Abstract
The wild boar (Sus scrofa) is a mammal with a broad distribution in the Eurasian territory and a potential reservoir for several zoonotic pathogens. Besides being part of the Mediterranean ecosystem and perpetuating these agents in the environment, this species is usually consumed in the Iberian Peninsula, representing a potential public health threat. Due to its extensive expansion and colonization of new geographical areas, which leads to increasing contact with humans, domestic animals, and wildlife, infectious disease assessments are crucial. During the last two decades (2001–2021), several researchers have studied wild boars to identify, understand, and predict potential health risks and disease outbreaks in animals and humans. North-eastern, central-eastern (mainly because of tuberculosis), and southern Portugal were the regions where most pathogens have been identified. Some agents with zoonotic importance (but with few reported data) should be the focus of future surveillance studies, such as Leptospira spp., Brucella spp., or Trichinella spp. This review aims to summarize the available information on pathogens (bacteria, viruses, and parasites) reported in wild boars, in Portugal, in the last two decades, with a particular focus on agents with zoonotic potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wildlife studies represent an essential part of infectious disease surveillance since most emerging zoonotic diseases come from wildlife (71.8%) (Jones et al. 2008; Figueiredo et al. 2020). Therefore, disease-tracing investigations, laboratory diagnostics, and molecular analysis performed in wild species are fundamental to understanding zoonotic diseases’ epidemiology and implementing public human and animal health measures (Vieira-Pinto et al. 2011a).
The Eurasian wild boar (Sus scrofa) is a mammalian species with wide distribution among the European and Asian continents, including Portugal. In the nineteenth century, wild boar populations suffered a considerable decrease. However, like other European ungulates, they are now massively increasing. The absence of wild boar predators, the growing population, and the abandonment of rural areas led to overabundant populations in some areas of Portugal (Lopes and Fonseca Borges 2004; Fredriksson-Ahomaa 2019). This game species presents remarkable adaptability to different habitats and food resources associated with a high reproductive capacity, contributing to the continuous occupation of new geographic regions (Fredriksson-Ahomaa 2019). Besides this natural expansion in Portugal, translocations between Portuguese hunting reserves and reintroductions from Spain have been particularly common in the last few decades (Lopes and Fonseca Borges 2004).
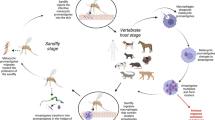
As a game species, wild boars are considered one of the best surveillance targets in Mediterranean habitats due to the accessibility for sampling and their abundance, as previously mentioned (Muñoz-Mendoza et al. 2013). Several authors have been discussing the role of wild boars as sources of zoonotic diseases worldwide, especially regarding globally recognized diseases such as tuberculosis, trichinellosis, or brucellosis (Meng and Lindsay 2009; Brown et al. 2018). Therefore, Portuguese researchers have been studying this species, reporting zoonotic agents for the first time (Mateus et al. 2021) or deepening our knowledge about the epidemiology of already-recognized pathogens (Santos et al. 2015). Figure 1 illustrates some examples and shedding and/or transmission pathways of several zoonotic pathogens. Most authors have also raised awareness of the importance of implementing public health measures to reduce the number of infections of wildlife origin (Vieira-Pinto et al. 2011a; Figueiredo et al. 2020; Mateus et al. 2021).
Locations and excretion pathways of some wild boar infectious agents (created with BioRender.com)
This review aims to summarize the available information on different agents isolated in S. scrofa in Portugal, considering the studies of the last two decades (2001–2021), with a particular focus on agents with zoonotic potential. To the authors’ knowledge, no similar (or recent) review has been made exclusively in Portugal, even though different general surveys and reviews have been done in different European countries and regions (Ruiz-Fons et al. 2008; Boadella et al. 2012; González-Barrio et al. 2015; Malmsten et al. 2018). Thus, the authors found an opportunity to develop this review.
Methods
Scopus®, PubMed®, and Google Scholar® were used to obtain an initial database of wild boar zoonoses reported in Portugal between 2001 and 2021 and start performing this review. An initial search was done in English and Portuguese, with the keywords: wild boar, Sus scrofa, zoonosis, zoonotic, and Portugal. All types of papers (original articles, book chapters, technical reports, and short communications) were included in our database of references. Articles unrelated to the subject or presenting information regarding other geographical areas were excluded from the database. Only articles containing information from the last two decades (2001–2021) were considered. Nevertheless, a few general references to some pathogens were promptly used to have a more complete perspective of some diseases and add relevant details.
