Abstract
Arbuscular mycorrhizal fungi (AMF) have a key role in natural and agricultural ecosystems affecting plant nutrition, soil biological activity and modifying the availability of nutrients. Thiamine (Vitamin B1) is an essential coenzyme which incorporated in a wide range of plant metabolic processes. Thus, this research aimed to study the possibility of mitigating the negative effect of salinity stress on white lupine plant via using AMF and Vit B1 through assessment growth, various physiological traits and yield components of the white lupine plant. AMF was added to the soil (0.0 and 7 g pot−1) and Vit B1was foliar applied to white lupine seedlings (0.0, 100 and 200 mgL−1) and watered by two levels of salinity (0.0 or 5000 mgL−1). Salinity stress (5000 mgL−1) resulted in significant reductions in growth photosynthetic pigments constituents, endogenous indole acetic acid (IAA), some elements & productivity of white lupine in comparison to control plants. While, increasing phenols, some osmolytes and sodium compared to control (plants irrigated by tap water). Adding AMF to soil with the recommended dose boosted white lupine growth, certain physiological aspects and productivity in white lupine plants under irrigation with saltwater (5000 mgL−1). Furthermore, exogenous Vit B1 treatment with 100 & 200 mgL−1 not only enhanced growth and seeds productivity of white lupine plants under normal irrigation but also, improved salinity tolerance by increasing white lupine growth and productivity via inmproving photosynthetic pigments, osmolytes levels and element contents compared to their corresponding controls. Finally it could be concluded that, 200 mgL−1Vit B1 wit AMF treatment shows superiority in inducing maximum improving white lupine plant salinity tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the most serious issues in the world’s arid and semi-arid climate areas is salinity. Saline water irrigation, inadequate drainage, irrigation techniques, increased transpiration and decreasing rainfall are from the main causes of increasing salt stress in agricultural soils. In addition to mineral imbalances, the lack of sufficient water accessible to plants—which is regarded as a secondary drought—also contributed to the negative impact of salt on plant growth (Debnath et al., 2018). About 20% of total agricultural area 33% of watered agricultural area are affected by high salt levels with 2 million ha (about 1%) being evaluated as degrading annually due to salinity (Ke et al., 2018). Salt is predicted to damage almost half of the arable agricultural area. by the year 2050 The unavoidable rise in global population demands a significant rise in agricultural productivity. By 2050, productivity must increase by 70% to fully feed the target populations, with totaling 2.3 billion individuals (Mir et al., 2021). Thus, efforts to enhance salinity tolerance in plant are cruicial to ensure targeted food protection globally. Elevated salt levels decrease growth and yield of different crops in various ways. Salinity stress caused immediate effect on plant cells, osmotic stress resulted from the presence of ions around roots preventing absorbtion of water by roots thus decreasing plant growth. Furthermore, secondary damage is ionic stress due to the imbalance of solutes in the cytosol and osmotic stress resulted in decreasing water availability in soil (Gupta and Huang 2014). Furthermore, these stressful conditions lead to overproduction of reactive oxygen species (ROS). Through the induction of lipid peroxidation, ROS causes damage to many cellular membranes as well as to DNA, chloroplast and protein (Mittova et al. 2002). Increased salinity contents hamper plant ability to absorb water and minerals such as potassium K+ and calcium Ca2+ while, increased sodium Na+ and chlorine Cl− absorption, affects directly on plant cells via toxic effects on cell membranes and metabolic activities of the cytosol (Hasegawa et al. 2000). These primary effects of salinity cause other secondary effects such as decreased cell expansion, production of assimilating as well as reduced membrane function and cytosolic metabolism and increased reactive oxygen species production (ROS) (Kapoor et al. 2015).
So, it is crucial to increase plant production under various abiotic challenges, which can be done by creating new cultivars with high yield potential. Additionally, implementing appropriate cultural techniques as fertilization with natural products such as biofertilizers in addition to use of different naturally present substances like plant growth regulators, vitamins, antioxidants amino acids and so on (Tzortzakis 2009). In this aspect, the use of Arbuscular mycorrhiza fungi (AMF) is a potential approach for improving crop productivity. The mechanism of intrinsic adaptation formed by plants evolves in combination with several soil microorganisms under natural conditions. Bacteria and fungi are among those microorganisms, which can improve plant performance under adverse environmental conditions (Aroca and Ruiz-Lozano 2009). AMF have reciprocal association with the roots of eighty percent of vascular plants (Smith and Read 2008). This AMF synergy is characterized as a more effective nutrient uptake and transfer system than roots alone. AM fungi’s extra-radical mycelium can also promote organically bound nitrogen (N) from plant residues. They can also break down certain complex minerals and organic substances in the soil and make it available to their hosts (Soliman et al. 2012). In addition, the relationship studied between an AM fungus and a plant in most cases makes the host plant more tolerant to environmental stresses (Dodd and Ruı’z-Lozano 2012). Arbuscula rmycorrhiza fungi are valuable in sustainable agriculture as they improve plant water relations so increase stress tolerance of host plants, improve disease resistance and increase mineral uptake via increasing accumulation of phosphorus and other low mobile mineral nutrients, which reduce fertilizers usage. AMF fungi might help plants to overcome the negative effect of salinity via improving mineral nutrient uptake, maintaining ion balance, stabilizing enzyme activities (Li et al. 2020), and improving water uptake, as well as improving antioxidant defense systems (Wu et al. 2014). Furthermore, AMF can improve the production of growth regulating substances such as indole acetic acid (IAA), improve photosynthesis, and boost osmotic adjustment under salinity stress via overproduction of osmolyte compounds as proline and sugars (Yamato et al. 2008) accompanied with the reduction in Na+ and Cl− uptake (Li et al. 2020).
