Abstract
The use of semiochemical-baited traps for detection, monitoring, and sampling bark beetles and woodboring beetles (BBWB) has rapidly increased since the early 2000s. Semiochemical-baited survey traps are used in generic (broad community level) and specific (targeted toward a species or group) surveys to detect nonnative and potentially invasive BBWB, monitor established populations of invasive or damaging native species, and as a tool to survey natural communities for various purposes. Along with expansion in use, much research on ways to improve the efficacy of trapping surveys for the detection of specific pests as well as BBWB in general has been conducted. In this review, we provide information on intrinsic and extrinsic factors and how they influence the efficacy of detecting BBWB in traps. Intrinsic factors, such as trap type and color, and other factors are described, as well as important extrinsic factors such as habitat selection, horizontal and vertical placement, and disturbance. When developing surveys, consideration of these factors should increase the species richness and/or abundance of BBWB captured in traps and increase the probability of detecting nonnative species that may be present. During generic surveys, deploying more than one trap type or color, using an array of lures, and trapping at different vertical and horizontal positions is beneficial and can increase the number of species captured. Specific surveys generally rely on predetermined protocols that provide recommendations on trap type, color, lure, and trap placement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key Message
-
Semiochemical-baited traps are important tools for detecting native and nonnative species.
-
Generic and specific surveys benefit from incorporating research findings on trapping efficacy.
-
Trap design, color, and killing agent are important intrinsic factors.
-
Site selection, habitat, disturbance, and spatial placement are important extrinsic factors.
-
Recommendations for optimally deploying traps will improve survey results.
Introduction
Bark beetles and woodboring beetles (BBWB) are important insects in forested ecosystems. They are key contributors to ecosystem processes (Müller et al. 2002; Ulyshen 2016), influence structure of stands (Feller and McKee 1999; Dodds et al. 2010a) and landscapes (Rodman et al. 2021), provide important food sources for invertebrate and vertebrate predators (Linsley 1959; Fayt et al. 2005), and can affect a wide array of ecosystem services (Embrey et al. 2012). Interest in BBWB has further increased because several species have been transported around the globe and introduced into new environments (Haack 2001; Barnouin et al. 2020; Marchioro et al. 2022b; Ruzzier et al. 2023), where some have become invasive pests, causing severe ecological and economic impacts threatening natural and managed forests (Seidl et al. 2018). Ecological impacts vary from changes to forest structure (Dodds and Orwig 2011; Haavik et al. 2018) to near or wholesale elimination of certain tree species in some forests (Klooster et al. 2014). Economic impacts can also be severe and wide ranging (Kovacs et al. 2010; Aukema et al. 2011; Kondo et al. 2017), especially considering the key value of urban and forest trees (Pearce 2001; McPherson et al. 2017). Thus, various techniques have been initiated to limit the movement of BBWB species into new environments, including treatment of goods and packing material with heat or pesticides prior to movement, product quarantines, and port inspections (Magarey et al. 2009; Nahrung et al. 2023). Even with these measures, BBWB continue to be intercepted in wood packaging used in global transport of containerized goods (Haack et al. 2022) resulting in a clear need to optimize the use of traps and lures for surveillance and early detection of nonnative BBWB.
Semiochemical-based forest insect surveys for BBWB have become commonplace across the globe (Suckling 2015) and represent an important second line of defense at ports of entry and surrounding high-risk areas (Fig. 1A) where nonnative species may gain footholds in new areas (Brockerhoff et al. 2006; Rassati et al. 2014; Rabaglia et al. 2019; Hoch et al. 2020; Mas et al. 2023; Melin et al. 2023). In addition, they are important tools for delineating the spatial extent of damaging BBWB species and monitoring population levels once a species becomes established in an area (Haack and Poland 2001; Dodds and de Groot 2012) (Fig. 1B). Outside of purely programmatic detection surveys, semiochemical-baited traps are also important tools to sample insect communities of both native and nonnative species for various ecological and/or conservation objectives (Sullivan et al. 2003; Gandhi et al. 2009; Larsson 2016; Dodds et al. 2019) (Fig. 1C). In fact, semiochemical-baited traps provide a cost-effective alternative to visual surveys because they can detect species that were previously unknown in an area (e.g., Webster et al. 2012b; Santos-Silva et al. 2020) or which are cryptic and not easily detectable by visual means. Traps do have limits, however, as it is generally unknown how well trap catches represent actual BBWB communities present in an area, and how well relative abundance relates to true abundance or rarity.
