Abstract
Several natural enemies are known as predators of the whitefly Bemisia tabaci, which is one of the most invasive pests worldwide and has developed high resistance to pesticides. However, biological control of this pest on tomato is often difficult because the plant’s glandular trichomes release substances that are toxic to arthropods and hinder the foraging of natural enemies. Therefore, adaptation of natural enemies to this crop is one of the selection criteria for potential biocontrol agents. We collected predatory mites from wild and feral tomato plants and found the species Amblyseius herbicolus and A. tamatavensis. Whereas the latter is known to feed on B. tabaci eggs, we investigated the ability of A. herbicolus to develop and reproduce when feeding on this prey stage, and assessed whether both species can feed and develop on B. tabaci crawlers. To verify the adaptation of these predators to tomato, we assessed their ability to disperse on tomato plants and their establishment on clean tomato plants with pollen as an alternative food. Finally, we evaluated whether the predators were effective in controlling B. tabaci on tomato plants with different pollen dosages as alternative food. We show that both predators fed and reproduced on B. tabaci immatures. A. herbicolus established and dispersed better on tomato plants supplemented with cattail pollen than A. tamatavensis and only A. herbicolus was able to control B. tabaci in two population dynamics experiments. Our results suggest that A. herbicolus is better adapted to tomato than A. tamatavensis and may therefore be a promising biocontrol agent on tomato.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato (Lycopersicon esculentum Miller) is among the most important crops in the world (Naika et al. 2005; Collins et al. 2022). One of the biggest challenges for tomato growers is the susceptibility of tomato plants to attacks by numerous pests and diseases (Picanço et al. 2007; Desneux et al. 2010; Hanssen et al. 2010; Simmons et al. 2018; Wakil et al. 2018; Jones 2021). A key pest of tomato is the whitefly Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) (Brown et al. 1995). This pest can seriously damage tomato plants by feeding on plant sap and by excreting honeydew on which fungi can grow, which decreases the rate of photosynthesis and consequently leads to significant yield reductions (Brown et al. 1995; Oliveira et al. 2001; Calvo et al. 2009). Moreover, B. tabaci can transmit a large number of plant-pathogenic viruses (Jones 2003; Navas-Castillo et al. 2011), including the devastating tomato yellow leaf curl virus (TYLCV) (Navot et al. 1991; Varma and Malathi 2003) and the tomato chlorosis virus (ToCV) (Wisler et al. 1998). Together, whiteflies and diseases can cause crop losses of up to 100% (Picó et al. 1996; Velasco et al. 2008). On tomato plants, whiteflies are usually controlled with a high number of pesticide applications from sowing to fruit harvesting (Picanço et al. 1997; Palumbo et al. 2001; Perring et al. 2018). Besides inflicting damage to human health and ecosystems worldwide, this excessive use of pesticides has led to severe and widespread resistance of whiteflies and other tomato pests (Palumbo et al. 2001; Horowitz et al. 2020). Moreover, a meta-analysis of field experiments showed that chemical pest control is often ineffective in the presence of natural enemies (Janssen and van Rijn 2021). Therefore, there is a growing demand for environmentally safer pest control methods in order to promote the long-term viability of agricultural systems (Pretty and Bharucha 2014; Pretty 2018).
One sustainable pest management practice is augmentative biological control; the release of large numbers of natural enemies to suppress pest densities (Van Lenteren 2000, 2012; van Lenteren et al. 2018). Various natural enemies, such as parasitoids, predatory mirids and predatory mites are commercially employed in augmentative biological control, but their success on tomato lags behind. Predatory mites, mostly from the family Phytoseiidae, are the dominant agents in commercial augmentative biological control in many countries, currently representing over 60% of the global market for arthropod natural enemies (van Lenteren 2012; Knapp et al. 2018). Amongst the factors that explain the success of predatory mites in biological control programmes are their generalist feeding habits, which enable then to control multiple pest species (Messelink et al. 2008a, 2012; Janssen and Sabelis 2015; Knapp et al. 2018) and their short generation times, allowing rapid numerical responses to increased pest densities (Helle and Sabelis 1985). Moreover, many of these predators also feed on plant-provided food such as pollen and nectar, which allows them to be introduced before the pest is present and to survive in the crop when target pest densities are low (van Rijn et al. 2002; Messelink et al. 2014; Janssen and Sabelis 2015).
Phytoseiid mites are often ineffective at controlling pests on tomato (van Haren et al. 1987; Gillespie and Quiring 1994; Cedola et al. 2001; Kennedy 2003; Legarrea et al. 2022). This is likely due to contact with glandular trichomes and their exudates (van Haren et al. 1987; Sato et al. 2011; van Houten et al. 2013; Legarrea et al. 2022). Glandular trichomes are outgrowths of the epidermis in which specialized metabolites are synthesized and stored (Schuurink and Tissier 2020). While trichomes act as a broad-spectrum direct defence against herbivores (Glas et al. 2012), they can also increase the mortality of natural enemies (Belcher and Thurston 1982; van Haren et al. 1987; Verheggen et al. 2009; Legarrea et al. 2022). In addition, trichomes can affect the density of herbivorous prey and hinder the establishment and dispersal of predators on the plant (van Lenteren et al. 1995; Simmons and Gurr 2005; Carrillo et al. 2008; Paspati et al. 2021; Legarrea et al. 2022). Therefore, finding phytoseiid mites that are adapted to tomato is a significant challenge.
In an attempt to find tomato-adapted predatory mites, we sampled tomato plants in the state of Minas Gerais, Brazil. Commercial tomato crops with pesticide applications usually do not harbour any predatory mites; therefore, predators were collected from untreated cultivated and feral tomato plants from gardens in urban and rural areas. Amblyseius herbicolus Chant (Acari: Phytoseiidae) was the most abundant species of predatory mites found. Other species were Amblyseius compositus Denmark and Muma, Propioseiopsis mexicanus (Garman) and Typhlodromus transvaalensis (Nesbitt), but they proved difficult to rear in the lab. Amblyseius herbicolus reproduces through thelytokous parthenogenesis; hence, populations consist of females only (De Moraes and Mesa 1988). It is a generalist predator, abundant in several crops and it can prey on several tomato pests such as immatures of B. tabaci, broad mites, and to some extent on spider mites (Rodríguez-Cruz et al. 2013; Cavalcante et al. 2015; Duarte et al. 2015). It is also able to feed on alternative food such as pollen (Rodríguez-Cruz et al. 2013; Marcossi et al. 2020). Another predatory mite found on tomato plants was Amblyseius tamatavensis Blommers (Acari: Phytoseiidae). This species has also shown potential to prey on B. tabaci eggs (Cavalcante et al. 2015, 2017; Barbosa et al. 2019) and reduced the densities of these whiteflies in the presence of pollen on bell pepper plants (Cavalcante et al. 2017). Amblyseius herbicolus is slightly larger than A. tamatavensis (Marina Barbosa, pers. comm.).
Although it is known that these predators can feed on B. tabaci, further experiments are still needed to determine their suitability to control this pest on tomato plants. Here, we report the ability of A. herbicolus to develop and reproduce when feeding on whitefly eggs on tomato leaves. Moreover, we measured oviposition and predation on B. tabaci crawlers by A. herbicolus and A. tamatavensis. To verify the adaptation of these predators to tomato, we assessed their ability to move on tomato plants and whether they can establish on clean tomato plants with pollen as alternative food. Finally, we evaluated whether these predators were effective in controlling B. tabaci on tomato plants using different pollen dosages as supplemental food.