Results and discussion
After carefully revising the published literature on the subject, the authors have found several relevant agents to be addressed in this article, due to their importance in public health or wildlife population health. A list of relevant agents reported in S. scrofa in Portugal is presented in Table 1.
Bacteria
In some southern-European countries, the wild boar is a critical host of animal tuberculosis (Mycobacterium bovis). Particularly in Portugal, wild boars are pointed as crucial in the surveillance and control of this disease (Duarte et al. 2008; Santos et al. 2009; Aranha et al. 2021), and some studies have been investigating this disease and drawing attention to this problem under a One Health approach. Molecular methods evidenced a predominant cluster between 2012 and 2016 in the Rosmaninhal area, near Castelo Branco, where this species contributed the most in relative risk (Reis et al. 2020). Also in Castelo Branco district, in Idanha-a-Nova, wild boars presented a higher bovine tuberculosis infection rate than the red deer (Cervus elaphus). Spoligotyping methods suggested transmission among different species (including livestock). Therefore, most authors believe that wild boars are more critical in the emerging and remerging of this disease than other ungulates, as red deers (Vieira-Pinto et al. 2011a). Group interactions between females contribute to an intraspecific spread of the disease. Furthermore, territorial expansion and uninspected translocations of wild boars between hunting areas (some of them in close contact with livestock holdings) contribute to the emergence of the disease in other regions and to interspecific spread (Santos et al. 2009). Recently, the first study using Geographic Information Systems and geostatistical analyses to evaluate the spatial–temporal occurrence of tuberculosis in large game was performed in Portugal. This study consistently confirmed what previous authors had pointed out, i.e., that wild boar acts as tuberculosis reservoir and plays a vital role in disease dispersion (Aranha et al. 2021). M. caprae and M. avium subsp. paratuberculosis were also isolated in wild boars in Portugal (Duarte et al. 2008; Matos et al. 2013).
Leptospirosis is a zoonotic disease caused by spirochaetes from the genus Leptospira, and S. scrofa seems to play a significant role in the epidemiology of this disease. In Northern Portugal, antibodies against nine serovars of Leptospira spp. were identified in wild boars. Moreover, these authors reported provenance and age as possible risk factors. Concretely, in Bragança, the seropositivity rate was significantly higher than in Vila Real, and adults present the highest seropositivity rate in the region of Trás-os-Montes, northern Portugal (Vale-Goncąlves et al. 2015).
Salmonellosis is a food-borne disease whose nomenclature is often complex due to the variety of species, subspecies, and serotypes belonging to the gender Salmonella (Starkey and Donnelly 2012). Vieira-Pinto et al. (2011b, c) reported a prevalence of 22.1% (17/77) of Salmonella spp. in S. scrofa fecal samples, identifying two distinct serovars: Salmonella typhimurium and Salmonella rissen.
Brucella suis is a zoonotic agent with a total of five biovars. Wild boars are a common source of the Brucella suis biovar 2 for outdoor domestic pigs in Europe, and this biovar has been detected in wild boars from Portugal (Ferreira et al. 2014, 2017). Humans can become infected by contact with infected animals, aerosols, or consuming contaminated food. Even though biovar 2 is recognized as having a low pathogenic potential in humans compared to other serovars or Brucella species, clinical disease was reported in hunters (Fredriksson-Ahomaa 2019). According to Muñoz et al. (2010), wild ungulates do not seem to generally represent a vital threat as brucellosis reservoirs in the Iberian Peninsula. However, wild boar is a significant hazard regarding B. suis biovar 2, which is particularly relevant to manage outdoor pig farms in Spain and Portugal.
Lyme borreliosis, caused by Borrelia burgdorferi sensu lato (s.l.) complex, is the most frequent tick-borne disease in Europe. Its notification is mandatory in Portugal, though some authors mentioned it as an underdiagnosed and underreported disease (Faria et al. 2015; Pereira et al. 2016). B. afzelii was first amplified from wild boar serum in Northern Portugal in 2015, confirming that wild boars are potential reservoirs for this bacteria (Faria et al. 2015). However, another study performed by Pereira et al. (2016) in the Center and South of Portugal found no agents of this complex in wild boars.