International port and surrounding area where traps can be deployed to survey for arriving or established nonnative bark beetles and woodborers (A), map depicting the initial (blue circles) and expanded (orange circles) trapping response in New York to delimit Sirex noctilio populations in the region (B), and multiple-funnel trap used to sample insect communities after a windstorm and salvage logging disturbance (C), photo credit—Shawn Fraver
Using traps to survey for nonnative BBWB generally falls into two broad categories, generic or specific surveys (Poland and Rassati 2019). Generic surveillance implements lures and traps to survey sites deemed high risk to detect invasive species early in the invasion. These surveys are becoming more common with examples from Australia, Canada, Czech Republic, Great Britain, Italy, New Zealand, Spain, and the USA (Brockerhoff et al. 2006; Bashford 2012; Rassati et al. 2014, 2015b; CFIA 2017; Inward 2019; Rabaglia et al. 2019; Fiala and Holuša 2023; Mas et al. 2023). Broad spectrum lures, such as ethanol, or multi-lure blends including several semiochemicals on a trap are usually employed (e.g., Fan et al. 2019; Roques et al. 2023) with the hope of maximizing species richness over abundance in collections. This same approach is often used for biodiversity and ecological studies focused on sampling at the community level (Sullivan et al. 2003; Gandhi et al. 2009; Dodds et al. 2019, 2023). Targeted surveys (i.e., specific surveys) that focus on only one species, a few species, or members of the same genera at the same time are the second type of BBWB surveys. Specific semiochemical lures and/or specific trapping protocols are used during these surveys often to track damaging insect populations (Billings and Upton 2010), but also when targeting rare or protected native species for conservation purposes (Žunič Kosi et al. 2017). The same approach is used when delimiting a population after detection, gaining a relative measure of abundance, and attempts to influence populations with mass trapping. In these surveys, abundance, while often difficult to interpret in relation to tree damage, is generally helpful. These specific surveys may also provide an opportunity to screen bycatch for other species of interest (Peck et al. 1997; Dodds and Ross 2002b; Skvarla and Holland 2011; DiGirolomo and Dodds 2014; Thurston et al. 2022).
Given the multiple potential uses of semiochemical-baited traps, an extensive testing of factors that may influence the efficacy of detecting BBWB in traps has been undertaken over the last 2 decades. Much of this information has not been consolidated. Our objective was to review and summarize the relevant literature on factors affecting BBWB trapping efficacy and provide natural resource professionals with guidance on ways to improve generic and specific surveys. With only one exception, Sirex noctilio F. (Hymenoptera: Siricidae), the focus will remain on bark and ambrosia beetles (Scolytinae), longhorned beetles (Cerambycidae), and jewel beetles (Buprestidae). These taxa were chosen due to their economic importance worldwide and the prevalence of important invasive species within these families. Early research that led to the development of semiochemical-baited trapping, mechanisms underlying trap efficacy, and factors related to successful trapping of these families will be covered. General guidelines for developing general and pest-specific surveys are also provided. We broadly reviewed the scientific literature and technical reports focused on semiochemical-baited traps and their applications, but without using specific criteria to select articles to include in this review.
Steps toward semiochemical-based trapping for BBWB
Early BBWB research observed patterns of saproxylic insect succession on host trees or logs, as well as specificity to plant tissues (Graham 1925; Savely 1939; Wallace 1953). Mechanisms for these patterns were generally focused on host condition including moisture and temperature, as well as size and decay class of host material. Specific chemical cues driving these patterns were largely unknown at the time, but the possibility of chemical host location cues were considered (Person 1931). Later, ethanol, a host volatile produced by injured and stressed trees (Kelsey and Joseph 2003) was observed and identified as a field attractant for many BBWB (Moeck 1970, 1971; Schroeder 1988). Consequently, ethanol-baited traps were some of the earliest semiochemical reliant surveys for BBWB (Roling and Kearby 1975; Montgomery and Wargo 1983). Ethanol is still the key lure for ambrosia beetles (Reding et al. 2011; Galko et al. 2014; Fiala and Holuša 2023) and an important synergist to other lures when targeting broader BBWB communities (Hanks and Millar 2013; Miller et al. 2015). Terpenes were also recognized as important attractants in the field (Jantz and Rudinsky 1966; Knopf and Pitman 1972; Bauer and Vité 1975; Chenier and Philogene 1989). In particular, Bauer and Vité (1975) recognized a synergistic effect of ethanol and alpha-pinene on trap catches of Trypodendron lineatum (Olivier). Alpha-pinene and ethanol have since been used extensively to sample BBWB communities (Schroeder 1988; Schroeder and Lindelöw 1989; Miller and Rabaglia 2009).