Materials and methods
Plant material
Tomato seeds (Solanum lycopersicum var. Aguamiel (EX V305), Limagrain®, imported by Vilmorin Seed Generation in Brazil) were sown in polystyrene trays (8 × 16 cells) in a commercial plant substrate enriched with macro and micronutrients (MECPLANT®-Mec Prec, Telêmeco Borba). Twenty days after germination, the seedlings were transplanted to plastic pots (2–3.5 L, depending on the experiment) with the same substrate. The plants were fertilized every week with a solution of 50 g of N-P-K (20-05-20) and 100 g of simple superphosphate in 20 L of water and they were tied to a bamboo stick with a string for support. The plants were kept inside cages in a room with natural light at 23 ± 3 °C and 70 ± 10% relative humidity. Cattail pollen used in all experiments was collected from Typha sp. plants on the campus of the Federal University of Viçosa. The pollen was dried in an oven at 60 °C for 24 h and stored in a freezer at − 20 °C according to recommendation (Hagedorn and Moeller 1968; Pernal and Currie 2000).
Rearing Bemisia tabaci
Rearing units of B. tabaci biotype B were started with individuals from a culture of the Laboratory of Integrated Pest Management of the Federal University of Viçosa, collected from cabbage and individuals obtained from tomato plants at the campus of the university. They were kept on tomato plants inside cages (0.5 × 0.5 × 1.0 m3). A new, clean tomato plant with at least four completely developed leaves was added to each cage every week. The oldest plants were removed from the cage once a month. The cages were kept in a room with natural light at 23 ± 3 °C and 70 ± 10% relative humidity.
Mite collection and rearing
To collect predatory mites, leaves attacked by pests were removed from selected plants in the field, placed in paper bags and kept in a cooler with ice packs to reduce predatory mite activity until laboratory evaluation. The leaves were inspected with a stereomicroscope and 11 adult predatory mites were found and transferred to arenas made of PVC sheets (15 × 10 cm) on top of foam pads (h = 3 cm), which were kept in plastic trays (29 × 14 × 4 cm) filled with water. The edges of the arenas were wrapped in wet tissue paper, serving as a barrier for mite escapes and as a water source (van Rijn and Tanigoshi 1999). Small pieces of cotton wool were placed on the arenas as oviposition sites and were covered with pieces of tent-shaped PVC sheet (1.5 cm2) for shelter. Initially, Typha sp. pollen and bee pollen (Santa Barbara®, Apiários Mackllani Ltda, Minas Gerais, Brazil) were supplied as food twice per week, until we noticed that Typha sp. pollen alone was sufficient to maintain laboratory cultures. After establishing a culture, microscope slides were made and the species was identified as A. herbicolus by Manoel Guedes Correa Gondim Junior from the Federal Rural University of Pernambuco (UFRPE).
A culture of A. tamatavensis was maintained in our laboratory and had been started with individuals found in our culture of Tetranychus urticae Koch (Acari: Tetranychidae) on tomato plants. The arenas of A. tamatavensis were made of black plastic dishes (Ø = 13 cm, h = 2 cm) placed on top of foam pads (h = 3 cm), which were kept in plastic trays (29 × 14 × 4 cm) filled with water. These predators were fed with cattail pollen and with the mite species Thyreophagus cracentiseta Astigmatina (Sarcoptiformes) (Barbosa, OConnor & Moraes) mixed with wheat bran once a week. The culture of T. cracentiseta was started with individuals obtained from the Laboratory of Acarology of the Escola Superior de Agricultura “Luiz de Queiroz” (ESALQ), in 2019. These astigmatids were reared on sterilized wheat bran in plastic pots (Ø = 10; h = 15 cm) covered with mesh (90 µm). Rearing arenas of all mite species were kept in a room at 24 ± 1 °C, 70 ± 10% RH, 12:12 L:D.
Oviposition and development of A. herbicolus on B. tabaci eggs
We first investigated whether A. herbicolus was able to reproduce and develop on B. tabaci eggs. Because it is already known that A. tamatavensis can do so (Cavalcante et al. 2015, 2017; Barbosa et al. 2019), we did not carry out such experiments with this latter species. Leaves of a tomato plant from the B. tabaci rearing (infested for c. 3 days) were removed and leaflets were cut into pieces. Each piece was examined with a stereomicroscope to ensure the presence of at least 20 fresh B. tabaci eggs (less than 48-h old, based on their light green coloration), and subsequently added to a Petri dish (Ø = 5 cm, 1.5 cm high). A piece of wet filter paper, serving as source of moisture, was placed inside each dish and the dishes were covered with transparent lids. As control, one mg of pollen with a clean piece of leaflet was placed in another set of those dishes. A predator larva was collected from the rearing unit with a fine brush and placed in each dish. The predators were checked once a day to verify survival and development until adulthood. After becoming adults, individuals were checked once a day to verify oviposition. Every day, the piece of leaf of each arena was replaced with a new piece with the same amount of fresh food, and predator eggs were removed to prevent cannibalism (Marcossi et al. 2020). Oviposition of the individuals surviving until adulthood was recorded during five more days. Fifteen and sixteen replicates were done for B. tabaci eggs and Typha sp. pollen respectively. The tests were performed in a room at 23 ± 3°C, 70 ± 10% relative humidity and 12 h of light. The effects of diet on survival and development were analysed with a Cox proportional hazards survival analysis of the survival package of R (Therneau 2020). To compare oviposition rates, data were taken from one to five days after the first individuals became adult, which were compared with a linear mixed effects model (LME, package “nlme”, Pinheiro et al. 2020) with diet and time as fixed factors and individual as random factor to correct for pseudoreplication. All models here and below were checked with normal error plots and plots of residuals against fitted values. Significance of factors and interactions were determined with likelihood ratio (L.R.), deviance, or F-tests. Contrasts among treatments were obtained with the package emmeans with a Tukey correction for multiple comparisons (Lenth 2019). All statistical analyses were done using the software R version 4.3.0 (R Core Team 2023).
Predation and oviposition of A. herbicolus on new and old eggs
We evaluated the predation and oviposition rates of A. herbicolus on young and old B. tabaci eggs (less and more than 48 h old, respectively). The experiment was carried out as above, but we used young females of A. herbicolus, aged between 4 and 6 days since becoming adults from an arena where they received pollen as food. Only ovipositing females were selected, with an egg visibly present in their opisthosoma. Predation and oviposition rates were verified every 24 h during 3 days. Eight replicates were done with exception of the treatment with pollen, which was replicated six times. The first day of oviposition was excluded from the analysis because of the possible effect of the previous pollen diet (Sabelis 1990). Oviposition and predation data were analysed with linear mixed effects models (LME) with replicate as random factor and diet and time as fixed factors.
Predation and oviposition of both predatory mites feeding on B. tabaci crawlers
We assessed the ability of A. herbicolus and A. tamatavensis to feed and reproduce on crawlers of B. tabaci on tomato leaf discs (Ø = 2 cm) arranged on wet tissue paper (Ø = 3 cm) and placed in Petri dishes as above. Twenty whitefly crawlers of up to 30 h old were placed on each leaf disc, which is sufficient to avoid prey depletion during the experiment. Typha sp. pollen placed on clean leaf discs was offered as food in a control treatment. Young, ovipositing females of each predator species, selected as above, were placed each in a separate Petri dish as above. The tests were performed in a room as above. Predation and oviposition rates were verified every 24 h during 4 days. After each evaluation, each predator was transferred to a Petri dish containing fresh prey. Fifteen replicates were done per treatment. The first day of oviposition was excluded as above. The numbers of crawlers consumed were analysed with a LME with replicate as random factor to correct for repeated measures, predator species as categorical factor and time as continuous factor. The interaction between predator species and time was also included but was not significant. The numbers of eggs produced on the different diets were compared within each predator species separately. Because of considerable non-normality of the errors, also after transformation, oviposition rates were summed per individual over the last three days of the experiment and compared with a GLM with a Poisson error distribution (log link). Nevertheless, we present oviposition data per day below to show possible trends through time.
