images, hyperintensity on diffusion-weighted images with corresponding hypointensity on apparent diffusion coefficient images (Fig. 1a). An ultrasound-guided core needle biopsy of the lesion showed spindle-cell proliferation, without evidence of dysplasia and malignancy, and immunohistochemistry revealed a diffusely cellular expression of S100 protein, suggesting the diagnosis of schwannoma. Laboratory tests and oncologic markers were within normal ranges. Under general anesthesia, a 5 cm incision over the mass was performed. The tumor was identified just below the left rectus abdominis sheath and was completely resected with preservation of both anterior and posterior muscle sheaths (Fig. 1b,c). Macroscopically, it appeared as a well-demarcated and solid tumor, without cystic degeneration. Microscopic findings revealed proliferation of spindle cells with twisted nuclei, arranged in intersecting or long fascicles. The tumor was composed exclusively of Antoni A areas, without cellular palisades, Verocay bodies or Antoni B areas (Fig. 2a). Mitotic activity was low (2 mitoses/10 hpf [high power field]), with mild nuclear atypia despite the high cellularity of the neoplasm. Immunohistochemically, the tumor displayed diffuse and strong positivity for S100 protein and SOX-10, diffuse positivity for CD34, negativity for c‑Kit, DOG‑1, calretinin, EMA (Epithelial Membrane Antigen), desmin, and TTF‑1, and retained the nuclear expression of Histone (H3K27me3) (Fig. 2b,c). The positivity for CD34, the intense positivity for S100 and SOX-10 and the negativity for c‑Kit, DOG1 and calretinin excluded the diagnosis of gastrointestinal stromal tumor and neurofibroma and were compatible with the diagnosis of “cellular schwannoma”. The post-operative course was regular. At 6‑month follow-up, the patient was doing well and an abdominal wall ultrasound revealed no signs of tumor recurrence.
Discussion
This study shows that schwannoma of the abdominal wall is an extremely rare tumor, with only few cases reported in the literature over the past decade. Sporadic schwannomas represent approximately 5% of all benign soft-tissue tumors; however, distinct autosomal-dominant diseases, such as neurofibromatosis‑2 and schwannomatosis, predispose to the growth of multiple tumors [20]. Most commonly, schwannoma develops in the retroperitoneum (32%), mediastinum (23%), head and neck region (18%), and upper and lower extremities (16%) [21], but cases of schwannoma in the thoracic [22] and abdominal wall [8,9,10,11,12,13,14,15,16,17,18,19] are also reported.
Benign schwannomas are often asymptomatic and may be incidentally discovered in a CT scan [8]; however, pain, paresthesia or weakness due to nerve compression may occur, depending on tumor size and location. Ultrasonography is the first-line diagnostic test for superficial soft tissue schwannomas. CT and MRI with gadolinium contrast permit a more detailed study, but still do not allow to distinguish with certainty schwannomas from other benign and malignant tumors. MRI can provide additional information on the cleavage planes. A homogenous isointense or low intensity signal relative to skeletal muscle on T1-weighted images, and a heterogeneous increased signal intensity with biphasic pattern on T2-weighted images are typical MRI features [23,24,25,26]. The eccentric position in relation to the nerve, the fusiform shape, the split-fat sign, and the MRI “target sign” can be useful in differentiating schwannoma from other neurogenic tumors [27]. However, sensitivity and specificity rates for diagnosis of malignant peripheral nerve sheath tumors (MPNST) based on imaging alone are 61% and 90%, respectively [28]. Therefore, a preoperative histological diagnosis remains essential for planning appropriate management. Percutaneous core needle biopsy is a simple and safe procedure, and can differentiate malignant from benign soft tissue tumors with a 96.3% sensitivity, 99.4% specificity, and 97.6% accuracy. Moreover, tumors subtype were correctly identified in 89.5% of benign tumors and 88.0% of soft tissue sarcomas [5]. This is critical, since desmoid and sarcoma still represent 45% and 40% of all abdominal wall neoplasms, respectively, and require a full-thickness abdominal wall resection with wide margin of normal tissue [29]. On the contrary, schwannoma requires a more conservative surgical approach, with a negligible risk of local recurrence in case of complete tumor removal [2, 30, 31].