Host volatiles became useful for broad surveys of BBWB, however, pheromones outperform host volatiles when the survey objectives are to detect or monitor the spread of a specific target species. The first bark beetle pheromones were identified from Ips confusus (LeConte) in 1966 (Silverstein et al. 1966). After that discovery, pheromone identification occurred across several genera of bark beetles. Identification of long-range pheromones in Cerambycidae began a few decades after bark beetles (e.g., Sakai et al. 1984; Noldt et al. 1995) and rapidly expanded in the early 2000s with identification of pheromones of native and nonnative species in North America (Lacey et al. 2004; Silk et al. 2007; Millar and Hanks 2017). The cross attractiveness of some identified cerambycid pheromones, especially within the same subfamily, made them especially useful for broader surveys (Hanks and Millar 2013), while specific pheromones could target individual (or a small number of) species of high value or concern (Silk et al. 2007; Rassati et al. 2021). Unlike sex pheromones of Lepidoptera (which are emitted by females, attract males only, and are highly species-specific) most pheromones identified in longhorn beetles are emitted by males, attract both sexes, and often several species. For example, 3-hydroxyhexan-2-one, 2,3-hexanediols, fuscumol, and fuscumol acetate are shared by species in different genera and subfamilies (Hanks and Millar 2016), making them especially useful for generic surveys. Identifying pheromones from Buprestidae has been even more challenging than for Cerambycidae. A pheromone identified from Agrilus planipennis Fairmaire represented the first time one was described in the family (Bartelt et al. 2007; Silk et al. 2011), but since then no important progress has been made on buprestid chemical ecology. Similarly, S. noctilio is the only economically important siricid for which lures have been specifically developed. However, none of the host volatiles and pheromones (Böröczky et al. 2009; Cooperband et al. 2012; Crook et al. 2012) identified for S. noctilio was effective as trap lures when tested in South Africa (Hurley et al. 2015).
In more recent years, it has become more common to combine host volatiles and pheromones on the same trap. For example, the combination of Ips bark beetle pheromones, including ipsenol and ipsdienol, and host volatiles, including ethanol and alpha-pinene, are effective for sampling large communities of BBWB in forests (Miller et al. 2011; Allison et al. 2013), urban and peri-urban areas (e.g., ports) (Rassati et al. 2015a, 2015b; Rabaglia et al. 2019). Similarly, traps baited with cerambycid pheromone blends have shown promise for capturing a larger array of cerambycid species across the Americas, Europe, and Asia (Hanks et al. 2012; Sweeney et al. 2014; Wickham et al. 2014; Fan et al. 2019; Santos-Silva et al. 2020; Roques et al. 2023), as well as other BBWB when combined with host volatiles (Sweeney et al. 2016; Rassati et al. 2019).
Mechanisms underlying trap efficacy
To develop an effective trap design, lure, and deployment strategy for detecting BBWB, it pays to “think like a beetle,” i.e., to understand their behavior (i.e., foraging, dispersal, mating, oviposition, etc.) and the factors affecting them, and to exploit them to our advantage. To survive and reproduce, most BBWB must find food, mates, and suitable brood hosts in a heterogeneous environment within a relatively short adult lifespan of a few days or weeks, and they do so with the aid of sophisticated sensory systems that detect and integrate olfactory and visual stimuli in their environment (Allison et al. 2004; Campbell and Borden 2009; Carrasco et al. 2015; Dodds et al. 2023). Other stimuli such as auditory (Rudinsky et al. 1973), gustatory/tactile cues (Ginzel and Hanks 2003), and even infrared (Evans 1964) can play a role in host/mate location and acceptance by BBWB, but it is chiefly olfactory and visual cues that have been exploited in the development of attractants and traps for BBWB surveys.