Establishment on tomato plants
Here, we investigated the ability of both predatory mite species to move and reproduce on tomato plants during seven days. Tomato plants were three weeks old (about 20 cm high) with three fully developed leaves. Pollen (1 mg) was placed on the release leaflet (the second leaflet from the insertion of the first leaf into the stem) at the beginning of the experiment and on the target leaflet (the leaflet opposite the release leaflet on the same leaf) on the fourth day. Eight young, ovipositing females of each predator species, selected as above, were released on the release leaflet of each plant (n = 11 plants). Wet cotton wool was placed around the base of the stem to confine predators on the plants. The position of the plants was randomized in a room at 23 ± 3 °C, 70 ± 10% relative humidity and 12 h of light. On the seventh day, the predatory mites were scored as dead or alive on the release and target leaflets and on the rest of the plant. Total numbers of dead plus alive predators of the two species on the entire plants were compared with a generalized linear model (GLM) with a Poisson error distribution (log link), numbers of alive individuals on the release plus target leaflet were log(x + 1) transformed and compared with a GLM with Gaussian error distribution (identity link), and the distribution of the alive individuals on the release and target leaflets were compared between the two species with a quasi-binomial GLM (logit link).
Establishment and population growth on tomato plants
We also tested the population growth and movement of the predatory mites on entire tomato plants with cattail pollen during twenty-eight days. Fifteen young, ovipositing females of A. herbicolus or A. tamatavensis, selected as above, were introduced individually on the lowest (= oldest) leaf from each four-leaf-stage tomato plant (16 plants per treatment). Typha sp. pollen (1 mg) was provided as food on the youngest, top leaves two times per week, taking care not to spill it on lower leaves. The plants were kept together outside (mean temperature 24.3 °C, range 18.7–27.7 °C; mean relative humidity 85%, range 59–96%), hence, could be infested by other organisms. During the experimental period, plants were allowed to develop from four to nine leaves, but the plant apices were subsequently removed, thus maintaining a maximum plant size of nine leaves. Four plants were evaluated per week by cutting them into pieces and storing the pieces in separate boxes according to the stratum of the plant. We assessed the densities of both predatory mites on three different plant strata (bottom, middle and top) under a stereo microscope (25 × magnification; Zeiss, Oberkochen, Germany). Moreover, the presence of pests and other organisms that had naturally invaded the plant was noted. Densities of the two predatory mite species were compared using a quasi-Poisson GLM (log link), with species, time and their interaction as factors. We also assessed differences in the proportions of the total population consisting of juveniles to detect differences in reproduction on the plants between the two species. This was done with a GLM with a binomial error distribution (logit link). Subsequently, we analysed the distribution of A. herbicolus (log(x + 1)-transformed numbers) over the three plant strata with a linear mixed effects model with time and strata as factors and individual plant as random factor.
Dynamics of B. tabaci, A. herbicolus and A. tamatavensis on tomato plants
We evaluated whether preventive releases of A. herbicolus and A. tamatavensis with the addition of pollen could prevent future infestations of B. tabaci. The experiment was carried out in 3 large outdoor screen cages (2 × 2 × 2 m–Howitec Netting®), each with 16 tomato plants (mean temperature 21.4 °C, range 14.8–25 °C; mean relative humidity 58%, range 33–82%). To prevent cross-contamination, the plants were spaced in the cage, and each plant pot was put in a dish with water, serving as barrier for dispersing mites. Initially, a tomato leaf disc (3 cm2) with 5 young, ovipositing female predatory mites (as above) was introduced on the bottom, middle and top leaf from each young (4-leaf stage) tomato plant in two cages, hence, in total 15 mites per plant. We also took care that the plants did not touch the mesh of the cages to prevent dispersal of predators. Plants in one cage received A. herbicolus, in a second cage, they received A. tamatavensis, and no predators were released in the third cage. One milligram of Typha sp. pollen was applied above the newly developed leaves of each plant twice per week, including to those in the cage without predators. Most of the pollen fell onto the new leaves, but some also fell onto the older leaves. Predatory mite densities on plants were not sampled during the first 3 weeks to avoid disturbance. Subsequently, we collected B. tabaci from the rearing cages using an aspirator. The whiteflies were placed in a refrigerator at 4 °C for 2 min to reduce their activity, and three pairs of female plus male whiteflies were then released on each plant in each cage inside a clip cage. Clip cages were placed on the third leaf of all plants and were removed after 2 days, leaving the adult whiteflies and their eggs behind. This procedure was repeated two weeks later, so a total of 6 pairs of whiteflies were released on each plant (three and five weeks after the release of predatory mites). Note that adult whiteflies were able to migrate among plants of the same treatment after the clip cages were removed, but juveniles were restricted to the plants. Although previous experiments with B. tabaci with another predatory mite species showed that the presence of predators on a plant only induces limited dispersal of adult whiteflies, which are not attacked by the predators (Meng et al. 2012), we did not analyse the numbers of adult whiteflies per plant. The numbers of predatory mites were evaluated weekly from the introduction of whiteflies (day 0) and the numbers of juvenile whiteflies seven days after the release of the first three pairs (day 7). The evaluations were carried out using a handheld magnifying glass and head magnifier with light, sampling all the leaves of each tomato plant. At the last sampling, the plants were cut and stored in separate boxes for evaluation in the laboratory under a stereo microscope. During this last evaluation, it was therefore also possible to assess the densities of whitefly eggs on the plants. Powdery mildew occurred during this experiment, became severe from the 7th week onwards, which forced us to end the experiment after thirty-two days after the introduction of B. tabaci. Numbers of juvenile whiteflies (log(x + 1)-transformed) were compared among treatments with an LME with predator treatment and time as factors and plant and cage as random factors to correct for pseudoreplication. Numbers of whitefly eggs were compared with a Kruskal–Wallis ANOVA with a Holm test for pairwise comparisons (package FSA, Ogle et al. 2022).
Dynamics of B. tabaci and A. herbicolus on tomato plants with high pollen supply
A second experiment was done to evaluate the capacity of A. herbicolus to reduce B. tabaci populations on tomato plants when supplying a larger amount of pollen distributed throughout the entire plant. It was conducted in six BugDorm-4F insect cages (0.5 × 0.5 × 1.0 m) inside a greenhouse. A tomato plant with four developed leaves and ca. 20 cm high was placed inside each cage. Half of the plants received fifteen young, ovipositing females (as above) of A. herbicolus. On the same day, three pairs of adults of B. tabaci (6 ± 1 days of age) were introduced in each of the six cages. All plants also received 15 mg of Typha sp. pollen distributed over all leaves of the plants using a brush. The experiment was evaluated twice a week. Whitefly adults were counted while they were sucked into a pipette tip (1000 µl; Nichiryo, Japan) connected to a transparent hose (Ø = 1 cm) closed with a mesh (size 170 μm) to avoid counting the same insect more than once. Subsequently, adult predatory mites were carefully counted on all leaves. Then, more pollen was added to the plants and the adult whiteflies were reintroduced to the cages by inserting the pointed part of the pipette tip into the soil. Plants without predatory mites were always evaluated first to avoid contamination of these control plants with predators. The experiment lasted for 61 days, and the temperature during the experiment ranged from 13 to 26 °C. Differences in numbers of whiteflies per plant between treatments were analysed with an LME with log(x + 1)-transformed numbers of whiteflies as dependent variable, time and treatment as fixed factors and replicate as random factor.