Among natural compounds used in improving plant tolerance to salinity stress are vitamins. Vitamins are organic chemicals which required in traces to maintain normal growth and development of all living organisms; they act as coenzymes of different enzymes thus playing very important role in plant metabolism control (Rady et al. 2011). Exogenous treatment of vitamins has significant effects in improving plant tolerance via regulating different biochemical processes and protecting plant from adverse influences of abiotic stress (Arrigoni et al. 1997). Vitamin B1 or thiamine is considered an essential coenzyme which incorporated in a wide range of metabolic processes such as the decarboxylation of α‑keto acids, like pyruvic acid and keto-glutamic acid which affect on the metabolism of fats and carbohydrates (Goyer 2010 and Sajjad et al. 2017). Kaya et al. (2016) found that the application of vitamin B1 improved growth, development and chemical components of maize plants under environmental stresses. Vitamin B1 induced an important function in plant reactions to water stress (Toscano et al. 2019). Exogenous application of Vit B1 can raise the antioxidant defense mechanism via involving the transketolase enzyme in the glycolytic cycle (El-Metwally and Sadak 2019).
White Lupine (Lupinus termis L.) is an important pulse crop that is grown in a variety of environments. Their seeds have nutritional components that are similar to soybean seeds and greater than other legumes seed. White lupine seeds are an influential protein and oil supplier (Prusinski 2017). Their seeds are a significant source of oil (5–13%) and protein (33–40%) with valuable amino acids patterns (Písaříková and Zralý 2009). Furthermore, husked seeds flour has a high content of protein, oil, total ash, crude fiber & carbohydrate (Mierlita et al. 2018). Bitter lupine seeds oil has high concentrations of antioxidants therefore, it is suitable for use in various food processing (El-Awadi et al. 2016 and Vogelsang-O’Dwyer et al. 2020). Furthermore, the lupine plant like other legume plants can fix atmospheric nitrogen which improves soil fertility, permeation and water storage (Wolko et al. 2011). Accordingly, plants have the potential of growing better in low fertility soils and are used as a supplement as green manure to improve its fertility.
Therefore, this investigation aimed to study the physiological role of AMF and/or Vit B1 treatment with different concentrations on white lupine plants grown under salinity stress.
Methods
A pot test was conducted on the greenhouse of the National Research Centre Dokki, Egypt during 2017/2018 and 2018/2019 winter seasons. Seeds of bitter lupine (Lupinus termis L.) cv., Giza 2 variety were gotten from Agriculture Research Centre, Egypt. They were inoculated using nitrogen fixing bacteria (Rhizobia). During November 2017 and November 2018, the seeds were planted in pots of 50 cm3. In the pots, the planting soil was composed of clay and sand soil with ratio of 2:1 to decrease compaction. Arbuscular mycorrhiza fungus (AMF) were obtained from The Agricultural Research Centre in Egypt. Inoculation of the Arbuscular mycorrhiza fungi (AMF) treatments was done by adding Glomus mosseae spp (VA-mycorrhizal fungus) to the soil of each pot at the rate of 1 g/pod. After 15 days, plants were thinned leaving 10 plants in each pot. Fertilization was added using superphosphate (5 g per pot), potassium sulfate (2.5 g/pot), and urea (6 g per pot). The experimental design was a factorial complete randomized design. AMF was added to half pots with the recommended dose (7 g per pot). Foliar application of different concentrations of Vit B1 (0.0, 100 & 200 mgL−1, denoted as B1 1, B1 2 and B1 3) were applied at 30 and 45 days. Pots were divided into two groups, each one irrigated with one of the following salt concentrations (0.0 or 5000 mgL−1). Plants were irrigated three times with equal amounts (liter/pot) of the salt solution followed by one with tap water to prevent the accumulation of salt around the root system. The preparation of salt mixture according to Stroganov (1962) equation as in (Table 1). The pots were irrigated with equal volumes of the various salinity levels.
Measurements
At 60 days of age, samples were taken to determine some growth parameters: plant height (cm), number of leaves per plant, fresh and dry weights of the shoot (g per plant) and some chemical analysis. Fresh samples were taken to determine photosynthetic pigments, IAA and phenol contents. Air-dried samples of white lupine plants were taken to analyze total soluble sugars, proline, free amino acids as well as some mineral contents (nitrogen, phosphorous, potassium, calcium, magnesium and sodium). Three plants per pot have been left for yield determination. At harvest time, yield and its related traits, i.e., number of pods per plant, number of seeds per pod, number of seeds per plant, dry weight of pods (g) and seeds per plant (g) and 100 seed weight (g). In addition to some nutritional components of the yielded seeds as carbohydrate%, protein%, total oil% and flavonoids as well as DPPD activities.
Chemical Analysis
Photosynthetic pigments, total chlorophyll a and b and carotenoid contents in fresh leaves were estimated using the method of Lichtenthaler and Buschmann (2001). Indole acetic acid contents (IAA) were determined by the method of Larsen et al. 1962). Total phenol content was measured as described by Danil and George (1972). Total soluble carbohydrates (TSS) were extracted according to Homme et al. (1992) and analyzed by Yemm and Willis (1956). Free amino acids and proline were extracted according to the method described by Vartainan et al. (1992). Free amino acid was determined with the ninhydrin reagent method Yemm and Cocking (1955). Proline was assayed according to the method described by Bates et al. (1973). Minerals content as nitrogen, phosphorous, potassium, calcium, magnesium and sodium) were determined according to the method described by Chapman and Pratt (1978). Nitrogen was determind using microkledahl method, P were determined using Spekol Spectrocolourimeter Carl Zeiss. While, Ca, K and Na contents were determined by the use of flame photometer, and Mg+2 was determined using atomic absorption spectrophotometer.
Nutritional values of the yielded seeds: Total carbohydrate determination was carried out according to Herbert et al. (1971). Flavonoid content of crude extract was determined by the Aluminium chloride colorimetric method (Chang et al. 2002). Oil of white lupines seeds was extracted according to Kates and Eberhardt (1957).
Statistical Analysis
The data were statistically analyzed according to Snedecor and Cochran (1989). A combined analysis of the two growing seasons was carried out. Means were compared by using the least significant difference (LSD) at 5% levels of probability (Duncan 1955).