The same authors found a prevalence of 13.3% of Anaplasma spp., another tick-borne bacteria, in Beja district (Pereira et al. 2016). These authors suggested that, depending on the studied areas, wild ungulates (including the wild boar) could or could not play an essential role in maintaining these bacteria (Pereira et al. 2016). The relation between human anaplasmosis and the its presence in wildlife remains unclear. Even though A. phagocytophilum is highly prevalent in in ticks and several wild and domestic species in Europe, human granulocytic anaplasmosis clinical cases are rarely reported compared to other parts of the world. However, some cases could have been misdiagnosed or not reported (Matei et al. 2019).
Wild animals can also shed to the environment another tick-borne bacteria: Coxiella burnetii. Even though domestic animals seem to be a more significant source of infection to humans, this highly depends on the geographic region and wild boar densities. Regarding the wild boar, infections during hunting activities should not be neglected (Anastácio 2019; Espí et al. 2021). Coxiella burnetii causes Q fever, an endemic disease in Portugal and Spain, a disease obligatory to notify since 1999 (Palmela et al. 2012; Espí et al. 2021).
Other bacteria, as Corynebacterium ulcerans, were only identified in Black Alentejano pig (S. scrofa domesticus) in Portugal. In this case, the agent was first identified as C. pseudotuberculosis by biochemical tests, electrophoresis, and PCR. However, after genome sequencing, it was reclassified as C. ulcerans (Oliveira et al. 2014; Viana et al. 2020). No reports were found in wild boars in Portugal, but infections associated to C. ulcerans and C. silvaticum were reported in other countries, as Germany (Eisenberg et al. 2014; Viana et al. 2020).
Virus
For the first time in Portugal, a study using wild boar carcasses for human consumption reported the presence of the hepatitis E virus (HEV), currently named Paslahepevirus balayani, in the liver (Mesquita et al. 2016). According to these authors, strains belonging to HEV genotype 3 subgenotype e are commonly present in swine and pointed to as important reservoirs for human disease. A very recent study in Spain suggests a high exposure of endangered Iberian lynx (Lynx pardinus) to this virus (specially HEV-3), being the wild boar the main wildlife reservoir of this disease in the country (Caballero-Gómez et al. 2022).
Porcine hokovirus, also known as porcine parvovirus 4, is intimately related to human parvovirus 4/5. Some authors mentioned a close relationship and common origin between these two agents. This agent was discovered in Northern Portugal in wild boar populations (Miranda et al. 2016). Even though its zoonotic potential is not fully recognized, authors suggest further research to understand this disease’s geographical distribution and epidemiology in the Iberian peninsula (Miranda et al. 2016).
Other relevant suid viruses include African swine fever (ASF) virus, classical swine fever (CSF) virus, and Aujeszky’s disease virus. In Portugal, the last ASF and CSF outbreak was in 1999 and 1985, respectively (DGAV 2018, 2022a). Particularly for ASF, a case was reported in September 2020 in a wild boar carcass in Germany (Federal Ministry of Food and Agriculture 2022). Aujeszky’s disease has a strict control plan in Portugal, including the wild boar in a big game health plan. Hunted wild boars are often analyzed for antibodies testing against Aujeszky’s disease (DGAV 2022b).
Parasites
Trichinella is a genus of parasites that infects a wide range of hosts from different taxonomic groups; animals are infected by ingesting tissues containing infective larvae. Wild boars represent the second primary source of Trichinella spp. to humans globally. In 2018, Trichinella spp. was identified in wild boars in Portugal, with 2 positive animals out of 739 (0.3%). Vieira-Pinto et al. (2021), who first reported T. britovi in wild boars in Portugal, mentioned that the number of animals analyzed in Portugal (n = 739) is much lower than in other countries, as Spain (n = 99,472). These authors believe that the number of tested animals is insufficient to give a trustworthy idea of the actual prevalence of this disease. Notwithstanding, consuming wild boars’ meat without proper cooking represents a significant health risk for humans (EFSA 2018; Vieira-Pinto et al. 2021).
Another potential danger from eating raw or undercooked boar meat is toxoplasmosis (Toxoplasma gondii). In Portugal, significant seroprevalences have been detected in wild boar: 100% and 20.6%, respectively, in 2011 (Lopes et al. 2011) and 2014 (Coelho et al. 2014). As intermediate hosts, wild boars play a relevant role in this disease’s epidemiology and its transmission to humans. Possibly, wild boars (and other wildlife) are exposed to sporulated oocysts due to the ingestion of water and food contaminated with felid feces, the definitive hosts of T. gondii (Lopes et al. 2011; Coelho et al. 2014).
