Abstract
Usutu virus (USUV) is a mosquito-borne virus belonging to the family Flaviviridae, genus Flavivirus. Natural transmission cycle of USUV involves mosquitoes and birds, so humans and other mammals are considered incidental hosts. In this study, USUV infection was diagnosed in all wild blackbirds, collected from July to September 2018 in a wildlife recovery center in the province of Bologna, in the Emilia-Romagna region, northern Italy. All blackbirds showed neurological clinical signs, such as overturning, pedaling, and incoordination. Moreover, the subjects died shortly after arriving at the hospitalization center. Virological investigations were performed by real-time PCR on frozen samples of the spleen, kidney, myocardium, and brain for the detection of Usutu (USUV) and West Nile (WNV) viruses. The small and large intestine were used as a matrix for the detection of Newcastle disease virus (NDV). All 56 subjects with neurological clinical signs were positive for USUV, only one subject (1.8%) tested positive for WNV, and no subject was positive for NDV. The most represented age class was class 1 J (58.9%), followed by class 3 (25.0%), and lastly from class 4 (16.1%). Most of the blackbirds before dying were in good (51.8%) and fair (39.3%) nutritional status, while only five subjects (8.9%) were cachectic. The USUV genomes detected in the blackbirds of this study fall within the sub-clade already called EU2 that has been detected since 2009 in the Emilia-Romagna region. Neurological clinical signs in USUV-affected blackbirds are still widely discussed and there are few works in the literature. Although our results require further studies, we believe them to be useful for understanding the clinical signs of Usutu virus in blackbirds, helping to increase the knowledge of this zoonotic agent in wild species and to understand its effect on the ecosystem. The goal of this study was to report—in the context of the regional passive surveillance program—the detection of USUV RNA in its most important amplifying host, the common blackbird, when showing clinical signs before death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Usutu virus (USUV) is a mosquito-borne virus belonging to the family Flaviviridae, genus Flavivirus. USUV is related to some important human pathogens, like Japanese encephalitis virus, Murray Valley encephalitis virus, Saint Louis encephalitis virus, and West Nile virus (WNV) (Bakonyi et al. 2004). Similarly to other flaviviruses, USUV is a spherical, small, enveloped virus with a single-stranded positive-sense RNA genome of ~ 12 kb (Vilibic-Cavlek et al. 2020). Its natural transmission cycle involves mosquitoes and birds, so humans and other mammals are considered “dead-end” incidental hosts (Vilibic-Cavlek et al. 2020). It was first identified in South Africa in 1959 (Woodall 1964), but received little attention until its emergence in Austria in 2001 (Weissenböck et al. 2002). USUV was later detected in other countries and now is considered endemic in at least 16 European countries (Vilibic-Cavlek et al. 2020). In Italy, it was first detected in 1996 on a retrospective analysis of archived tissue samples coming from dead birds of the Tuscany region, central Italy (Weissenböck et al. 2013). In the summers between 2006 and 2008, USUV infections were confirmed in two wild blackbirds and three captive owls in the Lombardy region, northwestern Italy (Manarolla et al. 2010). Phylogenetic analysis revealed 99.8–100% nucleotide identity of the Italian USUV strains in relation to those from other Central European countries (Manarolla et al. 2010). In 2007, a seroconversion was reported in sentinel chickens in the Emilia-Romagna region, northeastern Italy (Lelli et al. 2008). In the same region, other studies conducted in 2009 proved a co-circulation of USUV and WNV in wild birds and mosquitoes (Calzolari et al. 2010; Tamba et al. 2011).
Though bird mortalities were not reported in Africa, in Europe USUV appeared to be pathogenic for several bird species, especially blackbirds (Turdus merula), order Passeriformes, and great gray owls (Strix nebulosa), order Strigiformes (Weissenböck et al. 2002; Bakonyi et al. 2007; Manarolla et al. 2010). The disease in birds is characterized by encephalitis, myocardial degeneration, marked splenomegaly, mild hepatomegaly, and pulmonary hyperemia. Neurological clinical signs in USUV-affected blackbirds are still discussed and there are few works in the literature. This is due to the difficulty of finding wild birds in nature with clinical signs.