Results
Changes in Growth Criteria
Data presented in Table 2 show the effect of foliar treatment of Vit B1 without or with the addition of AMF to soil on growth criteria of white lupine plants grown under salinity stress. Results showed that, salinity stress (5000 mgL−1) caused significant reductions in all growth parameters of the white lupine plant (shoot length, no of leaves per plant, fresh and dry weights per plant, fresh and dry weight of root per plant) compared with the control plant, those plants irrigated with normal water (Table 2). While, the addition of AMF to soil increased the studied growth parameters of lupine plants (shoot length, no of leaves per plant, fresh and dry weights per plant, fresh and dry weight of root per plant) either grown under normal conditions or salinity stress as compared with those plants grown without AMF addition.
Furthermore, the obtained results also showed that foliar application of the lupine plant with different concentrations (100 and 200 mgL−1) of Vit B1 resulted in increases in the above-mentioned growth parameters in plants irrigated with normal water or those irrigated with saline water (5000 mgL−1). Different levels of Vit B1 increased significantly the above-mentioned growth criteria in plants grown without AMF and also caused more significant increases in plants grown with AMF. Moreover, within the above mentioned treatments, the obtained data indicate that using higher level of Vit B1 (200 mgL−1) resulted in better growth than using a lower level (100 mgL−1).
Changes in Photosynthetic Pigments
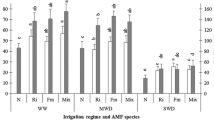
Salinity stress reduced photosynthetic pigments (Chlo a, Chlo b, carotenoids, total pigment and Chlo a/Chlo b,) contents of white lupine plant leaf as compared with plants irrigated with tap water (Fig. 1). The percentages of decreases were 9.47%, 18.52%, 20.16% and 12.79% of Chlo a, Chlo b, carotenoids and total pigments respectively. Meanwhile, increased Chlo a/Chlo b as compared with control plants. With respect to AMF addition to soil, data revealed that the addition of AMF caused significant increases in Chlo a, Chlo b, carotenoids and consequently total pigments of white lupine plants as compared with those grown without this addition either of plants irrigated with tap water or saline water. While decreasing the ratio of Chlo a/Chlo b either at normal irrigation or saline irrigated plants (Fig. 1). Moreover, foliar spraying of white lupine plants with Vit B1 with different concentrations (100 and 200 mgL−1) significantly improved all fractions of photosynthetic pigments, especially in plants subjected to salt stress either of plants grown without or with AMF as compared with those control plants. Meanwhile, decreased the ratio of Chlo a/Chlo b as compared with untreated control either under normal or salinity stressed conditions (Fig. 1).
Changes in Endogenous Indole Acetic Acid (IAA) and Phenolics Contents
The effect of foliar treatment of Vit B1 in the absence and presence of AMF on lupine plants grown under normal and salinity stress conditions are presented in Fig. 2. Salinity stress reduced endogenous IAA contents of white lupine leaves as compared with plants irrigated with tap water (control), in the mean time increased phenolic contents as compared with normal irrigated plants. The addition of AMF to the soil increased significantly not only endogenous IAA but also phenolics contents of white lupine plants compared with plants grown in soil without AMF amended either of normal irrigated and salt-stressed irrigated plants (Fig. 2). Furthermore, exogenous treatment as foliar spraying of lupine plants with Vit B1 with different concentrations (100 and 200 mgL−1) caused significant increases in endogenous IAA and phenolic contents with comparison by their corresponding untreated plants. A higher level (200 mgL−1) of Vit B1 was more effective than a lower level (100 mgL−1) in increasing IAA and phenol contents of white lupine plants grown under normal and stressed conditions (0.0 and 5000 mgL−1) in soil without or with AMFamendement.
Changes in Some Osmolytes
The effect of salinity stress and different treatments of Vit B1 in the absence and presence of MF on some compatible organic solutes (total soluble sugars TSS, proline and free amino acids) are presented in Table 3. Subjecting white lupine plant to salinity stress caused significant increases in the studied compatible solutes. Moreover adding AMF to soil with te recommended dose and foliar treatment of Vit B1 caused more significant increases in TSS, proline and free amino acids as compared with those plants without the addition of AMF (Table 3). Plants accumulate higher amounts of compatible solutes under the effect of salinity.
Changes in Some Macronutrients and Sodium Contents
The mineral contents of white termis plants in response to Vit B1 foliar treatment in absence and presence of AMF under different salinity levels are presented in Table 4. Irrigation of white lupine plant with 5000 mgL−1 salinity level caused significant decreases in the contents of nitrogen (N), phosphorus (P3+), potassium (K+), calcium (Ca2+), magnesium (Mg++),. In the same time, sodium contents increased significantly under salinity stress. On the other hand, amended AMF to the soil could improve te mineral contents of white lupine plant by increasing significantly N, P, K. Ca and Mg contents, while decreasing sodium contents as compared with plants grown in absence of AMF. Moreover, foliar treatment of different concentrations of Vit B1 caused significant increases in mineral contents (N, P, K, Ca, Mg), while decreasing sodium contents of white lupine plants irrigated with tap water and could alleviate the negative effect of salinity by improving the contents of the studied mineral as compared with their corresponding untreated controls (Table 4).
Yield and Its Component Traits
Results of the effect of foliar treatment of Vit B1 without or with the addition of AMF to soil on yield and its attributes of lupine termis plants grown under salinity stress are presented in (Table 5). Data showed that salinity stress (5000 mg L−1) caused a marked reduction in all yield attributes of the lupine plant (No of pods per plant, No of seeds per pod, weight of pods/plant, weight of seeds/plant and weight of 100 seeds)as compared with the control plant (those irrigated with normal water). On the other hand, the addition of AMF to soil with the recommended dose increased yield and yield attributes of lupine plants either grown under normal conditions or salinity stress conditions.