Results
Oviposition and development of A. herbicolus on B. tabaci eggs
Nineteen out of twenty juvenile A. herbicolus survived on a diet of B. tabaci eggs, whereas all twenty juveniles survived on a pollen diet (Fig. 1), but development was significantly faster on a diet of pollen than on whitefly eggs (Fig. 1; Cox proportional hazards: Likelihood ratio (L.R.) = 11.67, d.f. = 1, p < 0.001). There was no significant difference in oviposition through time between the two diets (Fig. 1, interaction of diet with time: L.R. = 0.84, d.f. = 1, p = 0.36) and there was no significant effect of diet on oviposition (Fig. 1, LME, L.R. = 2.86, d.f. = 1, p = 0.091). Per diet, oviposition rates varied significantly through time (Fig. 1, LME, L.R. = 137.9, d.f. = 1, p < 0.0001).
Juvenile development, survival and average oviposition rates of Amblyseius herbicolus fed with Bemisia tabaci eggs or Typha sp. pollen. Juvenile development is shown as the mean (± s.e.) cumulative proportion of adults as a function of time (B. tabaci: white circles; Pollen: white squares). Total survival is the final cumulative proportion that reached adulthood on day 6 (open circles and squares for a pollen or whitefly egg diet, respectively). Average oviposition rates (± s.e.) of A. herbicolus on the 4th-9th day are given by black circles (B. tabaci) and black squares (Pollen)
Predation and oviposition of A. herbicolus on new and old eggs
The predation rate on old eggs was significantly lower than on young eggs (Fig. S1a, LME, L.R. = 70.5, d.f. = 1, p < 0.0001). The oviposition of A. herbicolus also differed significantly among diets through time (Fig. S1b; LME, interaction of diet with time: L.R. = 18.8.27, d.f. = 2, p = 0.016). On the third day, oviposition of A. herbicolus on a diet of old B. tabaci eggs was lower than when feeding on young eggs or pollen (Fig. S1b).
Predation and oviposition of both predator mites feeding on B. tabaci crawlers
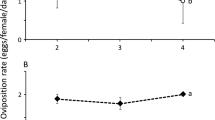
The average consumption of B. tabaci crawlers did not differ significantly between the two predator species (Fig. 2a; LME: L.R. = 2.04, d.f. = 1, p = 0.15) or among days (L.R. = 1.85, d.f. = 1, p = 0.17). The two predator species produced more eggs on a diet of pollen than on a diet of crawlers (Fig. 2b), but this difference was not significant for A. tamatavensis (GLM, A. herbicolus: Deviance = 8.37, d.f. = 1, p = 0.0038; A. tamatavensis: Deviance = 3.53, d.f. = 1, p = 0.060).
Average (± s.e.) predation (a) and oviposition rates (b) of A. herbicolus (circles) and A. tamatavensis (squares) on the 2nd–4th day of feeding on crawlers of B. tabaci (white symbols) or Typha sp. pollen (black symbols). The average predation rate (a) did not differ significantly between the two species and different letters to the right of the data in (b) indicate significant differences between diets per species (a, b for A. herbicolus, x for A. tamatavensis)
Establishment on tomato plants
The two species showed no differences in dispersal between leaflets (Fig. 3a; GLM: F1,19 = 1.28, p = 0.27). The total number of alive plus dead predators after 7 days did not differ significantly between predator species (Fig. 3a; GLM: Deviance = 0.0047, d.f. = 1, p = 0.95). There was also no significant difference in the number of alive predators of the two species on the release plus target leaflets (Fig. 3a; GLM: F1,20 = 0.15, p = 0.7).
a Average numbers (± s.e.) of alive and dead predators found on the release and target leaflets or on the rest of the plants after 7 days. Negative (downwards) error bars are of the categories, positive (upward) error bars are of the total. Dispersal of the two species did not differ significantly. b Average densities (± s.e.) of juveniles (white symbols) and adults (black symbols) of the predatory mites A. herbicolus (circles) and A. tamatavensis (squares) on tomato plants during 28 days when only pollen was offered as food, in a second experiment, different from that presented in (a). Total numbers of A. herbicolus were significantly higher than of A. tamatavensis (GLM, indicated with a, b at the right), as was the proportion of juveniles (x, y). (c) Average densities (± s.e.) of all motile stage of the predatory mite A. herbicolus in three different plant strata in the same experiment as (b). Densities in strata with different letters in the legend differed significantly (contrasts through model simplification after GLM; p < 0.05)
Establishment and population growth on tomato plants
In a test on whole tomato plants during 28 days, the numbers of adults plus juveniles of A. herbicolus were significantly higher than those of A. tamatavensis (Fig. 3b; GLM: F1,30 = 22.4, p < 0.0001) and the numbers of predators of both species varied through time (F1,29 = 14.7, p < 0.001). The proportion of juveniles of A. herbicolus was also significantly higher than that of A. tamatavensis (Fig. 3b; GLM: F1,27 = 31.2, p < 0.001). Whereas A. tamatavensis remained only in the lowest stratum (data not shown), A. herbicolus spread somewhat towards the other strata near the end of the experiment. However, the distribution of the numbers of A. herbicolus over the three strata differed significantly (lme: L.R. = 62.0, d.f. = 2, p < 0.0001), with numbers in the top and middle stratum being much lower than in the bottom stratum (Fig. 3c). Besides the predatory mites, we found whiteflies, some thrips, astigmatid mites, iolinid mites, powdery mildew and some spiders on the plants (Suppl. Table 1).
Dynamics of B. tabaci, A. herbicolus and A. tamatavensis on tomato plants
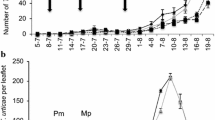
There was a significant difference in densities of immature B. tabaci among the three cages through time (LME: interaction of treatment with time: L.R. = 6.87, d.f. = 2, p = 0.032). Amblyseius herbicolus delayed the increase of whiteflies compared to the treatment with A. tamatavensis and without predators, but only significantly so at 7 and 14 days after the beginning of the experiment (Fig. 4a). Tomato plants with A. herbicolus had significantly lower whitefly egg densities than plants without predators, but did not differ from plants with A. tamatavensis (Fig. 4a; Kruskal–Wallis ANOVA, Chi2 = 7.96, d.f = 2, p = 0.019). Whereas A. tamatavensis disappeared from all plants, populations of A. herbicolus persisted (Fig. 4b).
Population dynamics of B. tabaci and the predatory mites A. herbicolus or A. tamatavensis on tomato plants. Vertical arrows indicate the days that the plants were infested with 3 pairs of whiteflies. a Average densities (± SE) of immature whiteflies and average numbers of whitefly eggs on the 28th day in the presence of A. herbicolus (circles), A. tamatavensis (triangles) and without predators (squares). For egg densities at 28 days (right-hand points), different letters indicate significant differences among treatments (Kruskal–Wallis rank sum test with Holm method correction; p < 0.05). Asterisks and n.s. above the data points show significance and non-significance of difference between the two treatments with predators from the control (contrasts after LME). b Average densities (± s.e.) of all mobile stages of both predatory mites
Dynamics of B. tabaci and A. herbicolus on tomato plants with high pollen supply
In the experiment with higher pollen supply, the density of B. tabaci in the presence of A. herbicolus was significantly lower through time than in their absence (Fig. 5a; LME: interaction of predator presence with time: L.R. = 18.5, d.f. = 1, p < 0.001). The difference in whitefly densities between plants with and without predators became apparent and significant from day 47 onwards, when a new generation of adult whiteflies appeared on the plants (Fig. 5a). At the end of the experiment, there were about 91% more adult whiteflies on plants without predatory mites than on plants with A. herbicolus. Predator densities increased until reaching an average of 120 predators per plant around the 37th day, after which when they began to decrease. At the end of the experiment, there were still about forty predatory mites per plant (Fig. 5b).