The zoonotic potential of USUV has been reported in a growing number of human cases and needs to be considered (Cadar et al. 2017). Clinical cases of neuroinvasive disease and USUV fever, as well as seroconversion in blood donors, were reported in Europe since 2009 (Vilibic-Cavlek et al. 2020). A study by Grottola et al. (2017) highlighted that USUV infection may not be a sporadic event in humans, with 6.57% of patients who developed USUV antibodies in a province (Modena) adjacent to the study area of this work. So, researching for the death diagnosis of wild birds’ unusual mortality events—that proved to be a useful practice to detect the virus circulation—must be maintained and encouraged, due to the importance of the health surveillance of this potentially zoonotic virus.
This study was conducted as part of the regional passive surveillance program, which is performed every year in the Emilia-Romagna region to verify the circulation of Flavivirus and Paramyxovirus in avifauna.
The study’s goal was to report the detection of USUV RNA in its most important amplifying host (Clè et al. 2019), the common blackbird, when showing clinical signs before death, contributing to increase the knowledge of zoonotic agents in wild species and understand their effect on the ecosystem.
Materials and methods
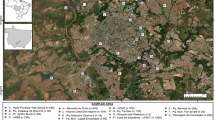
The study involved 56 wild blackbirds (Turdus merula) collected between July 1 and September 20, 2018 in the province of Bologna, mainly in green areas, e.g., garden and small urban parks, in Bologna (Fig. 1). The study area map was created using the open-source software QGIS 3.10. and edited with the software Inkscape.
The alive subjects were easily caught by hand because of their inability to fly and their clinical signs, i.e., overturning, pedaling, and incoordination. All of them died a few minutes after arriving at the recovery center. Dead animals were frozen at − 20 °C by the recovery center and delivered to the Department of Veterinary Medical Sciences of the University of Bologna.
Upon arrival of each bird, a form containing the identification number of the subject, coordinates of the place of collection, date of collection, age class (estimated by observing the plumage and body development using the Euring code), and nutrition status was filled out (Table 1). After external inspection the carcass was positioned in the dorsal position, then the subcutis was unglued to examine the pectoral muscles and evaluate the nutritional status (Table 1), which was estimated based on visibility and palpability of the sternal keel. In case “good status,” the sternal keel was not visible, in case “fair status,” it appeared slightly visible and palpable, and in case “cachectic status,” it was protruding with − atrophy of the pectoral muscles.
We proceeded opening the coelomic cavity and exposing the thoracic and abdominal cavity. After inspecting the cavities and the conditions of the internal organs, sex was determined observing the gonads and then recorded (Table 1). For some young subjects (class 1 J) with immature gonads, it was not possible to record the sex, thus they were identified as “not determinable” (“ND”). After this investigation, spleen, kidney, intestines, and myocardium were collected and stored at −80 °C until the biomolecular tests. The skull was then opened using the parietal bone as an opening point and exerting pressure with scissors between the foramen magnum and the supraoccipital bone. After observing the potential gross pathological findings on the brain structures, the autopsy was completed with sampling the brain and storing it at −80 °C until biomolecular tests.
The spleen, kidney, myocardium, and brain were tested for WNV and USUV genomes, while small and large intestines for NDV (Newcastle Disease Virus) genome.