Moreover, the obtained results also showed that foliar application of white lupine plant with different concentrations of vitamin B1 resulted in increases in the above-mentioned yield and yield attributes in plants irrigated with normal water or those irrigated with saline water either without and with the addition of AMF (Table 5). Data clearly show that higher level of Vit B1 was more effective than lower one in increasing white termis productivity under the studied conditions.
Changes in Chemical Constituents of Lupine Seeds
Table 6 shows that subjecting white lupine plants to salinity stress (5000 mgL−1) caused marked decreases in carbohydrate percentage meanwhile increased markedly protein%, flavonoids content, oil % and DPPH% activities. Meanwhile, addition of AMF to the soil increased Carbohydrates%, protein%, flavonoid and oil% of seeds of while lupine plant either grown under normal irrigation or salinity irrigated water. Furthermore, foliar treatment of white lupine plant with different concentrations of Vit B1 (100 and 200 mgL−1) caused marked increases in different studied nutritional components and antioxidant activities percentages of white lupines seeds as compared with their corresponding controls without or with the addition of AMF treatment either under normal and salinity stressed conditions.
Discussion
Abiotic stress, especially saline stress, drastically impact growth, metabolic activities, yield and the nutritional value of while lupine seeds. Addition of AMF as well as Vit B1 to lessen the unfavorable consequences of saline stress on white lupine plants were studied. In the present research, salinity stress (5000 mg L−1) impacted significant reductions in lupine growth parameters of the white lupine plant. The results were obtained by El-Bassiouny et al. (2017), Ahammed et al. (2018), Ramadan et al. (2019), Sadak et al. (2020), Sadak and Talaat (2021) and Sadak (2022) are concurrent with our obtained data. These reductions in growth criteria could result from reduced water uptake, disorders in various physiological activities, nutrient deficiency and the reduced role of increased Na+ and Cl− aggregation around the root (Everado et al. 1975). Moreover, salt stress causes osmotic stress which causes stressed plants water balance disturbances and in turn, leads to stomatal closure, decreases in photosynthesis, toxic ions aggregation thus growth reductions. Moreover, it was suggested that salinity inhibitory impact on growth might be due to the reduction of cell division or the inhibition of both cell elongation and activity of meristematic tissues (Shalata and Neumann 2001). Salinity stress reduced Chlo a, Chlo b, carotenoids, total pigment and Chlo a/Chlo b of lupine plant leaves compared to those irrigated with tap water (Fig. 1). Those data are similar to those in chickpea (Abd Elhamid et al. 2018) and wheat plants (Sadak et al. 2019). These reductions caused by salinity stress on photosynthetic pigments components could be ascribed to salt deleterious impact on biosynthesis, improving their destruction (Kumar et al. 2012) and/or causing serious injury to chloroplast thylakoids (Camejo et al. 2006). Salinity improved chlorophyllase activity and inhibited protein de-novo formation, which links chlorophyll (Jaleel et al. 2007). Also, K+ is known as a stimulator of several enzymes which are necessary for photosynthesis. So, decreases in K+ content caused inhibition of photosynthesis and, ultimately, decreased growth (Salisbury and Ross 1992). Plants subjected to salinity had lower Chlo levels, while the Chlo a/b improved, owing to chlorophyll b breakdown being faster than chlorophyll a (Fig. 1). These are supported by the fact that transformation to a is the initial step in the destruction of chlorophyll b (Fang et al. 1998). Chlo a/b increment was related to variations in Chlo a & b constituents that have decreased contents of light-harvesting proteins (Loggini et al. 1999). Indole acetic acid as an endogenous bioregulator preserves the defensive function in plant cells towards stress through antagonist or complementary actions by other hormones, gibberellic, cytokinins and abscisic acid which is termed, signaling crosstalk. Salt reduced endogenous IAA while increasing phenolic levels of lupine (Fig. 1). That reduction might be explained by salt’s role in boosting IAA degradation or lowering its formation (Bano and Samina 2010). Jasim et al. (2016) and Sadak (2019) confirmed these results. Meanwhile, the increment of phenolic of the lupine plant can alleviate the adverse salt result. The increased reactivity of phenolics as H2 or electron donors reflects antioxidant protective strategies (Huang et al. 2005). That mechanism causes the unpaired electron to be stabilized also delocalized (Michalak 2006). Salinity stress increased the studied compatible solutes. Plants accumulate higher amounts of compatible solutes under the effect of salinity stress, these compounds shield the plant from stress via membrane stabilization, tertiary structures of proteins and enzymes. Osmoprotectants (TSS, proline and free amino acids) have a major effect on cell acclimation to varying unfavorable environmental stress by enhancing osmosis in the cytoplasm, balancing proteins and membranes, and sustaining greater water level required to plant growth and cell activities (Michalak 2006). Increment TSS improves turgor up-keeping and maintains cell membrane. Proline buildup is thought to be a signal of stress in several plants, serving as an osmotic protective and aiding in cell turgor stability (Lee et al. 2008). Moreover, the higher proline level might result from a proline oxidase activity decrease. Also, it is proposed as C and N supplier for quick recovery and stabilization. Proline is a scavenging osmolyte that neutralizes dangerous ROS; (Matysik et al. 2002). Quench of singlet oxygen (1O2) and chemical interaction with OH radicals are two techniques that proline lowers ROS harm. Free amino acid buildup correlated with stress can be a component of the adapting method that helps with osmotic balance. Macro element contents of white lupine plant were decreased in plants irrigated with salt water but sodium content was increased. These reductions in mineral contents with the increased sodium contents was confirmed earlier by Sadak and Abd Elhamid (2013) in flax plant, Rady et al. (2015) on soybean and Sadak et al. (2017) on chickpea plant. Munns (2002) stated that, saline stress might influence plant via three mechanisms, the first is water deficiency stress by decreasing water potential in root, second phyto-toxicity of Na+ and Cl−1 and third, nutrient imbalances resulted from their improper uptake. Moreover, Na+ ions interfere with K+ ions for the binding sites necessary for biochemical activities. The increased levels of Na+ with the reductions in K+ contents, in response to salinity stress, caused marked decreases in K+/Na+. These might cause