Currently, the term malignant schwannoma is obsolete. Rather, tumors deriving from Schwan cells are now defined as malignant peripheral nerve sheath tumors (MPNST) and those with melanocytic differentiation are defined malignant melanotic nerve sheath tumors (MMMST). These tumors have an aggressive clinical behavior, early age at presentation, and are frequently associated with autosomal dominant syndromes [6, 7, 32]. Macroscopically, schwannoma appears as a well-circumscribed mass with rounded or ovoid shape and smooth surface. At microscopic analysis two alternating patterns of growth, “Antoni A” and “Antoni B” are usually described. The highly cellular “Antoni A” areas are composed of spindle cells organized to form long bundles with nuclear palisading and occasionally “Verocay bodies”, formed by two compact rows of well-aligned nuclei separated by fibrillary cell processes. The myxoid “Antoni B” pattern is looser, less ordered, with areas of microcystic and xanthomatous change, inflammatory cells, and hyalinization with partially thrombosed vessels. Mitotic activity is rare. Immunohistochemically, schwannoma demonstrates diffuse positivity for S100 protein and SOX-10 [33, 34]. Several morphologic variants of schwannoma are well recognized, including cellular schwannoma, plexiform schwannoma, epithelioid schwannoma, “ancient” schwannoma, and mixed forms of schwannoma with neurofibroma. The “Antoni A” and “Antoni B” areas are present in different proportion [35]. In our case, the tumor was composed exclusively of Antoni A areas. Schwannomas composed exclusively or predominantly of “Antoni A” areas are defined “cellular schwannomas”, characterized by a high cellularity, fascicular pattern, occasional nuclear hypercromasia and atypia, and mitotic activity (usually <4 mitoses/10 hpf). The histopathological findings of degenerative changes such as calcification, interstitial fibrosis, hemosiderin deposition, cystic and vascular hyaline degeneration characterize another subtype of schwannoma, defined as “ancient” [8, 9, 11, 13, 16, 27]. The histological features of high cellularity, fascicular pattern, occasional nuclear hypercromasia and atypia, mitotic activity can lead to an erroneous diagnosis of MPNST. However, the cellularity too high compared to the number of mitoses, the perivascular hyalinization, occasional “Antoni B” areas and strong positivity for S100 protein and SOX-10, suggest a benign diagnosis. Since well-differentiated leiomyosarcoma can also show nuclear palisading aspects and positivity for S100 protein, SOX-10 positivity, which is never expressed in leiomyosarcoma, is useful for the differential diagnosis [36].
In conclusion, we reported a rare case of cellular schwannoma of the abdominal wall and compared our findings and principal features of this benign tumor with the current literature. We underlined the importance of preoperative imaging and percutaneous core needle biopsy to avoid undertreatment or overtreatment. Patients who are preoperatively diagnosed with desmoid tumor or sarcoma of the abdominal wall should preferably be managed in referral centers where a multidisciplinary approach can be provided by a specialized team.
References
Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10.000 patients with soft tissue sarcoma. Ann Surg. 2014;260:416–21.
Smith HG, Tzanis D, Messiou C, et al. The management of soft tissue tumours of the abdominal wall. Eur J Surg Oncol. 2017;43(9):1647–55.
Kransdorf MJ. Benign soft-tissue tumors in a large referral population: distribution of specific diagnoses by age, sex, and location. AJR Am J Roentgenol. 1995;164(2):395–402.
Salzer JL. Schwann cell myelination. Cold Spring Harb Perspect Biol. 2015;7(8):a20529.
Strauss DC, Qureshi YA, Hayes AJ, et al. The role of core needle biopsy in the diagnosis of suspected soft tissue tumours. J Surg Oncol. 2010;102(5):523–9.
Choi JH, Ro JY. The 2020 WHO classification of tumors of soft tissue: selected changes and new entities. Adv Anat Pathol. 2021;28(1):44–58.
Kallen ME, Hornick JL. The 2020 WHO classification: what’s new in soft tissue tumor pathology? Am J Surg Pathol. 2021;45(1):e1–e23.
Bhatia RK, Banerjea A, Ram M, et al. Benign ancient schwannoma of the abdominal wall: an unwanted birthday present. BMC Surg. 2010;10:1.
Mishra A, Hamadto M, Azzabi M, et al. Abdominal wall schwannoma: case report and review of the literature. Case Rep Radiol. 2013;2013:456863.
Balzarotti R, Rondelli F, Barizzi J, et al. Symptomatic schwannoma of the abdominal wall: a case report and review of the literature. Oncol Lett. 2015;9(3):1095–8.
Ginesu GC, Puledda M, Feo CF, et al. Abdominal wall Schwannoma. J Gastrointest Surg. 2016;20(10):1781–3.
Basu S. Benign schwannoma of the abdominal wall: a rare case report and a review of literature. Ind J Case Rep. 2018;4(3):250–2.
Lam R, Hunt BL, Arreola-Owen O. Abdominal Wall Schwannoma. Fed Pract. 2019;36(3):129–33.