In 2013, Echinococcus intermedius (Echinococcus granulosus “pig strain,” G7) was found in wolves in Portugal, and it was firstly hypothesized the importance of domestic pigs and wild boars in its transmission to wolves (Guerra et al. 2013). In 2021, Echinococcus ortleppi was identified in wild boar in Portugal (and in Europe) by Mateus et al. (2021). The taxonomic organization and terminology of Echinococcus spp. is still under development. However, most authors classify E. ortleppi as part of the E. granulosus s. l. complex, responsible for cystic echinococcosis. Even though Portugal is considered endemic for this disease, no reports have been made specifically on wildlife (Eckert et al. 2002; Mateus et al. 2021).
Some coprological studies detected parasites in wild boars, as Trichuris suis or Ascaris suum. The zoonotic potential of these agents is still being discussed in the scientific community (Nejsum et al. 2012). Nevertheless, the pathogenicity of these agents is a matter of concern, especially in nearby pig farms, due to the risk of introducing these agents in these facilities (Dos Santos 2013; Bernardino 2017). Moreover, regarding protozoans, Eimeria spp. and Balantidium coli have been identified more than once in wild boars parasitology assessments (Dos Santos 2013; Figueiredo et al. 2020).
Fasciolosis is an emerging or re-emerging zoonotic disease worldwide. In Galicia (NW Spain), wild boar is assumed as a possible reservoir of Fasciola hepatica, due to a high prevalence of infection, the normal development of adult forms in the liver, and the viability of embryonated eggs (Mezo et al. 2013). These authors also suggested the role of this species as a secondary reservoir, with the potential to infect intermediate hosts. In Portugal, in Tapada Nacional de Mafra (TNM), De Sousa et al. (2003) reported a high prevalence of F. hepatica (60.8%) in wild boars. In TNM, wild boars live in a fenced area, cohabiting it with fallow deers (Dama dama), which generally have a high prevalence of this parasitosis, probably resulting in cross-species parasite transmission. Although pig fasciolosis is common in geographical regions outside Europe (particularly South America and Africa), it is considered a rare disease in the European continent in both domestic pigs and wild boars (Mas-Coma et al. 2003).
A tick-borne protozoan, Theileria spp., responsible for piroplasmosis, was identified in 20% of the wild boars (n = 65) from Beja district. Besides its zoonotic potential, this agent may cause substantial economic losses in livestock, being S. scrofa one of the ungulates responsible for its maintenance in the south of Portugal (Pereira et al. 2016, 2018).
Ixodes ricinus (Faria et al. 2015), Hyalomma lusitanicum, and Rhipicephalus sanguineus (Dos Santos 2013) are ectoparasites and vectors of different agents mentioned in the previous paragraphs, which have been reported in Portugal.
Finally, considering Sarcocystis spp., a high prevalence (73.8%) has been found in wild boars from north-eastern Portugal. However, all positive cases were identified as S. miescheriana (which is not reported as a zoonotic agent) (Coelho et al. 2015).
Conclusions
This review highlights the importance of continuous monitoring of the wild boar populations in the Portuguese territory, not only because of the variety of agents mentioned but also due to the significant significance of this species as a reservoir of multiple diseases. It is imperative to implement health measures when working with these wild animals, consuming their meat or during hunting activities, and several authors suggested it in their studies. These measures include (but are not limited to) a health inspection of the carcasses as soon as possible, implement good individual practices and the use of protective equipment when eviscerating the carcasses, and prohibit the disposal of viscera into the environment (or its consumption by dogs and other animals), by creating a program to collect those by-products (Figueiredo et al. 2020; Koutsoumanis et al. 2020; Reis et al. 2020; Gomes-Neves et al. 2021; Mateus et al. 2021; Vieira-Pinto et al. 2021).
As illustrated in Fig. 2, the north-eastern, central-eastern (particularly for tuberculosis), and southern Portugal should be part of intensive and continuous monitoring of relevant pathogens, also due to the abundance of wild boar’s populations and the proximity to other ungulates (as the red deer or roe deer) or livestock. Some agents with zoonotic importance (but with little data) should be the focus of future surveillance studies as Leptospira spp., Brucella spp., or Trichinella spp. Notwithstanding, the variety of agents already detected in this species in Portugal, during the last two decades, reveals the importance of further research and continuous monitoring of wild boar populations, under an One Health perspective.