disturbances in the accumulation of Na+ in plant organs (Rady et al. 2015). This improving effect on Na+ ion contents resulted from salinity stress was concurrent by decreases in phosphorus and potassium ions contents and this result might be due to the antagonism of phosphorus and potassium ions versus sodium ions (Munns 2002). Data showed that salt (5000 mg L−1) caused a marked reduction in all yield attributes of the lupine plant as compared with the control plant. Regarding reducing salt role (5000 mg/L) on yield indices of white lupine, those decreases are reflected in growth decreases (Table 2), and photosynthetic pigments (Fig. 1) reductions. Moreover, the reductions in chlorophyll content caused decreased photosynthesis activity, causing lower carbohydrates to build up thus reducing transportation from leaves to the new seeds (Anjum et al. 2003). Table 6 shows that subjecting lupine plants to salinity stress caused marked decreases in carbohydrate percentage meanwhile increased markedly protein%, flavonoids content, oil % and DPPH% activities. Sadak et al. (2015) confirmed those results. Carbohydrates reduction mostly resulted from decreases in Chlo a, b, carotenoids (Fig. 1). Seeds carbohydrate variations are useful due to relation tovarious processes such as photosynthesis, transfer, & respiration (Sadak et al. 2015). Salt reduced Chls levels causing decreases in photosynthetic activity. Thus, lower carbohydrates build up in mature leaves and thus decrease carbohydrate transport from leaves to the seeds. Salinity stress increased protein and flavonoids contents of the yielded seeds. Flavonoids, including flavones, flavanols and condensed tannins, are secondary metabolites, the antioxidant activity of which depends on the presence of free OH groups, especially 3‑OH (Geetha et al. 2003). The higher levels of flavonoids could indicate some types of defense against salt stress since salinity stress was accompanied by higher ROS levels (Geetha et al. 2003).
While in Table 2 the increased effect of AMF on lupine plants compared with plants grown without AMF. Those data on fungi are in agreement with Hafez et al. (2013) and Bakry et al. (2016) on olive and flax. These increases might reflect via AM role in various nutrient uptake (N, Ca, K, Cu, Zn, S, P) (Sharifi et al. 2007). Applying AMF enhances growth and influences nutrient allocation and transport among stem and root, resulting in improved dry weight of shoot (Smith and Read 2008). Data presented in Fig. 1 revealed that adding AMF increased significantly Chlo a, Chlo b, carotenoids & total pigments in lupine plants compared to those that grow without AMF. Furthermore, the addition of AMF to the soil increased significantly endogenous IAA and phenolics contents in white lupine either in normal irrigated and salt-stressed irrigated plants (Fig. 2). It was hypothesized that the altered IAA balance with AMF aided plant development and helped in improving growth and yield. While decreasing the ratio of Chlo a/Chlo b under tap irrigation or saline irrigated (Fig. 1). Many scientists assumed the AMF, symbiosis role in improving Chlo a, Chlo b, carotenoids and total pigments (Augé 2001). The addition of AMF to soil with the recommended dose increased lupine yield either at normal or salinity stress conditions. Those increments resulted from AMF role in nutriments uptake like N, Ca, K, Cu. Zn, S, and P (Sharifi et al. 2007). Applying AMF enhances plant development & influences nutrient allocation and transport along the stem and root resulting in higher dry weight of the shoot (Smith and Read 2008). The stimulating effect of AMF soil addition on seeds carbohydrate may be growth increases & photosynthetic pigments (Table 2 and Fig. 1). Moreover, enhanced photosynthetic production boosted carbohydrates production of the leaves and consequently improved carbohydrate transfer from leaves to seeds.
In response to Vit B1, Khafagy et al. (2017) observed that the treated white lupine plants with vitamin B1 led to significantly enhanced growth criteria. Vit B1 is the main function of coenzyme thiamine pyrophosphate which plays a significant role in the balance of carbon metabolism in plants. Ghafar et al. (2019) found that foliar spray of thiamine (50 mM) enhanced plant growth under water deficit. They referred to this growth improvement as thiamine-induced raise in the photosynthetic pigments and total phenolic contents of Trifolium repens L. plants under water stress. Foliar spraying to white lupine plants with thiamine vitamin with different concentrations significantly improved photosynthetic pigments, in plants subjected to salt stress either of plants grown without or with AMF compared to those control plants. While decreasing the ratio of Chlo a/Chlo b as compared with untreated control (Fig. 1). The role of vitamins was to enhance chlorophyll biosynthesis or decreased degradation. This enhancement was generally because of the defending role of vitamins which led to a decrease in oxidative stress. The positive role of thiamine on chlorophyll was stated in many plants because thiamine enhances or assists re synthesize chlorophyll (Hamada and Khulaef 2000 and El Karamany et al. 2022). El-Awadi et al. (2016) reported that thiamine treatment is mostly associated with suitable regulation of photosynthesis and energy-providing reactions in plants. Exogenous treatment as foliar spraying of lupine plants with thiamine vitamin significantly increased endogenous IAA and phenolic levels. Vit B1 greater concentration was effective (100 mg/l) in increasing lupine plant IAA and phenolic at normal and stressed (0.0 and 5000 mg/l) in soil without or with AMF. These increases might be due to the role of Vitamins in IAA biosynthesis and retarding its degradation (El Hariri et al. 2010 and El Karamany et al. 2022). The obtained data proved that Vit B1 treatment has an important function in the regulating of carbon metabolism and protein synthesizes as an effective coenzyme in the metabolic passageways of these biochemical. Accumulation of TSS by thiamine treatment in drought-stressed wheat plants could be a significant adaption reply to drought (Ashraf and Harris 2004). Similar alleviating responses of the wheat plant on TSS, FAA and Pro accumulation were noticed in Zea maize plants (Kaya et al. 2016). In addition, Vitamin B could act as an antioxidant in improving plant tolerance. Vitamins have lately been stated to be powerful antioxidants with a special ability to quench ROS (Shimasaki and Fukunoto 1998). Treating white lupines by vit B1 increased markedly different nutritional components of lupine seeds. The results of the variations of flavonoids of the lupine plant as affected by Vit B1 treatments (Table 6). Vit B1 addition to lupine caused increases in flavonoids. These findings suggest that Vit B1 as a bioactive chemical can be considered an activator for the synthesis of secondary metabolites (flavonoids).