Biomolecular tests
The organ samples (myocardium, brain, kidney, and spleen) were pooled and tissue specimens were homogenized in 25 ml of PBS in a Stomacher Lab-Blender 80 mL (INTERSCIENCE—France) for 2 min. Viral RNA was extracted from tissue homogenate in 96-well plates using the BioSprint® 96 One-For-All Vet kit (Qiagen) and the BioSprint 96 workstation (Qiagen) according to the manufacturer’s instructions. The RNA extracted was tested by real-time RT-PCRs to detect WNV (Tang et al. 2006; Del Amo et al. 2013) and USUV RNAs (Cavrini et al. 2011). The samples tested positive were further tested with traditional PCRs to obtain amplicons for sequencing: they were submitted to a Pan-flavivirus protocol targeting the NS5 gene (Scaramozzino et al. 2001) and to two specific protocols directed to the gene E of WNV (Lanciotti et al. 2000) and USUV (Manarolla et al. 2010). The amplicons were Sanger sequenced, and the obtained sequences (including the two primers) were aligned and inspected to detect mutations. The consensus sequence was then utilized for a BLAST search (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Subsequently, then sequences obtained in this work were aligned with MAFFT (Katoh et al. 2019) with selected homologous sequences of USUV deposed in GenBank. Sequences not covering the entire length of our amplicons were excluded. We selected the sequences according to geographic origin, favoring sequences detected multiple times in different areas, identical sequences were screened with the ElimDupes tool (https://www.hiv.lanl.gov/content/sequence/elimdupesv2/elimdupes.html). Maximum likelihood trees were constructed by IQ-TREE software (Nguyen et al. 2015) with the ultrafast bootstrap approximation (Minh et al. 2013) and visualized by iTOL (Letunic and Bork 2021). We selected the best-fit substitution models with Modelfinder (Kalyaanamoorthy et al. 2017), obtaining the K3P + R2 for the NS5 dataset and TNe + G4 for the E dataset.
To diagnose and pathotype NDV directly in intestines, we performed the method based on RT-PCR and pyrosequencing analysis described by De Battisti et al. (2013).
Results
All blackbirds tested negative for NDV and tested positive for USUV RNA, one blackbird (1.8%) was positive for both WNV and USUV (Table 1).
In this study, the sampling was concentrated mainly in August with 32 blackbirds (57.1%) examined, followed by July with 13 subjects (23.2%), and finally September with 11 blackbirds (19.6%) (Table 1).
The most represented age class was class 1 J (33/56—58.9%), consisting of blackbirds fledged but flying so weakly that they couldn’t have flown far from the nest, followed by class 3 (14/56—25.0%) represented by subjects that certainly hatched during the current calendar year, and lastly from class 4 (9/56—16.1%), subjects born before that, but the exact year is unknown (Table 1). Additionally, most of the blackbirds before dying were in good (29/56—51.8%) and fair (22/56—39.3%) nutritional status, while only five subjects (5/56—8.9%) were cachectic (Table 1).
Biomolecular results
Twenty-five amplicons of the USUV NS5 gene (215 bp) and 30 of the E gene (425 bp) were obtained and analyzed. All the NS5 sequences were similar and only three synonymous mutations were registered. One of these was represented by an A in position 93 in two sequences, while the others all carried a G. The presence of a G in position 93 was common in this group but is absent in homologous sequences deposed in the GenBank database. The most similar sequences present in it derive from mosquitoes of the Emilia-Romagna region (2009–2010), with an identity of 99.59% with our consensus sequence (GB accessions: HM138707, HM138708, HM138710, HM138714, HM138718, JF834546, JF834549, JF834550, JF834556, JF834560).
In the partial gene E sequences, two mutations were found, one of which is not synonymous, i.e., C > A in position 320 that causes a threonine > asparagine substitution in the amino acid sequence. This mutation is not present in other sequences deposited in GenBank: the most similar sequences in it, with a 100% identity with our consensus sequence, are those coming from Emilia-Romagna mosquitoes in 2010 (GB accessions: JF834599, JF834606, JF834623, JF834626, JF834673) and from one human case in the same region in 2009 (GB accession: JF826447).
In the two phylogenetic trees, obtained by the NS5 and E datasets, the sequences obtained in this work were grouped, with other Italian sequences, in a well-supported branch within the European clade (EU) (Fig. 2). Both datasets indicate that the genomes detected in blackbirds are very similar to each other and belong to the EU2 sub-clade of USUV that has been detected since 2009 in the Emilia-Romagna region (Calzolari et al. 2017).
Midpoint rotted maximum likelihood trees obtained by the NS5 (a) and E datasets (b). The bootstraps over 70% was shown near the nodes. The GenBank accessions were reported with numerosity and origin of identical sequences in GB. In the two phylogenetic trees, the sequences obtained in this work were grouped, with other Italian sequences, in a well-supported branch within the European clade (EU)
Discussion
Passive surveillance on synanthropic birds is essential for the early detection of mosquito transmitted Flavivirus. We examined 56 wild blackbirds collected from a wildlife recovery center in three provinces in northern Italy between July and September 2018. We observed that the blackbirds died in good and fair nutritional conditions, with a higher occurrence in the 1 J age group and female subjects. The greater involvement of the 1 J class and so of the younglings can be justified by a greater exposure to mosquito bites for they are unable to fly, lack of a complete plumage, and have a higher susceptibility due to the maternal antibodies decay.