Spatial distribution of some of the wild boar’s infectious diseases (2001–2021) in Portugal (de Sousa et al. 2003; Santos et al. 2009; Muñoz et al. 2010; Lopes et al. 2011; Vieira-Pinto et al. 2011a, b, 2021; Cunha et al. 2012; Soeiro 2013; Coelho et al. 2014; Faria et al. 2015; Vale-Goncąlves et al. 2015; Pereira et al. 2016; Mesquita et al. 2016; Miranda et al. 2016; Bernardino 2017; Anastácio 2019; Figueiredo et al. 2020; Aranha et al. 2021; Mateus et al. 2021) (Created with BioRender.com)
References
Adlhoch C, Kaiser M, Ellerbrok H, Pauli G (2010) High prevalence of porcine hokovirus in German wild boar populations. Virol J 7:1–4. https://doi.org/10.1186/1743-422X-7-171/FIGURES/1
Anastácio SF (2019) Coxiella burnetii and Q fever an emergent zoonosis in Portugal? - Doctoral Thesis, Faculdade de Farmácia da Universidade de Coimbra, Coimbra, Portugal
Aranha J, Abrantes AC, Gonçalves R et al (2021) GIS as an epidemiological tool to monitor the spatial–temporal distribution of tuberculosis in large game in a high-risk area in Portugal. Animals 11:2374. https://doi.org/10.3390/ANI11082374
Atreya R, Bülte M, Gerlach G-F et al (2014) Facts, myths and hypotheses on the zoonotic nature of Mycobacterium avium subspecies paratuberculosis. Int J Med Microbiol 304:858–867. https://doi.org/10.1016/j.ijmm.2014.07.006
Bernardino S (2017) Estudos sobre parasitismo gastrointestinal e pulmonar em javalis e veados caçados em montarias do centro e sul de portugal continental. Faculdade de Medicina Veterinária - ULisboa
Boadella M, Ruiz-Fons JF, Vicente J et al (2012) Seroprevalence evolution of selected pathogens in Iberian wild boar. Transbound Emerg Dis 59:395–404. https://doi.org/10.1111/J.1865-1682.2011.01285.X
Brown VR, Bowen RA, Bosco-Lauth AM (2018) Zoonotic pathogens from feral swine that pose a significant threat to public health. Transbound Emerg Dis. https://doi.org/10.1111/tbed.12820
Caballero-Gómez J, Rivero-Juarez A, Zorrilla I et al (2022) Hepatitis E virus in the endangered Iberian lynx (Lynx pardinus). Transbound Emerg Dis. https://doi.org/10.1111/tbed.14624
Coelho C, Gomes J, Inácio J et al (2015) Unraveling Sarcocystis miescheriana and Sarcocystis suihominis infections in wild boar. Vet Parasitol 212:100–104. https://doi.org/10.1016/j.vetpar.2015.08.015
Coelho C, Vieira-Pinto M, Faria AS et al (2014) Serological evidence of Toxoplasma gondii in hunted wild boar from Portugal. Vet Parasitol 202:310–312. https://doi.org/10.1016/j.vetpar.2014.03.013
Cortez Nunes F, Letra Mateus T, Taillieu E et al (2022) Molecular detection of Helicobacter spp. and Fusobacterium gastrosuis in pigs and wild boars and its association with gastric histopathological alterations. Vet Res 53:78. https://doi.org/10.1186/s13567-022-01101-5
Cunha MV, Matos F, Canto A et al (2012) Implications and challenges of tuberculosis in wildlife ungulates in Portugal: a molecular epidemiology perspective. Res Vet Sci 92:225–235. https://doi.org/10.1016/j.rvsc.2011.03.009
De Sousa CB, Madeira De Carvalho L, Fazendeiro I et al (2003) Contribution for the knowledge of wild boar (Sus scrofa L.) helmintic fauna in Tapada Nacional de Mafra, an enclosured hunting area. Rev Iber Parasitol 64
Dias D, Caetano T, Torres RT et al (2019) Shiga toxin-producing Escherichia coli in wild ungulates. Sci Total Environ 651:203–209. https://doi.org/10.1016/j.scitotenv.2018.09.162
Direção Geral de Alimentação e Veterinária (DGAV) (2018) Peste suína africana. https://www.dgav.pt/animais/conteudo/animais-de-producao/suinos/saude-animal/doencas-dos-suinos/peste-suina-africana/. Accessed 20 Jun 2022
Direção Geral de Alimentação e Veterinária (DGAV) (2022a) Peste Suína Clássica. https://www.dgav.pt/animais/conteudo/animais-de-producao/suinos/saude-animal/doencas-dos-suinos/peste-suina-classica/. Accessed 20 Jun 2022a