Conclusions
In conclusion, salinity reduced growth and biochemical parameters. Mycorrihyzae treatment significantly improved growth & productivity by improving photosynthetic pigments, IAA, phenolic, total soluble sugars, proline and free amino acids contents. Moreover, the lupine plant treated by Arbuscularmycorrhizaand Vitamin B1 has higher nutritional constituents of yielded lupine seeds as carbohydrate%, protein%, flavonoids and oil%.
Abbreviations
- AMF:
-
Arbuscularmycorrhiza
- IAA:
-
Indole acetic acid contents
- ROS:
-
Reactive oxygen species
- TSS:
-
Total soluble carbohydrates
- Vit B1:
-
Vitamin B1
References
Ahammed GJ, Li Y, Li X, Han WY, Chen Sh (2018) Epigallocatechin-3-gallate alleviates salinity-retarded seed germination and oxidative stress in tomato. J Plant Growth Reg 37:1349–1356. https://doi.org/10.1007/s00344-018-9849-0
Anjum F, Yaseen M, Rasul E et al (2003) Water stress in barley. I. Effect on chemical composition and chlorophyll content. Pakistan J Agric Sci 40:45–49
Aroca R, Ruiz-Lozano JM (2009) Induction of plant tolerance to semi-arid environments by beneficial soil microorganisms.A review. In: Lichtfouse E (ed) Climate change, intercropping, pest control and beneficial microorganisms, sustainable agriculture reviews, vol 2. Springer, pp 121–135
Arrigoni O, Calabrese C, De Gara L, Bitonti MB, Liso R (1997) Correlation between changes in cell ascorbate and growth of Lupinu salbus seedlings. J Plant Physiol 150:302–308
Augé RM (2001) Water relation, drought and vesicular arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Bakry AB, Sadak M, Abd Allah MM, El-Razik TMA, Dawood MG (2016) Maximizing the performance, productivity and quality traits of two flax cultivars by using some bio-fertilizers under newly reclaimed sandy soil. Res J Pharm Biol Chem Sci 7(6):429–441
Bano A, Samina Y (2010) Role of phytohormones under induced drought stress in wheat. Pak J Bot 42:2579–2587
Bates LS, Waldan RP, Teare LD (1973) Rapid determination of free proline under water stress studies. Plant Soil 39:205–207
Camejo D, Jiménez A, Alarcón JJ, Torres W, Gómez JM, Sevilla F (2006) Changes in photosynthetic parameters and antioxidantactivities following heat-shock treatment intomato plants. Funct Plant Biol 33:177–187
Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Chapman HD, Pratt PF (1978) Methods of Analysis for Soils, Plant and Water. California University, Division of Agricultural Sciences, pp 50–169
Danil AD, George CM (1972) Peach seed dormancy in relation to endogenous inhibitors and applied growth substances. Am Soc Hort Sci J 17:621–624
Debnath M, Ashwath N, Hill CB, Callahan DL, Dias DA, Jayasinghe NS, Midmore DJ, Roessner U (2018) Comparative metabolic and ionomic profiling of two cultivars of Stevia rebaudiana Bert. (Bertoni) grown under salinity stress. Plant Physiol Biochem 129:56–70
Dodd IC, Ruı’z-Lozano JM (2012) Microbial enhancement of crop resource use efficiency. Curr Opin Biotech 23:236–242
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
El Hariri DM, Sadak MS, El-Bassiouny HMS (2010) Response of flax cultivars to ascorbic acid and α–tocopherol under salinity stress conditions. Int J Acad Res 2(4):101–109
El Karamany MF, Sadak MS, Bakhoum GS, Omar HAA, Bakry BA (2022) Pyridoxine improving effect on yield, chemical and nutritional value of Egyptian clover plant. Asian J Plant Sci 21(3):360–373. https://doi.org/10.3923/ajps.2022.360.373
El-Awadi ME, Abdel-Baky YR, Sadak S, Mervat AA, Amin AA, Dawood MG (2016) Physiological response of Lupinustermisto trans-cinammic acid and benzoic acid treatments under sandy soil conditions. Res J Pharm Biol Chem Sci 7(4):1012–1024
El-Bassiouny HMS, Abd El-Monem AA, Sadak M, Badr NM (2017) Amelioration of the adverse effects of salinity stress by using ascorbic acid in sunflower cultivars. Bull NRC 41(2):233–249
El-Metwally IM, Sadak MS (2019) Physiological role of thiamine and weed control treatments on faba bean and associated weeds grown under salt affected soil. Bull Nat Res Centre 43:105
Elhamid AME, Sadak MS, Tawfik MM (2018) Glutathione treatment alleviate salinity adverse effects on growth, some biochemical aspects, yield quantity and nutritional value of chickpea plant. Sci Fed J Glob Warming 2(2):1–11
Everado AN, Stozy HL, Mehuzs RG (1975) Effect of soil osmotic potential produced with two salts onplant water potential. Plant Soil 42(9):619–657
Fang Z, Bouwkamp J, Solomos T (1998) Chlorophyllaseactivities and chlorophyll degradation during leaf senescence in non-yellowing mutant and wild-type of Phaseolus vulgaris L. J Experim Bot 49:503–510
Geetha S, Sai-Ram M, Mongia SS, Singh V, Ilavazha-gan G et al (2003) Evaluation of antioxidant activity of leaf extract of sea buckthorn (Hippoph aerhamnoides L.) on chromium (VI) induced oxidative stress in albino rats. J Ethnopharmacol 87:247–251
Ghafar MA, Akram NA, Ashraf M, Ashraf MY, Sadiq MM (2019) Thiamin-induced variations in oxidative defense processes in white clover (Trifolium repens L.) under water deficit stress. Turk J Bot 43:58–66
Goyer A (2010) Thiamine in plants: aspects of its metabolism and functions. Phytochemistry 71:1615–1624