The genomes detected in the blackbirds of this study fall within the sub-clade already called EU2 (Fig. 2), as most of the viruses identified in the Emilia-Romagna region during the epidemiological surveillance (Calzolari et al. 2017), indicating so the probable persistence of the virus in this region over the years. This data seems confirmed by the presence of mutations typical of the EU2 sub-clade and not found in other deposited sequences.
All the animals examined showed neurological clinical signs, like pedaling, overturning, and incoordination. Of the blackbirds, 91.1% were in good nutritional state, this supports the hypothesis of an acute and fast process. Clinical signs are rarely described in the USUV’s mortality studies about wildlife due to the rapid disease process (Steinmetz et al. 2011) and the difficulty of collecting wild animals (Chvala et al. 2007). Some studies hypothesized that the neurological disorders preceding death were due to USUV by detecting it in the affected animals’ tissues (Steinmetz et al. 2011; Garigliany et al. 2017; Weidinger et al. 2020).
The USUV, despite being able to infect various species of birds, was confirmed to be an extremely pathogenic virus particularly for blackbirds, with a consequent negative impact on the species’ population trend. Lühken et al. (2017) estimated USUV infection in southwestern Germany caused high mortality rates in blackbirds, calculating their further decrease of the 15.7%, while no effect was found in 14 other bird species. More data on avian hosts are needed to clarify the epidemiology of USUV, investigate the ecological consequences and understand if the sharp decline in avian hosts could increase the risk of infection in humans. The high mortality rate among blackbirds might increase the chance of USUV spillover into humans because the decrease of the natural host’s population could induce a modification of feeding pattern in mosquitoes (Kilpatrick et al. 2006; Molaei et al. 2006). We must also consider how climate change, causing an increase of the disease’s vectors and of the pressure on the susceptible species, which play an important role in the epidemiology of vector-borne diseases (Paz 2015).
We confirmed the detection of the USUV genome in all blackbirds deceased with neurological clinical signs. The cause of death was probably multiorgan failure and brain lesions may have also played a key role (Chvala et al. 2004).
Unfortunately, we could not analyze fresh subjects with cytological and histopathological techniques, observe the pathological findings of a USUV infection in tissues and cells, or IHC technique that allows detecting the virus presence within affected tissues. This study focuses on the detection of viruses circulating in Italy (USUV, WNV, and NCD), sought within the regional passive surveillance program, and which can cause neurological clinical signs in avifauna. For further studies, it would be useful to search for other pathogens that can generate overlapping clinical pictures (e.g., Avian malaria or other Flaviviruses circulating in other countries, such as the Bagaza virus—BAGV), associating cytological, histopathological, and IHC techniques to the molecular methods.
We reported a case study in a limited area and with a convenience sampling within the regional passive surveillance program. This approach is commonly adopted for this type of study (e.g., Austria, Chvala et al. 2007; Becker et al. 2012). However, it should be emphasized that based on the limitations of the opportunistic sampling, we could not make any claims about the USUV inference and incidence in space or time.
Despite our findings call for further studies and have sampling limits, we deem them important as the Usutu virus positivity found in all subjects with clinical signs can indicate a mechanism through which USUV might affect bird mortality. By undermining the functionality of the neural system, we believe USUV might increase the risk of bird collisions with the surrounding environment and anthropogenic elements (e.g., buildings, cars). Understanding the mechanisms of transmission, the pathogenicity and the clinical symptomatology of Usutu virus in blackbirds would help to increase the knowledge of this zoonotic agent in wild species and understand its effect on the ecosystem.
Change history
24 July 2022
Missing Open Access funding information has been added in the Funding Note.