Direção Geral de Alimentação e Veterinária (DGAV) (2022b) Doença de Aujeszky. https://www.dgav.pt/animais/conteudo/animais-de-producao/suinos/saude-animal/doencas-dos-suinos/doenca-de-aujeszky/. Accessed 20 Jun 2022b
Dos Santos D (2013) Caracterização do parasitismo de ungulados silvestres e aspectos da sua epidemiologia na tapada nacional de mafra, concelho de mafra. Universidade de Lisboa, Portugal
Duarte EL, Domingos M, Amado A, Botelho A (2008) Spoligotype diversity of mycobacterium bovis and mycobacterium caprae animal isolates. Vet Microbiol 130:415–421. https://doi.org/10.1016/j.vetmic.2008.02.012
Eckert J, Gemmell MA, Meslin F-X, Pawłowski ZS (2002) World Health Organization World Organisation for Animal Health WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern (Eckert J et al. (eds)). World Organisation for Animal Health. https://iris.who.int/handle/10665/42427
EFSA (2018) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA Journal 16. https://doi.org/10.2903/J.EFSA.2018.5500
Eisenberg T, Kutzer P, Peters M, Sing A, Contzen M, Rau J (2014) Nontoxigenic tox-bearing Corynebacterium ulcerans infection among game animals, Germany. Emerg Infect Dis 20(3):448–452
Espí A, Del Cerro A, Oleaga Á et al (2021) One health approach: an overview of Q fever in livestock, wildlife and humans in Asturias (northwestern Spain). Animals 11:1–12. https://doi.org/10.3390/ani11051395
Faria AS, Paiva-Cardoso MN, Nunes M et al (2015) First detection of Borrelia burgdorferi sensu lato DNA in serum of the wild boar (Sus scrofa) in Northern Portugal by nested-PCR. EcoHealth 12:183–187. https://doi.org/10.1007/s10393-014-0973-4
Federal Ministry of Food and Agriculture (2022) African swine fever (ASF): information on cases in Germany. In: BMEL. https://www.bmel.de/EN/topics/animals/animal-health/african-swine-fever.html. Accessed 20 Jun 2022
Ferreira AC, Tenreiro R, Corrêa de Sá MI, Dias R (2014) Complete genome sequences of three Iberian Brucella suis biovar 2 strains isolated from wild boars. Genome Announc 2. https://doi.org/10.1128/GENOMEA.00618-14
Ferreira AC, Tenreiro R, da Sá MIC, Dias R (2017) Evolution and genome specialisation of Brucella suis biovar 2 Iberian lineages. BMC Genomics 18. https://doi.org/10.1186/s12864-017-4113-8
Figueiredo AM, Valente AM, Fonseca C et al (2020) Endoparasite diversity of the main wild ungulates in Portugal. Wildlife Biol 2020. https://doi.org/10.2981/wlb.00657
Fredriksson-Ahomaa M (2019) Wild boar: a reservoir of foodborne zoonoses. Foodborne Pathog Dis 16:153–165. https://doi.org/10.1089/FPD.2018.2512
Gomes-Neves E, Abrantes AC, Vieira-Pinto M, Müller A (2021) Wild game meat—a microbiological safety and hygiene challenge? Current Clinical Microbiology Reports 2021 8:2 8:31–39. https://doi.org/10.1007/S40588-021-00158-8
Gonçalves D, Pereira-Vaz J, Duque V et al (2018) First serological evidence on endemicity of HEV infection in wild boar (Sus scrofa) populations from Portugal. Virol Sin 33:197–200. https://doi.org/10.1007/s12250-018-0008-3
González-Barrio D, Martín-Hernando MP, Ruiz-Fons F (2015) Shedding patterns of endemic Eurasian wild boar (Sus scrofa) pathogens. Res Vet Sci 102:206–211. https://doi.org/10.1016/J.RVSC.2015.08.014
Guerra D, Armua-Fernandez MT, Silva M, Bravo I, Santos N, Deplazes P, Madeira de Carvalho LM (2013) Taeniid species of the Iberian wolf (Canis lupus signatus) in Portugal with special focus on Echinococcus spp. Int J Parasitol Parasites Wildl 2:50–53. https://doi.org/10.1016/j.ijppaw.2012.11.007
Jones KE, Patel NG, Levy MA et al (2008) Global trends in emerging infectious diseases. Nature 451:7181/451:990–993. https://doi.org/10.1038/nature06536