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics. https://doi.org/10.1155/2014/701596
Hafez OM, Saleh MA, El-Lethy SR (2013) Response of some seedlings olive cultivars to foliar spray of yeast and garlic extracts with or without vascular arbuscularmycorrhizal fungi. World Appl Sci J 24(9):1119–1129
Hamada AM, Khulaef EM (2000) Simulative effects of ascorbic acid, thiamin or pyridoxine on Viciafabagrowth and some related metabolic activities. Pak J Biol Sci 3(8):1330–1332
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Ann Rev Plant Physiol Plant Mol Biol 51:463–499
Herbert DP, Phipps J, Strange RE (1971) Chemical analysis of microbial cells. Method Microbiol 5:209–344. https://doi.org/10.1016/S0580-9517(08)70641-X
Homme PM, Gonzalez B, Billard J (1992) Carbohydrate content, frutane and sucrose enzyme activities in roots, stubble and leaves of rye grass (Lolium perenne L.) as affected by sources/link modification after cutting. J Plant Physiol 140:282–291
Huang C, He W, Guo J, Chang X, Su P, Zhang L (2005) Increased sensitivity to salt stress in ascorbate-deficient Arabidopsis mutant. J Exp Bot 56:3041–3049
Jaleel CA, Manivannan P, Lakshmanan GMA (2007) NaCl as a physiological modulator of proline metabolism andantioxidant potential in Phyllanthu samarus. Comptes Rendus Biol 330:806–813
Jasim AH, Abo Al Timmen WM, Abid AS (2016) Effect of salt stress on plant growth and free endogenous hormones of primed radish (Raphanus sativus, L.) seeds with salicylic acid. Int J Chem Tech Res 9(06):339–346 (CODEN)
Kapoor D, Sharma R, Handa N, Kaur H, Rattan A, Yadav P (2015) Redox homeostasis in plants under abiotic stress: role of electroncarries, energy metabolism mediators and protein aceous thiols. Front Environ Sci 3:13. https://doi.org/10.3389/fenvs.2015.00013
Kates M, Eberhardt FM (1957) Isolation and fractionation of leaf phospholipids. Can J Bot 35:895–905
Kaya C, Ashraf M, Sonmez O, Tuna AL, Polat T, Aydemir S (2016) Exogenous application of thiamine promotes growth and antioxidative defense system at initial phases of development in salt-stressed plants of two maize cultivars differing in salinity tolerance. Acta Physiol Plant 37:1741–1743
Ke Q, Ye J, Wang B, Ren J, Yin L, Deng X, Wang S (2018) Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front Plant Sci 9:1–11
Khafagy MA, Mohamed ZAA, Farouk S, Amrajaa HK (2017) Effect of pre-treatment of barley grain on germination and seedling growth under drought stress. Adv Appl Sci 2:33–42
Kumar S, Singh R, Nayyar H (2012) α‑Tocopherol application modulates the response of wheat (Triticum aestivumL.) seedlings to elevated temperatures by mitigation of stress injury and enhancement of antioxidants. J Plant Growth Regul 32(2):307–314
Larsen P, Harbo A, Klungron S, Ashein TA (1962) On the biosynthesis of some indole compounds in Acetobacter Xylinum. Physiol Plant 15:552–565
Lee G, Carrow RN, Duncan RR, Eiteman MA, Rieger MW (2008) Synthesis of organic osmolytes and salt tolerance mechanisms in Paspalum vaginatum. Environ Experiment Bot 63:19–27
Li Z, Wu N, Meng S, Wu F, Liu T (2020) Arbuscular mycorrhizal fungi (AMF) enhance the tolerance of Euonymus maackii Rupr at a moderate level of salinity. PLoS ONE 15:e231497. https://doi.org/10.1371/journal.pone.0231497
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. In: Wrolstad RE, Acree TE, An H, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Sporns P (eds) Current protocols in food analytical chemistry (CPFA). John Wiley & Sons, New York, pp F4.3.1–F4.3.8
Loggini B, Scartazza A, Brugnoli E, Navariizzo FF (1999) Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol 119:1091–1099
Matysik J, Alia, Bhalu B, Mohanty P (2002) Molecularmechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15(4):523–530
Mierlita D, Simeanu D, Pop IM, Criste F, Pop C, Simeanu C, Lup F (2018) Chemical composition and nutritional evaluation of the lupine seeds (Lupinus albus L.) from low-alkaloid varieties. Rev Chim. https://doi.org/10.37358/RC.18.2.6126
Mir RA, Aryendu, Somasundaram R (2021) Salicylic acid and salt stress tolerance in plants: a review. J Stress Physiol Biochem 17(3):32–50
Mittova V, Tal M, Volokita M, Guy M (2002) Salt stress induces up-regulation of an efficient chloroplast antioxidant system in the salt tolerant wild tomato species Lycopersicon pennellii but not in the cultivated species. J Plant Physiol 115:393–400
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 28:239–250
Písaříková B, Zralý Z (2009) Nutritional value of lupine in the diets for pigs (a review). Acta Vet Brno 78:399–409
Prusinski J (2017) White lupin (Lupinus albus L.)—nutritional and health values in human nutrition—a review. Czech J Food Sci 35:95–105. https://doi.org/10.17221/114/2016-CJFS
Rady MM, Sadak MS, El-Bassiouny HMS, Abd El-Monem AA (2011) Alleviation the adverse effects of salinity stress in sunflower cultivars using nicotinamide and α‑tocopherol. Aust J Basic Appl Sci 5(10):342–355
Rady MM, Sadak MS, Safaa El-Lethy R, Abd El-Hamid E, Abdelhamid MT (2015) Exogenous α‑tocopherol has a beneficial effect on Glycine max (L.) plants irrigated with diluted sea water. J Hortic Sci Biotechnol 90(2):195–202