References
Bakonyi T, Gould EA, Kolodziejek J, Weissenböck H, Nowotny N (2004) Complete genome analysis and molecular characterization of Usutu virus that emerged in Austria in 2001: comparison with the South African strain SAAR-1776 and other Flaviviruses. Virology 328:301–310
Bakonyi T, Erdelyi K, Ursu K, Ferenczi E, Csorgo T, Lussy H, Chvala S, Bukovsky C, Meister T, Weissenbock H et al (2007) Emergence of Usutu virus in Hungary. J Clin Microbiol 45:3870–3874
Becker N, Jöst H, Ziegler U, Eiden M, Höper D, Emmerich P, Fichet-Calvet E, Ehichioya DU, Czajka C, Gabriel M, Hoffmann B, Beer M, Tenner-Racz K, Racz P, Günther S, Wink M, Bosch S, Konrad A, Pfeffer M, Groschup MH, Schmidt-Chanasit J (2012) Epizootic emergence of Usutu virus in wild and captive birds in Germany. PLoS One 7(2):e32604
Cadar D, Lühken R, van der Jeugd H, Garigliany M, Ziegler U, Keller M, Lahoreau J, Lachmann L, Becker N, Kik M, Oude Munnink BB, Bosch S, Tannich E, Linden A, Schmidt V, Koopmans MP, R"ks J, Desmecht D, Groschup MH, Reusken C, Schmidt-Chanasit J, (2017) Widespread activity of multiple lineages of Usutu virus, western Europe, 2016. Euro Surveill 22(4):30452
Calzolari M, Bonilauri P, Bellini R, Albieri A, Defilippo F, Maioli G, Galletti G, Gelati A, Barbieri I, Tamba M et al (2010) Evidence of simultaneous circulation of West Nile and Usutu viruses in mosquitoes sampled in Emilia-Romagna Region (Italy) in 2009. PLoS One 5:e14324
Calzolari M, Chiapponi C, Bonilauri P, Lelli D, Baioni L, Barbieri I, Lavazza A, Pongolini S, Dottori M, Moreno A (2017) Co-circulation of two Usutu virus strains in northern Italy between 2009 and 2014. Infect Genet Evol 51:255–262
Cavrini F, Della Pepa ME, Gaibani P, Pierro AM, Rossini G, Landini MP, Sambri V (2011) A rapid and specific real-time RT-PCR assay to identify usutu virus in human plasma, serum, and cerebrospinal fluid. J Clin Virol 50:221–223
Chvala S, Kolodziejek J, Nowotny N, Weissenböck H (2004) Pathology and viral distribution in fatal usutu virus infections of birds from the 2001 and 2002 outbreaks in Austria. J Comp Pathol 131:176–185
Chvala S, Bakonyi T, Bukovsky C, Meister T, Brugger K, Rubel F, Nowotny N, Weissenbo ̈ck H, (2007) Monitoring of Usutu virus activity and spread by using dead bird surveillance in Austria, 2003–2005. Vet Microbiol 122:237–245
Clé M, Beck C, Salinas S, Lecollinet S, Gutierrez S, Van de Perre P, Baldet T, Foulongne V, Simonin Y (2019) Usutu virus: a new threat?. Epidemiol Infect 147:1–11
De Battisti C, Salomoni A, Ormelli S, Monne I, Capua I, Cattoli G (2013) Rapid pathotyping of newcastle disease virus by pyrosequencing. J Virol Methods 188:13–20
Del Amo J, Sotelo E, Fernández-Pinero J, Gallardo C, Llorente F, Agüero M, Jiménez-Clavero MA (2013) A novel quantitative multiplex real-time RT-PCR for the simultaneous detection and differentiation of West Nile virus lineages 1 and 2, and of Usutu virus. J Virol Methods 189:321–327
Garigliany M, Linden A, Gilliau G, Levy E, Sarlet M, Franssen M, Benzarti E, Derouaux A, Francis F, Desmecht D (2017) Usutu virus, Belgium, 2016. Infect Genet Evol 48:116–119
Grottola A, Marcacci M, Tagliazucchi S, Gennari W, Di Gennaro A, Orsini M, Monaco F, Marchegiano P, Marini V, Meacci M, Rumpianesi F, Lorusso A, Pecorari M, Savini G (2017) Usutu virus infections in humans: a retrospective analysis in the municipality of Modena. Italy Clin Microbiol Infect 23(1):33–37
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14(6):587–589
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20(4):1160–1166
Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P (2006) West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol 4
Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT (2000) Rapid detection of west nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol 38(11):4066–4071
Lelli R, Savini G, Teodori L, Filipponi G, Di Gennaro A, Leone A, Di Gialleonardo L, Venturi L, Caporale V (2008) Serological evidence of USUTU virus occurrence in north-eastern Italy. Zoonoses Public Hlth 55:361–367
Lühken R, Jöst H, Cadar D, Thomas SM, Bosch S, Tannich E, Becker N, Ziegler U, Lachmann L, Schmidt-Chanasit J (2017) Distribution of Usutu virus in Germany and its effect on breeding bird populations. Emerg Infect Dis 23:1991–1998
Letunic I, Bork P (2021) Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49:293–296
Manarolla G, Bakonyi T, Gallazzi D, Crosta L, Weissenböck H, Dorrestein GM, Nowotny N (2010) Usutu virus in wild birds in northern Italy. Vet Microbiol 141:159–163
Minh BQ, Nguyen MA, von Haeseler A (2013) Ultrafast approximation for phylogenetic bootstrap. Mol Biol Evol 30(5):1188–1195
Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck CR (2006) Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis 12:468–474
Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32(1):268–274
Paz S (2015) Climate change impacts on West Nile virus transmission in a global context. Phil Trans R Soc B 370:20130561
Scaramozzino N, Crance JM, Jouan A, DeBriel DA, Stoll F, Garin D (2001) Comparison of Flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-PCR assay for detection of Flaviviruses targeted to a conserved region of the NS5 gene sequences. J Clin Microbiol 39:1922–1927
Steinmetz HW, Eulenberger U, Robert N, Hoop R, Nowotny N (2011) Emergence and establishment of Usutu virus infection in wild and captive avian species in and around Zurich, Switzerland—genomic and pathologic comparison to other Central European outbreaks. Vet Microbiol 6
Tamba M, Bonilauri P, Bellini R, Calzolari M, Albieri A, Sambri V, Dottori M, Angelini P (2011) Detection of Usutu virus within a West Nile virus surveillance program in northern Italy. Vector-Borne Zoonotic Dis 11:551–557
Tang Y, Anne Hapip C, Liu B, Fang CT (2006) Highly sensitive TaqMan RT-PCR assay for detection and quantification of both lineages of West Nile virus RNA. J Clin Virol 36:177–182
Vilibic-Cavlek T, Petrovic T, Savic V, Barbic L, Tabain I, Stevanovic V, Klobucar A, Mrzljak A, Ilic M, Bogdanic M et al (2020) Epidemiology of Usutu virus: the European scenario. Pathogens 9
Weidinger P, Kolodziejek J, Bakonyi T et al (2020) Different dynamics of Usutu virus infections in Austria and Hungary, 2017–2018. Transbound Emerg Dis 67:298–307
Weissenböck H, Kolodziejek J, Url A, Lussy H, Rebel-Bauder B, Nowotny N (2002) Emergence of Usutu virus, an African Mosquito-borne Flavivirus of the Japanese Encephalitis Virus Group, Central Europe. Emerg Infect Dis 8:652–656
Weissenböck H, Bakonyi T, Rossi G, Mani P, Nowotny N (2013) Usutu virus, Italy, 1996. Emerg Infect Dis 19:274
Woodall JP (1964) The viruses isolated from arthropods at the East African Virus Research Institute in the 26 years ending December 1963. Proc E Afr Acad 2:141–146
Acknowledgements
We thank all the staff of the “Otus” wildlife treatment and rehabilitation center (LIPU-Bologna), in particular Nadia Caselli for her willingness to provide us with the exact data of the findings of the blackbirds examined and Caterina Cicero for her help with organ sampling.
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Musto, C., Tamba, M., Calzolari, M. et al. Usutu virus in blackbirds (Turdus merula) with clinical signs, a case study from northern Italy. Eur J Wildl Res 68, 23 (2022). https://doi.org/10.1007/s10344-022-01572-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-022-01572-z