Koutsoumanis K, Allende A, Alvarez-Ord A et al (2020) Evaluation of public and animal health risks in case of a delayed post-mortem inspection in ungulates. EFSA Journal 18:e06307. https://doi.org/10.2903/J.EFSA.2020.6307
Lopes AP, Sargo R, Rodrigues M, Cardoso L (2011) High seroprevalence of antibodies to Toxoplasma gondii in wild animals from Portugal. Parasitol Res 108:1163–1169. https://doi.org/10.1007/s00436-010-2158-6
Lopes FJV, Fonseca Borges JM (2004) Wild Boar in Portugal Galemys 16:243–251
Malmsten A, Magnusson U, Ruiz-Fons F et al (2018) A serologic survey of pathogens in wild boar (Sus scrofa) in Sweden. 54:229–237. https://doi.org/10.7589/2017-05-120
Mas-Coma S, Bargues MD, Valero MA, Fuentes M, v, (2003) Adaptation capacities of Fasciola hepatica and their relationships with human fascioliasis: from below sea level up to the very high altitude. In: Combes C, Jourdane J (eds) Taxonomy, ecology and evolution of metazoan parasites. Presses Universitaires de Perpignan, Perpignan, pp 81–123
Matei IA, Estrada-Peña A, Cutler SJ et al (2019) A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasit Vectors 12:599. https://doi.org/10.1186/s13071-019-3852-6
Mateus TL, Gargaté MJ, Vilares A et al (2021) First report of echinococcus ortleppi in free-living wild boar (Sus scrofa) from Portugal. Microorganisms 9. https://doi.org/10.3390/microorganisms9061256
Matos AC, Figueira L, Martins MH et al (2013) Granulomatous lesions and Mycobacterium avium subsp. paratuberculosis in Portuguese Wild Boars (Sus scrofa). J Comp Pathol 148:85. https://doi.org/10.1016/J.JCPA.2012.11.158
Meng XJ, Lindsay DS (2009) Wild boars as sources for infectious diseases in livestock and humans. Philosophical Transactions of the Royal Society b: Biological Sciences 364:2697–2707. https://doi.org/10.1098/RSTB.2009.0086
Mesquita JR, Oliveira RMS, Coelho C et al (2016) Hepatitis E virus in sylvatic and captive wild boar from Portugal. Transbound Emerg Dis 63:574–578. https://doi.org/10.1111/TBED.12297
Mezo M, González-Warleta M, Castro-Hermida JA et al (2013) The wild boar (Sus scrofa Linnaeus, 1758) as secondary reservoir of Fasciola hepatica in Galicia (NW Spain). Vet Parasitol 198:274–283. https://doi.org/10.1016/J.VETPAR.2013.09.009
Miranda C, Coelho C, Vieira-Pinto M, Thompson G (2016) Porcine hokovirus in wild boar in Portugal. Arch Virol 161:981–984. https://doi.org/10.1007/s00705-015-2730-6
Muñoz PM, Boadella M, Arnal M et al (2010) Spatial distribution and risk factors of Brucellosis in Iberian wild ungulates. BMC Infect Dis 10:1–14. https://doi.org/10.1186/1471-2334-10-46/TABLES/5
Muñoz-Mendoza M, Marreros N, Boadella M et al (2013) Wild boar tuberculosis in Iberian Atlantic Spain: a different picture from Mediterranean habitats. BMC Vet Res 9. https://doi.org/10.1186/1746-6148-9-176
Nejsum P, Betson M, Bendall RP et al (2012) Assessing the zoonotic potential of Ascaris suum and Trichuris suis: looking to the future from an analysis of the past. J Helminthol 86:148–155. https://doi.org/10.1017/S0022149X12000193
Oliveira M, Barroco C, Mottola C et al (2014) First report of Corynebacterium pseudotuberculosis from caseous lymphadenitis lesions in black Alentejano pig (Sus scrofa domesticus). BMC Vet Res 10:1–5. https://doi.org/10.1186/S12917-014-0218-3/FIGURES/2
Palmela C, Badura R, Valadas E (2012) Acute Q fever in Portugal. Epidemiological and clinical features of 32 hospitalised patients. Germs 2:43. https://doi.org/10.11599/GERMS.2012.1013
Pereira A, Parreira R, Cotão AJ et al (2018) Tick-borne bacteria and protozoa detected in ticks collected from domestic animals and wildlife in central and southern Portugal. Ticks Tick Borne Dis 9:225–234. https://doi.org/10.1016/j.ttbdis.2017.09.008