Population dynamics of B. tabaci and A. herbicolus on tomato plants with a high amount of pollen, spread over the plants. a Average densities (± s.e.) of adult whiteflies in the presence of A. herbicolus and without predators (Control). The vertical arrow indicates the day that the plants were infested with 3 pairs of whiteflies. Different letters in the legend indicate significant differences of densities through time. Asterisks indicate significant differences between treatments per time step (contrasts through model simplification after LME): *p < 0.05. b Average densities (± s.e.) of all mobile stages of A. herbicolus
Discussion
We show that A. herbicolus is able to develop on B. tabaci eggs (Fig. 1), and both A. herbicolus and A. tamatavensis fed and reproduced on a diet of B. tabaci crawlers (Fig. 2). However, A. herbicolus established and dispersed better on tomato plants supplemented with cattail pollen than did A. tamatavensis (Fig. 3b, c) and only A. herbicolus significantly reduced B. tabaci densities in the two population dynamics experiments (Figs. 4a and 5a). These results suggest that A. herbicolus is better adapted to tomato plants than A. tamatavensis, and thus, it may be a more efficient biocontrol agent on tomato.
Predation rates of A. herbicolus and A. tamatavensis on crawlers were lower than those reported for A. swirskii, which is commonly used for whitefly control (Nomikou et al. 2001, 2002, 2004; Calvo et al. 2015; Janssen and Sabelis 2015), but the host plant used in these papers differed from ours. The oviposition rate of A. herbicolus fed with crawlers was lower than that of A. tamatavensis, however, the eggs produced by A. herbicolus will only result in female offspring, which will increase the intrinsic growth rate of this species compared to sexual species with the same oviposition rate (Cicolani 1979). Our findings show that both predatory mites can efficiently reproduce when feeding on crawlers and cattail pollen, which is contrary to the results of Cavalcante et al. (2015), who reported low oviposition rates for A. tamatavensis when fed with cattail pollen. The ability of predatory mites to feed on alternative resources allows their introduction and persistence in crops with low pest densities or before pest invasion, potentially resulting in an increase in predator densities and enhanced pest control (McMurtry and Johnson 1965; Kennett et al. 1979; van Rijn and Sabelis 1993; Nomikou et al. 2010; Delisle et al. 2015; Duarte et al. 2015; Leman and Messelink 2015).
An important characteristic when selecting natural enemies is their adaptability to the host plant. Negative effects of tomato plants on the survival of phytoseiids have been observed frequently due to contact with glandular trichomes and their exudates (van Haren et al. 1987; Paspati et al. 2021; Legarrea et al. 2022, but see Castañé et al. 2022 for an exception). Secondary metabolites, such as acyl sugars present in tomato trichomes can be highly toxic to mites and can accumulate on their bodies after walking on tomato stems (Chatzivasileiadis and Sabelis 1997). Whereas our results showed no differences in the capacity of A. tamatavensis and A. herbicolus to move from leaflet to leaflet of the same leaf (Fig. 3a), they did differ significantly in their capacity to move from leaf to leaf and in their reproduction on entire tomato plants (Fig. 3b). This was probably caused by the higher density of trichomes on tomato stems than on leaves (van Haren et al. 1987). Although A. herbicolus initially showed a high mortality rate in the experiment on entire plants, their numbers increased about 4.2 times during the experiment, with a high number of juveniles (Fig. 3b). Possibly, A. herbicolus can adapt its behaviour to tomato defences, as was demonstrated for other predators on tomato (Drukker et al. 1997; Wheeler and Krimmel 2015).
Whereas A. tamatavensis was always found close to their point of release, A. herbicolus was found in the different strata of the plant, but still 96.8% were found in the lowest stratum (Fig. 3c). Similar results were found by van Houten et al. (2013) for Amblydromalus limonicus. In this last study, predatory mites only successfully established on the plants after degradation of tomato trichomes as a result of Aculops lycopersici infestations. A recent study showed that predatory mites do not only perform better on tomato plants lacking defensive hairs, but also that they can suppress herbivore densities better and faster on these plants without trichomes (Legarrea et al. 2022). Based on the limited mobility of mites on tomato plants due to the high density of trichomes on tomato stems (van Haren et al. 1987), different strategies could be used to increase the densities of A. herbicolus on plants. One possibility is increasing the frequency of release of the predators on different parts of the plant. This approach could improve establishment of the predators on various plant parts without the need to cover long distances on the plant.
In the first population dynamics experiment in which we tested both predators with a low dosage of pollen, A. herbicolus was better able to control B. tabaci than A. tamatavensis. Whiteflies were absent during the first 21 days; thus, the only food available for predators was cattail pollen, which was supplied on the newly developed leaves. Hence, predators had to move to these new leaves. This resulted in the extinction of A. tamatavensis from the plants and a reduction in the densities of A. herbicolus (Fig. 4b). Nevertheless, A. herbicolus was able to reduce immature whitefly densities (Fig. 4a). Moreover, the plants with A. herbicolus had c. half the numbers of whitefly eggs at the end of this experiment as the treatment without predators, although this difference was not significant. As a considerable number of whiteflies were still present on the plants at the end of the experiment, control by A. herbicolus was not considered satisfactory. We therefore decided to test this predator with higher quantities of pollen, which was distributed over the plants. Thus, the predator would not need to move frequently to other parts of the plant, thus preventing contact with the stem trichomes and consequently reducing the initial mortality. Indeed, when a larger supply of pollen was distributed throughout the plant, A. herbicolus substantially reduced B. tabaci populations (Fig. 5a). This was certainly due to the high number of predatory mites, with final densities that were on average eight times higher than the release density (Fig. 5b). It is highly likely that the differences in whitefly densities between the two population dynamics experiments were caused by the amount and distribution of the pollen offered. With the availability of commercial equipment to apply pollen throughout a crop (Pijnakker et al. 2014, 2016), the distribution of pollen has become easy. Nevertheless, the densities of A. herbicolus began to decrease after the thirty-seventh day despite the presence of pollen throughout the experiment. This was possibly caused by the supply of excess pollen, which resulted in growth of fungus on the uneaten pollen, which may have decreased pollen quality on plants. It is therefore necessary to investigate the ideal pollen dosage to be supplied to A. herbicolus and other natural enemies to prevent an excess supply of pollen, also because predators may concentrate on feeding on pollen, decreasing their predation of the pest. Besides pollen, there are other alternative foods/prey items that can support predatory mite populations in crops, such as species of astigmatid mites (Messelink et al. 2008a). These mites have particular potential as an alternative food for predatory mites because they can be produced at low costs (Pirayeshfar et al. 2020). The use of astigmatid mites in greenhouse crops is being increasingly explored (Messelink et al. 2014) and further experiments should evaluate the potential of astigmatid mites as alternative food for A. herbicolus on tomato.