Ramadan AA, Abd Elhamid E, Sadak M (2019) Comparative study for the effect of arginine and sodium nitroprusside on sunflower plants grown under salinity stress conditions. Bull Natl Res Centre 43:118
Sadak M (2019) Physiological role of trehalose on enhancing salinity tolerance of wheat plant. Bull Natl Res Cent 43:38. https://doi.org/10.1186/s42269-019-0098-6
Sadak MS (2022) Biochemical responses of white termis to pyridoxine and mycorrhizae treatment under salinity stress. Egypt J Chem 65(10):429–439. https://doi.org/10.21608/EJCHEM.2022.118032.5319
Sadak M, Abd Elhamid E (2013) Physiological response of flax cultivars to the effect of salinity and salicylic acid. J Appl Sci Res 9(6):3573–3581
Sadak M, Talaat IM (2021) Attenuation of negative effects of saline stress in wheat plant by chitosan and calcium carbonate. Bull Natl Res Cent 45:136. https://doi.org/10.1186/s42269-021-00596-w
Sadak MS, Abdelhamid MR, Schmidhalter U (2015) Effect of foliar application of aminoacids on plant yield and some physiological parameters in faba bean plants irrigated with seawater. Acta Biol Colombiana 20(1):141–152
Sadak M, Abd Elhamid E, Ahmed M (2017) Glutathione induced antioxidant protection against salinity stress in chickpea (Cicer arietinum L.) plant. Egypt J Bot 57(2):293–302
Sadak MS, Bakry AB, Taha MH (2019). Physiological role of trehalose on growth, some biochemical aspects and yield of two flax varieties grown under drought stress. Plant Archives 19(2):215–225
Sadak MS, Abd El-Hameid AR, Zaki FSA, Dawood MG, El-Awadi ME (2020) Physiological and biochemical responses of soybean (Glycine max L.) to cysteine application under sea salt stress. Bull Natl Res Cent 44:1. https://doi.org/10.1186/s42269-019-0259-7
Sajjad A, Saad S, Khan AA (2017) Effects of thiamine hydrochloride on plant growth and nutrient uptake of mustard. Indian J Appl Pure Biol 32:265–277
Salisbury FB, Ross CW (1992) Mineral nutrients. In: PlantPhysiology. Wadsworth Inc, Belmont, pp 116–135
Shalata A, Neumann P (2001) Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot 52(364):2207–2211
Sharifi M, Ghorbanli M, Ebrahimzadeh H (2007) Improved growth of salinity-stressed soybean after inoculation with pre-treated mycorrhizal fungi. J Plant Physiol 164:1144–1151
Shimasaki K, Fukunoto Y (1998) Effects of B vitamins and benzylaminopurine on adventitious shoot formation from hypocotyls segments of snapdragon (Antirrhinum majus L.). Plant Biotechnol 15(4):239–240
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, San Diego
Snedecor GW, Cochran WG (1989) Statistical methods, 8th edn. Iowa State University Press, Ames
Soliman AS, Shanan NT, Massoud ON, Swelim DM (2012) Improving salinity tolerance of Acacia saligna (Labill.) plant by arbuscularmycorrhizal fungi and Rhizobium inoculation. J Biotechnol 11(5):1259–1266
Stroganov BP (1962) Physiological basis of the salt tolerance of plants (under different types of soil salinization). Izd.Akad.Nauk, Moscow
Toscano S, Ferrante A, Romano D (2019) Response of Mediterranean ornamental plants to drought stress. Horticulturae 5(6):1–20
Tzortzakis NG (2009) Influence of NaCl and calcium foliar spray on lettuce and endive growth using nutrient film technique. Int J Veg Sci 15:1–13
Vartainan N, Hervochon P, Marcotte L, Larher F (1992) Proline accumulation during drought rhizogenesis in Brassica napusvar. oleifera. Plant Physiol 140:623–628
Vogelsang-O’Dwyer M, Bez J, Petersen IL, Joehnke MS, Detzel A, Busch M, Krueger M, Ispiryan L, O’Mahony JA, Arendt EK, Zannini E (2020) Techno-functional, nutritional and environmental performance of protein isolates from blue lupine and white lupine. Foods 9(2):230. https://doi.org/10.3390/foods9020230
Wolko B, Clements JC, Naganowska B, Nelson MN, Yang H (2011) Lupinus. In: Kole C (ed) Wild crop relatives: genomic and breeding resources. Springer, Berlin, Heidelberg, pp 153–206
Wu QS, Ying-Ning Z, Abd Allah EF (2014) Mycorrhizal association and ROS in plants. In: Ahmad P (ed) Oxidative damage to plants. Academic Press; Elsevier, New York, pp 453–475
Yamato M, Ikeda S, Iwase K (2008) Community of arbuscularmycorrhizal fungi in a coastal vegetation on Okinawa Island and effect of the isolated fungi on growth of sorghum under salt-treated conditions. Mycorrhiza 18:241–249
Yemm EW, Cocking EC (1955) The determination of amino acids with ninhydrin. Analyst 80:209–213
Yemm EW, Willis AJ (1956) The respiration of barley plants IX. the metabolism of roots during the assimilation of nitrogen. New Phytol 55(2):229–252 (http://www.jstor.org/stable/2429868)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M. S. Sadak, designed and performed the experiment, responsible for all physiological and biochemical, responsible for statistical analysis and also wrote and reviewed the manuscript. The author has read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
M. S. Sadak declares that he has no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sadak, M.S. Physiological Role of Arbuscular Mycorrhizae and Vitamin B1 on Productivity and Physio-Biochemical Traits of White Lupine (Lupinus termis L.) Under Salt Stress. Gesunde Pflanzen 75, 1885–1896 (2023). https://doi.org/10.1007/s10343-023-00855-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-023-00855-y