Paramythiotis D, Pagkou D, Moysidis M, et al. External oblique muscle Schwannoma: a rare anatomical presentation. Case Rep Surg. 2019; https://doi.org/10.1155/2019/9290821.
Tarchouli M, Essarghini M, Qamouss O, et al. Abdominal wall schwannoma: a case report. Gastroenterol Hepatol Bed Bench. 2020;13(1):95–100.
Alsahwan AG, Felemban JM, Al-Othman A, et al. Symptomatic abdominal wall Schwannoma mimicking infected Subcutanous soft tissue lesion. A case report. Int J Surg Case Rep. 2021;81:105751. https://doi.org/10.1016/j.ijscr.2021.105751.
Lazaros GA, Panagiotides HC, Ioakimidis DE, et al. A muscle mass in a patient with polymyalgia rheumatica. J Clin Rheumatol. 2001;7(6):395–9.
Khorgami Z, Nasiri S, Rezakhanlu F, et al. Malignant schwannoma of anterior abdominal wall: report of a case. J Clin Med Res. 2009;1(4):233–6.
Liu Y, Chen X, Wang T, et al. Imaging observations of a schwannoma of low malignant potential in the anterior abdominal wall: a case report. Oncol Lett. 2014;8(3):1159–62.
Evans DG, Bowers NL, Tobi S, et al. Schwannomatosis: a genetic and epidemiological study. J Neurol Neurosurg Psychiatry. 2018;89(11):1215–9.
White W, Shiu MH, Rosenblum MK, et al. Cellular schwannoma. A clinicopathologic study of 57 patients and 58 tumors. Cancer. 1990;66(6):1266–75.
Urakawa T, Kawakita N, Nagahata Y. A case of benign schwannoma of the thoracic wall mimicking a malignant tumor. Kobe J Med Sci. 1993;39(4):123–31.
Koga H, Matsumoto S, Manabe J, et al. Definition of the target sign and its use for the diagnosis of schwannomas. Clin Orthop Relat Res. 2007;464:224–9.
Beaman FD, Kransdorf MJ, Menke DM. Schwannoma: radiologic-pathologic correlation. Radiographics. 2004;24(5):1477–81.
Murphey MD, Smith WS, Smith SE, et al. From the archives of the AFIP. Imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlation. Radiographics. 1999;19(5):1253–80.
Lin J, Martel W. Cross-sectional imaging of peripheral nerve sheath tumors: characteristic signs on CT, MR imaging, and sonography. AJR Am J Roentgenol. 2001;176(1):75–82.
Albert P, Patel J, Badawy K, et al. Peripheral nerve Schwannoma: a review of varying clinical presentations and imaging findings. J Foot Ankle Surg. 2017;56(3):632–7.
Wasa J, Nishida Y, Tsukushi S, et al. MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. AJR Am J Roentgenol. 2010;194(6):1568–74.
Stojadinovic A, Hoos A, Karpoff HM, et al. Soft tissue tumors of the abdominal wall. Analysis of disease patterns and treatment. Arch Surg. 2001;136:70–9.
Wilder F, D’Angelo S, Crago AM. Soft tissue tumors of the trunk: management of local disease in the breast and chest and abdominal walls. J Surg Oncol. 2015;111(5):546–52.
Pencavel T, Strauss DC, Thomas JM, et al. The surgical management of soft tissue tumours arising in the abdominal wall. Eur J Surg Oncol. 2010;36(5):489–95.
James AW, Shurell E, Singh A, et al. Malignant peripheral nerve sheath tumor. Surg Oncol Clin N Am. 2016;25(4):789–802.
Elder DE, Massi D, Scolyer RA, Willemze R. WHO classification of skin tumours: WHO classification of tumours. 4th ed. Vol. 11;2020.
Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. WHO classification of tumours of soft tissue and Bone: WHO classification of tumours. 4th ed. Vol. 5;2020.
Meyer A, Billings SD. What’s new in nerve sheath tumors. Virchows Arch. 2020;476(1):65–80.
Pekmezci M, Reuss DE, Hirbe AC, et al. Morphologic and immunohistochemical features of malignant peripheral nerve sheath tumors and cellular schwannomas. Mod Pathol. 2015;28(2):187–200.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Panici Tonucci, A. Sironi, E. Pisa, B. Di Venosa and L. Bonavina declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Internal review board approval HSD 2020-077.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Panici Tonucci, T., Sironi, A., Pisa, E. et al. Schwannoma of the abdominal wall: updated literature review. Eur Surg 54, 74–79 (2022). https://doi.org/10.1007/s10353-021-00720-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-021-00720-0