To the best of our knowledge, our results are the first to show that a phytoseiid mite can effectively control B. tabaci on tomato plants when supplemented with pollen. Moreover, the fact that A. herbicolus reproduces through thelytokous parthenogenesis can facilitate their persistence in a crop compared to non-thelytokous species because one individual can give rise to an entire new population. Moreover, thelytoky may be advantageous because adaptive traits of strains will not be diluted due to outcrossing with other strains of this species in the field (Hoy 1985; Hoy and Cave 1986). Amblyseius herbicolus is also able to feed on other tomato pests, such as Aculops lycopersici, Polyphagotarsonemus latus and also somewhat on Tetranychus urticae (Rodríguez-Cruz et al. 2013; Cavalcante et al. 2015; Duarte et al. 2015; Cardoso 2019), and this can reduce the number of natural enemies to be introduced in the crop, thus reducing costs (Messelink et al. 2008b). In addition, feeding on more than one pest species can result in better control of those pests through an increase in predator densities (Messelink et al. 2008b; Holt and Bonsall 2017).
Taken together, our results demonstrate the potential of A. herbicolus to control B. tabaci on tomato plants, both when releasing the predator together with the pest, and when introduced before the pest. Our results do not support the use of A. tamatavensis to control B. tabaci on tomato plants. Further studies are needed to investigate the possibility of A. herbicolus controlling whitefly populations in the field.
Author contributions
ACC, IM, MMF and AJ designed experiments, experiments were carried out by ACC, IM, MMF, LSF and MOK, data were analysed by ACC, IM, MMF and AJ, and the ms was written by ACC, IM, MMF, AP, TVMG and AJ. All authors read and approved the final manuscript.
References
Barbosa MFC, Poletti M, Poletti EC (2019) Functional response of Amblyseius tamatavensis Blommers (Mesostigmata: Phytoseiidae) to eggs of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) on five host plants. Biol Control 138:104030. https://doi.org/10.1016/j.biocontrol.2019.104030
Belcher DW, Thurston R (1982) Inhibition of movement of larvae of the convergent lady beetle by leaf trichomes of tobacco. Environ Entomol 11:91–94. https://doi.org/10.1093/ee/11.1.91
Brown JK, Frohlich DR, Rosell RC (1995) The sweetpotato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex? Annu Rev Entomol 40:511–534. https://doi.org/10.1146/annurev.en.40.010195.002455
Calvo F, Bolckmans K, Belda J (2009) Development of a biological control-based integrated pest management method for Bemisia tabaci for protected sweet pepper crops. Entomol Exp Appl 133:9–18. https://doi.org/10.1111/j.1570-7458.2009.00896.x
Calvo FJ, Knapp M, van Houten YM et al (2015) Amblyseius swirskii: What made this predatory mite such a successful biocontrol agent? Exp Appl Acarol 65:419–433. https://doi.org/10.1007/s10493-014-9873-0
Cardoso AC (2019) Search for predatory mites to control tomato pests. MSc Dissertation, Federal University of Viçosa
Carrillo D, Peña JE, Capinera JL (2008) Effect of host plants on successful parasitism by Haeckeliania sperata (Hymenoptera: Trichogrammatidae) on Diaprepes abbreviatus (Coleoptera: Curculionidae) eggs. Environ Entomol 37:1565–1572. https://doi.org/10.1603/0046-225X-37.6.1565
Castañé C, Alomar O, Rocha A et al (2022) Control of Aculops lycopersici with the predatory mite Transeius montdorensis. InSects 13:1116. https://doi.org/10.3390/insects13121116
Cavalcante ACC, Dos Santos VLV, Rossi LC, de Moraes GJ (2015) Potential of five Brazilian populations of Phytoseiidae (Acari) for the biological control of Bemisia tabaci (Insecta: Hemiptera). J Econ Entomol 108:29–33. https://doi.org/10.1093/jee/tou003
Cavalcante ACC, Mandro ME, Paes ER, de Moraes GJ (2017) Amblyseius tamatavensis Blommers (Acari: Phytoseiidae) a candidate for biological control of Bemisia tabaci (Gennadius) biotype B (Hemiptera: Aleyrodidae) in Brazil. Int J Acarol 43:10–15. https://doi.org/10.1080/01647954.2016.1225816
Cedola CV, Sanchez NE, Liljesthrom GG (2001) Effect of tomato leaf hairiness on functional and numerical response of Neoseiulus californicus (Acari: Phytoseiidae). Exp Appl Acarol 25:819–831. https://doi.org/10.1023/A:1020499624661
Chatzivasileiadis EA, Sabelis MW (1997) Toxicity of methyl ketones from tomato trichomes to Tetranychus urticae Koch. Exp Appl Acarol 21:473–484. https://doi.org/10.1023/A:1018436113892
Cicolani B (1979) The intrinsic rate of natural increase in dung macrochelid mites, predators of Musca domestica eggs. Ital J Zool 46:171–178. https://doi.org/10.1080/11250007909440296
Collins EJ, Bowyer C, Tsouza A, Chopra M (2022) Tomatoes: an extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation. Biology 11:239. https://doi.org/10.3390/biology11020239
De Moraes G, Mesa N (1988) Mites of the family Phytoseiidae (Acari) in Colombia, with descriptions of three new species. Int J Acarol 14:71–88. https://doi.org/10.1080/01647958808683790
Delisle JF, Shipp L, Brodeur J (2015) Apple pollen as a supplemental food source for the control of western flower thrips by two predatory mites, Amblyseius swirskii and Neoseiulus cucumeris (Acari: Phytoseiidae), on potted chrysanthemum. Exp Appl Acarol 65:495–509. https://doi.org/10.1007/s10493-014-9863-2
Desneux N, Wajnberg E, Wyckhuys KA et al (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J Pest Sci 83:197–215. https://doi.org/10.1007/s10340-010-0321-6
Drukker B, Janssen A, Ravensberg W, Sabelis MW (1997) Improved control capacity of the mite predator Phytoseiulus persimilis (Acari: Phytoseiidae) on tomato. Exp Appl Acarol 21:507–518. https://doi.org/10.1023/B:APPA.0000018885.35044.c6
Duarte MV, Venzon M, de Bittencourt MCS et al (2015) Alternative food promotes broad mite control on chilli pepper plants. Biocontrol 60:817–825. https://doi.org/10.1007/s10526-015-9688-x
Gillespie DR, Quiring D (1994) Reproduction and longevity of the predatory mite, Phytoseiulus persimilis (Acari: Phytoseiidae) and its prey, Tetranychus urticae (Acari: Tetranychidae) on different host plants. J Entomol Soc Br Columbia 91:3–8.