Pereira A, Parreira R, Nunes M et al (2016) Molecular detection of tick-borne bacteria and protozoa in cervids and wild boars from Portugal. Parasit Vectors 9. https://doi.org/10.1186/s13071-016-1535-0
Reis AC, Tenreiro R, Albuquerque T et al (2020) Long-term molecular surveillance provides clues on a cattle origin for Mycobacterium bovis in Portugal. Sci Rep 10. https://doi.org/10.1038/s41598-020-77713-8
Ruiz-Fons F, Segalés J, Gortázar C (2008) A review of viral diseases of the European wild boar: effects of population dynamics and reservoir rôle. Vet J 176:158–169. https://doi.org/10.1016/J.TVJL.2007.02.017
Santos N, Almeida V, Gortázar C, Correia-Neves M (2015) Patterns of Mycobacterium tuberculosis-complex excretion and characterisation of super-shedders in naturally-infected wild boar and red deer. Vet Res 46. https://doi.org/10.1186/s13567-015-0270-4
Santos N, Correla-Neves M, Ghebremichael S et al (2009) Epidemiology of Mycobacterium bovis infection in wild boar (Sus scrofa) from Portugal. J Wildl Dis 45:1048–1061. https://doi.org/10.7589/0090-3558-45.4.1048
Soeiro V (2013) Pseudorabies and tuberculosis: serologic evidence of infection in wild boar populations in southeast Portugal and associated risks for Iberian lynx conservation. Universidade de Évora
Starkey SR, Donnelly TM (2012) Salmonellosis. Clinical veterinary advisor: birds and exotic pets 726–729. https://doi.org/10.1016/B978-1-4160-3969-3.00422-4
Vale-Goncąlves HM, Cabral JA, Faria MC et al (2015) Prevalence of Leptospira antibodies in wild boars (Sus scrofa) from Northern Portugal: risk factor analysis. Epidemiol Infect 143:2126–2130. https://doi.org/10.1017/S0950268814003331
Viana MVC, Profeta R, da Silva AL et al (2020) Taxonomic classification of strain PO100/5 shows a broader geographic distribution and genetic markers of the recently described Corynebacterium silvaticum. PLoS One 15. https://doi.org/10.1371/JOURNAL.PONE.0244210
Vieira-Pinto M, Fernandes ARG, Santos MH, Marucci G (2021) Trichinella britovi infection in wild boar in Portugal. Zoonoses Public Health 68:103–109. https://doi.org/10.1111/zph.12800
Vieira-Pinto M, Alberto J, Aranha J et al (2011a) Combined evaluation of bovine tuberculosis in wild boar (Sus scrofa) and red deer (Cervus elaphus) from Central-East Portugal. Eur J Wildl Res 57:1189–1201. https://doi.org/10.1007/s10344-011-0532-z
Vieira-Pinto M, Morais L, Caleja C et al (2011b) Salmonella sp. in game (Sus scrofa and Oryctolagus cuniculus). Foodborne Pathog Dis 8:739–740. https://doi.org/10.1089/fpd.2010.0742
Vieira-Pinto M, Morais L, Caleja C et al (2011c) Salmonella spp. in wild boar (Sus scrofa): a public and animal health concern. Game meat hygiene in focus 131–136. https://doi.org/10.3920/978-90-8686-723-3_10
Funding
Open access funding provided by FCT|FCCN (b-on). This work was supported by National Funds by FCT—Portuguese Foundation for Science and Technology (FCT) under the grant 2021.04520.BD. The authors of the research unit CITAB (Jota Baptista, C. and Oliveira, P.A.) received funding from FCT; reference of the project UIDB/04033/2020. The author of the research unit CECAV (Seixas, F.) received funding from FCT; reference of the projects UIDB/CVT/00772/2020 and LA/P/0059/2020.
Author information
Authors and Affiliations
Contributions
Conceptualization and writing—original draft preparation: Catarina Jota Baptista; writing—review and editing and supervision: Fernanda Seixas, José M. Gonzalo-Orden, and Paula A. Oliveira.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jota Baptista, C., Seixas, F., Gonzalo-Orden, J.M. et al. Wild boar (Sus scrofa) as a potential reservoir of infectious agents in Portugal: a review of two decades (2001–2021). Eur J Wildl Res 69, 101 (2023). https://doi.org/10.1007/s10344-023-01732-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-023-01732-9