Glas JJ, Schimmel BC, Alba JM et al (2012) Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int J Mol Sci 13:17077–17103. https://doi.org/10.3390/ijms131217077
Hagedorn HH, Moeller FE (1968) Effect of the age of pollen used in pollen supplements on their nutritive value for the honeybee. I. Effect on thoracic weight, development of hypopharyngeal glands, and brood rearing. J Apic Res 7:89–95. https://doi.org/10.1080/00218839.1968.11100195
Hanssen IM, Lapidot M, Thomma BP (2010) Emerging viral diseases of tomato crops. Mol Plant Microbe Interact 23:539–548. https://doi.org/10.1094/MPMI-23-5-0539
Helle W, Sabelis MW (eds) (1985) Spider mites: Their biology, natural enemies and control. Elsevier, Amsterdam
Holt RD, Bonsall MB (2017) Apparent competition. Annu Rev Ecol Evol Syst 48:447–471. https://doi.org/10.1146/annurev-ecolsys-110316-022628
Horowitz AR, Ghanim M, Roditakis E et al (2020) Insecticide resistance and its management in Bemisia tabaci species. J Pest Sci 93:893–910. https://doi.org/10.1007/s10340-020-01210-0
Hoy MA (1985) Recent advances in genetics and genetic improvement of the Phytoseiidae. Annu Rev Entomol 30:345–370. https://doi.org/10.1146/annurev.en.30.010185.002021
Hoy MA, Cave FE (1986) Screening for thelytoky in the parahaploid phytoseiid, Metaseiulus occidentalis (Nesbitt). Exp Appl Acarol 2:273–276. https://doi.org/10.1007/BF01193959
Janssen A, Sabelis MW (2015) Alternative food and biological control by generalist predatory mites: the case of Amblyseius swirskii. Exp Appl Acarol 65:413–418. https://doi.org/10.1007/s10493-015-9901-8
Janssen A, van Rijn PCJ (2021) Pesticides do not significantly reduce arthropod pest densities in the presence of natural enemies. Ecol Lett 24:2010–2024. https://doi.org/10.1111/ele.13819
Jones DR (2003) Plant viruses transmitted by whiteflies. Eur J Plant Pathol 109:195–219. https://doi.org/10.1023/A:1022846630513
Jones RA (2021) Global plant virus disease pandemics and epidemics. Plants 10:233. https://doi.org/10.3390/plants10020233
Kennedy GG (2003) Tomato, pests, parasitoids, and predators: tritrophic interactions involving the genus Lycopersicon. Annu Rev Entomol 48:51–72. https://doi.org/10.1146/annurev.ento.48.091801.112733
Kennett CE, Flaherty DL, Hoffmann RW (1979) Effect of wind-borne pollens on the population dynamics of Amblyseius hibisci [Acarina: Phytoseiidae]. Entomophaga 24:83–98. https://doi.org/10.1007/BF02377513
Knapp M, van Houten Y, van Baal E, Groot T (2018) Use of predatory mites in commercial biocontrol: current status and future prospects. Acarologia 58:72–82. https://doi.org/10.24349/acarologia/20184275
Legarrea S, Janssen A, Dong L et al (2022) Enhanced top-down control of herbivore population growth on plants with impaired defences. Funct Ecol 36:2859–2872. https://doi.org/10.1111/1365-2435.14175
Leman A, Messelink GJ (2015) Supplemental food that supports both predator and pest: a risk for biological control? Exp Appl Acarol 65:511–524. https://doi.org/10.1007/s10493-014-9859-y
Lenth R (2019) Emmeans: estimated marginal means, aka least-squares means. https://CRAN.R-project.org/package=emmeans
Marcossi Í, Fonseca MM, Carbajal PAF et al (2020) High-quality alternative food reduces cannibalism in the predatory mite Amblyseius herbicolus (Acari: Phytoseiidae). Exp Appl Acarol 81:189–200. https://doi.org/10.1007/s10493-020-00500-7
McMurtry J, Johnson H (1965) Some factors influencing the abundance of the predaceous mite Amblyseius hibisci in southern California (Acarina: Phytoseiidae). Ann Entomol Soc Am 58:49–56. https://doi.org/10.1093/aesa/58.1.49
Meng RX, Sabelis MW, Janssen A (2012) Limited predator-induced dispersal in whiteflies. PLoS ONE 7:e45487. https://doi.org/10.1371/journal.pone.0045487
Messelink G, Ramakers P, Cortez J, Janssen A (2008a) How to enhance pest control by generalist predatory mites in greenhouse crops. In: Procceedings of the Third International Symposium on Biological Control of Arthropods, Christchurch, 8–13 February, 2009, pp 309–318
Messelink GJ, Bennison J, Alomar O et al (2014) Approaches to conserving natural enemy populations in greenhouse crops: current methods and future prospects. Biocontrol 59:377–393. https://doi.org/10.1007/s10526-014-9579-6
Messelink GJ, Sabelis MW, Janssen A (2012) Generalist predators, food web complexities and biological pest control in greenhouse crops. In: Larramendy ML, Soloneski S (eds) Integrated Pest Management and pest control - current and future tactics. InTech, Rijeka, pp 191–214
Messelink GJ, van Maanen R, van Steenpaal SEF, Janssen A (2008b) Biological control of thrips and whiteflies by a shared predator: two pests are better than one. Biol Control 44:372–379. https://doi.org/10.1016/j.biocontrol.2007.10.017
Naika S, van Lidth de Jeude J, de Goffau M, et al (2005) Cultivation of tomato: production, processing and marketing. In: Agrodok, 4th ed. Agromisa Foundation and CTA, Wageningen
Navas-Castillo J, Fiallo-Olivé E, Sánchez-Campos S (2011) Emerging virus diseases transmitted by whiteflies. Annu Rev Phytopathol 49:219–248. https://doi.org/10.1146/annurev-phyto-072910-095235
Navot N, Pichersky E, Zeidan M et al (1991) Tomato yellow leaf curl virus: a whitefly-transmitted geminivirus with a single genomic component. Virology 185:151–161. https://doi.org/10.1016/0042-6822(91)90763-2
Nomikou M, Janssen A, Schraag R, Sabelis MW (2001) Phytoseiid predators as potential biological control agents for Bemisia tabaci. Exp Appl Acarol 25:271–291. https://doi.org/10.1023/A:1017976725685
Nomikou M, Janssen A, Schraag R, Sabelis MW (2002) Phytoseiid predators suppress populations of Bemisia tabaci on cucumber plants with alternative food. Exp Appl Acarol 27:57–68. https://doi.org/10.1023/A:1021559421344
Nomikou M, Janssen A, Schraag R, Sabelis MW (2004) Vulnerability of Bemisia tabaci immatures to phytoseiid predators: consequences for oviposition and influence of alternative food. Entomol Exp Appl 110:95–102
Nomikou M, Sabelis MW, Janssen A (2010) Pollen subsidies promote whitefly control through the numerical response of predatory mites. Biocontrol 55:253–260. https://doi.org/10.1007/s10526-009-9233-x
Ogle DH, Doll JC, Wheeler P, Dinno A (2022) FSA: Fisheries Stock Analysis. https://github.com/fishR-Core-Team/FSA
Oliveira MRV, Henneberry TJ, Anderson P (2001) History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot 20:709–723
Palumbo JC, Horowitz AR, Prabhaker N (2001) Insecticidal control and resistance management for Bemisia tabaci. Crop Prot 20:739–765. https://doi.org/10.1016/S0261-2194(01)00117-X
Paspati A, Rambla JL, Gresa MPL et al (2021) Tomato trichomes are deadly hurdles limiting the establishment of Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae). Biol Control 157:104572. https://doi.org/10.1016/j.biocontrol.2021.104572
Pernal SF, Currie RW (2000) Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie 31:387–409. https://doi.org/10.1051/apido:2000130
Perring TM, Stansly PA, Liu T et al (2018) Whiteflies: Biology, ecology, and management. In: Wakil W, Brust GE, Perring TM (eds) Sustainable management of arthropod pests of tomato. Academic Press, London, pp 73–110. https://doi.org/10.1016/B978-0-12-802441-6.00004-8
Picanço M, Bacci L, Crespo A et al (2007) Effect of integrated pest management practices on tomato production and conservation of natural enemies. Agric for Entomol 9:327–335. https://doi.org/10.1111/j.1461-9563.2007.00346.x
Picanço M, Faleiro FG, Pallini Filho A, Matioli AL (1997) Perdas na produtividade do tomateiro em sistemas alternativos de controle fitossanitário. Hortic Bras 15:88–91
Picó B, Díez MJ, Nuez F (1996) Viral diseases causing the greatest economic losses to the tomato crop. II. The tomato yellow leaf curl virus–a review. Sci Hortic 67:151–196. https://doi.org/10.1016/S0304-4238(96)00945-4
Pijnakker J, Arijs Y, de Sousa A, Wackers F (2016) The use of Typha angustifolia (cattail) pollen to establish the predatory mites Amblyseius swirskii, Iphiseius degenerans, Euseius ovalis and Euseius gallicus in glasshouse crops. IOBC-WPRS Bull 120:47–54
Pijnakker J, de Souza A, Wäckers F (2014) Euseius gallicus, a bodyguard for roses. IOBC-WPRS Bull 102:191–195
Pinheiro J, Bates D, DebRoy S, et al (2020) NLME: linear and nonlinear mixed effects models. In: HttpCRANR-Proj. http://CRAN.R-project.org/package=nlme
Pirayeshfar F, Safavi SA, Sarraf Moayeri HR, Messelink GJ (2020) The potential of highly nutritious frozen stages of Tyrophagus putrescentiae as a supplemental food source for the predatory mite Amblyseius swirskii. Biocontrol Sci Technol 30:403–417. https://doi.org/10.1080/09583157.2020.1722798
Pretty J (2018) Intensification for redesigned and sustainable agricultural systems. Science 362:eaav0294. https://doi.org/10.1126/science.aav0294
Pretty J, Bharucha ZP (2014) Sustainable intensification in agricultural systems. Ann Bot 114:1571–1596. https://doi.org/10.1093/aob/mcu205
R Core Team (2023) R: a language and environment for statistical computing. http://www.R-project.org
Rodríguez-Cruz FA, Venzon M, Pinto CMF (2013) Performance of Amblyseius herbicolus on broad mites and on castor bean and sunnhemp pollen. Exp Appl Acarol 60:497–507. https://doi.org/10.1007/s10493-013-9665-y
Sabelis MW (1990) How to analyze prey preference when prey density varies? A new method to discriminate between effects of gut fullness and prey type composition. Oecologia 82:289–298. https://doi.org/10.1007/BF00317473
Sato MM, de Moraes GJ, Haddad ML, Wekesa VW (2011) Effect of trichomes on the predation of Tetranychus urticae (Acari: Tetranychidae) by Phytoseiulus macropilis (Acari: Phytoseiidae) on tomato, and the interference of webbing. Exp Appl Acarol 54:21–32. https://doi.org/10.1007/s10493-011-9426-8
Schuurink R, Tissier A (2020) Glandular trichomes: micro-organs with model status? New Phytol 225:2251–2266. https://doi.org/10.1111/nph.16283
Simmons AM, Wakil W, Qayyum MA et al (2018) Lepidopterous pests: Biology, ecology, and management. In: Wakil W, Brust GE, Perring TM (eds) Sustainable management of arthropod pests of tomato. Academic Press, San Diego, pp 131–162
Simmons AT, Gurr GM (2005) Trichomes of Lycopersicon species and their hybrids: effects on pests and natural enemies. Agric for Entomol 7:265–276. https://doi.org/10.1111/j.1461-9555.2005.00271.x
Therneau TM (2020) A package for survival analysis in R
van Haren R, Steenhuis M, Sabelis M, De Ponti O (1987) Tomato stem trichomes and dispersal success of Phytoseiulus persimilis relative to its prey Tetranychus urticae. Exp Appl Acarol 3:115–121
van Houten YM, Glas JJ, Hoogerbrugge H et al (2013) Herbivory-associated degradation of tomato trichomes and its impact on biological control of Aculops lycopersici. Exp Appl Acarol 60:127–138. https://doi.org/10.1007/s10493-012-9638-6
van Lenteren J (2000) Success in biological control of arthropods by augmentation of natural enemies. In: Gurr G, Wratten S (eds) Biological control: measures of success. Springer, Dordrecht, pp 77–103
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 57:1–20. https://doi.org/10.1007/s10526-011-9395-1
van Lenteren JC, Bolckmans K, Köhl J et al (2018) Biological control using invertebrates and microorganisms: plenty of new opportunities. Biocontrol 63:39–59. https://doi.org/10.1007/s10526-017-9801-4
van Lenteren J, Hua LZ, Kamerman J, Rumei X (1995) The parasite-host relationship between Encarsia formosa (Hym., Aphelinidae) and Trialeurodes vaporariorum (Hom., Aleyrodidae) XXVI. Leaf hairs reduce the capacity of Encarsia to control greenhouse whitefly on cucumber. J Appl Entomol 119:553–559. https://doi.org/10.1111/j.1439-0418.1995.tb01335.x
van Rijn PCJ, Sabelis MW (1993) Does alternative food always enhance biological control? The effect of pollen on the interaction between western flower thrips and its predators. IOBCWPRS Bull 16:123–125
van Rijn PCJ, Tanigoshi LK (1999) Pollen as food for the predatory mites Iphiseius degenerans and Neoseiulus cucumeris (Acari: Phytoseiidae): dietary range and life history. Exp Appl Acarol 23:785–802. https://doi.org/10.1023/A:1006227704122
van Rijn PCJ, van Houten YM, Sabelis MW (2002) How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology 83:2664–2679. https://doi.org/10.1890/0012-9658(2002)083[2664:HPBFPF]2.0.CO;2
Varma A, Malathi V (2003) Emerging geminivirus problems: a serious threat to crop production. Ann Appl Biol 142:145–164. https://doi.org/10.1111/j.1744-7348.2003.tb00240.x
Velasco L, Simón B, Janssen D, Cenis J (2008) Incidences and progression of tomato chlorosis virus disease and tomato yellow leaf curl virus disease in tomato under different greenhouse covers in southeast Spain. Ann Appl Biol 153:335–344. https://doi.org/10.1111/j.1744-7348.2008.00262.x
Verheggen FJ, Capella Q, Schwartzberg EG et al (2009) Tomato-aphid-hoverfly: a tritrophic interaction incompatible for pest management. Arthropod-Plant Interact 3:141–149. https://doi.org/10.1007/s11829-009-9065-8
Wakil W, Brust GE, Perring TM (2018) Tomato and management of associated arthropod pests: past, present, and future. In: Wakil W, Brust GE, Perring TM (eds) Sustainable management of arthropod pests of tomato. Academic Press, San Diego, pp 3–12. https://doi.org/10.1016/B978-0-12-802441-6.00001-2
Wheeler AG, Krimmel BA (2015) Mirid (Hemiptera: Heteroptera) specialists of sticky plants: adaptations, interactions, and ecological implications. Annu Rev Entomol 60:393–414. https://doi.org/10.1146/annurev-ento-010814-020932
Wisler G, Li R, Liu H-Y et al (1998) Tomato chlorosis virus: a new whitefly-transmitted, phloem-limited, bipartite closterovirus of tomato. Phytopathology 88:402–409. https://doi.org/10.1094/PHYTO.1998.88.5.402
Acknowledgements
We thank Koppert the Netherlands for financial support, Manoel Guedes Correa Gondim Junior for identification of A. herbicolus, Felipe Lemos for the Typha sp pollen, Marcelo Coutinho Picanço for the B. tabaci biotype B and Jussara Mencalha for assistance with collecting predators from tomato plants. The associate editor and two anonymous reviewers are thanked for constructive comments.
Funding
This research was supported by Koppert the Netherlands and the Brazilian research funding agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Jay Rosenheim.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cardoso, A.C., Marcossi, Í., Fonseca, M.M. et al. A predatory mite as potential biological control agent of Bemisia tabaci on tomato plants. J Pest Sci (2024). https://doi.org/10.1007/s10340-024-01809-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10340-024-01809-7