Olfaction
As discussed above, olfactory cues used by BBWB include volatiles emitted from host- and non-host trees (Metcalf and Kogan 1987; Bruce and Pickett 2011; Xu and Turlings 2018) and sex- or aggregation pheromones of both conspecifics and heterospecifics (Lu et al. 2007; Hanks and Millar 2016), the latter of which may serve as kairomones (e.g., Allison and Borden 2001). Plant volatiles play an important role in insect host location and are important for some BBWB (Moeck 1970; Brattli et al. 1998; Pureswaran and Borden 2005; McCullough et al. 2009; Tluczek et al. 2011; Flaherty et al. 2013; Silk et al. 2020). Plants share many of the same volatile compounds such as terpenes and sesquiterpenes and the relative amounts of these compounds may vary among species (Pureswaran et al. 2004), plant tissues (e.g., Zeneli et al. 2001), seasons (e.g., Zhang et al. 1999), time of day (e.g., Martin et al. 2003), stages of growth (e.g., Johnson et al. 2004), levels of moisture stress, and levels of herbivory (Dicke and van Loon 2000). It is the particular ratio of these common volatiles, rather than unique species-specific volatiles, that allows most insects to discriminate between host and non-host plants, or stressed versus healthy trees, by integrating signals from antennal olfactory receptors in the central nervous system (Bruce et al. 2005). Recognition and avoidance of non-host volatiles may be as important as positive response to host odors to a beetle seeking a suitable host in a forest composed of many different tree and plant species (Zhang and Schlyter 2003, 2004; Byers et al. 2004; Campbell and Borden 2009). For example, odors from nonhost trees inhibit response of some bark and ambrosia beetles to their hosts (Schroeder and Lindelöw 1989; Schroeder 1992).
Experiments in wind tunnels have shown that beetles orient to pheromone sources using positive optomotor anemotaxis (Fadamiro et al. 1998) as previously shown for moths (Kennedy et al. 1980; Kuenen and Baker 1982; Baker et al. 1984; Cardé 1984; Cardé and Willis 2008). When insects detect their sex or aggregation pheromone they initiate upwind flight and maintain a constant velocity of image motion across the eyes (optomotor anemotaxis) which results in a constant ground velocity independent of wind speed (Murlis et al. 1992). Variation in pheromone concentration in the plume (i.e., pockets of air with and without pheromone) caused by turbulence downwind of the source is necessary for upwind flight and also induces the zig-zag pattern (casting) (Elkinton and Cardé 1984; Murlis et al. 1992). The attractive radius or active space [defined as the distance from the pheromone source at which the odor concentration is sufficient to produce a response in the receiving organism (Elkinton and Cardé 1984)] of a pheromone-baited trap is affected by a number of factors such as pheromone release rate, wind speed, and turbulence, which affect dispersion of the odor plume and concentration of attractant in the odor filaments downwind (Schlyter 1992; Thistle et al. 2011; Bouwer et al. 2020). Turbulence produced by the trap itself can affect pheromone dispersion and trap performance (Wyatt et al. 1993; Cooperband and Cardé 2006). Estimates of the attractive radius of BBWB pheromone-baited traps, from mark-release-recapture or trap spacing-interference experiments, have ranged from 10 to 500 m (Schlyter 1992; Dodds and Ross 2002a; Maki et al. 2011; Torres-Vila et al. 2013; Jactel et al. 2019; Parker et al. 2020; Wittman et al. 2021).
Semiochemical release rate is an important factor that can affect BBWB trap catches (e.g., de Groot and Zylstra 1995; Ross and Daterman 1998; Borden and Miller 2000; Erbilgin et al. 2003; Grommes et al. 2023), yet optimal release rates are unknown for most species. Higher release rates, especially for host volatiles, are believed to generally increase insect capture by dispersing higher attractant concentrations around and downwind from a trap. High release host volatile lures are often components of generic trapping surveys for this reason. Nonetheless, the relationship between trap catch and release rate is not always positively linear, as high release rates can have a repellant effect on some species. For example, in Lepidoptera (e.g., Baker et al. 1981; Baker and Roelofs 1981) there is usually an optimal release rate of pheromone or host volatiles above or below which attraction and trap catches decline. Some BBWB have also demonstrated this behavior (e.g., Klimetzek et al. 1986; Ross and Daterman 1998; Erbilgin et al. 2003; Grommes et al. 2023), while others show limited differences among release rates (Sun et al. 2004) or a positive response to tested release rates but no upper limit detected (Fatzinger 1985; Franklin and Grégoire 2001; Gallego et al. 2008). As shown for ambrosia beetles, such preferences may correspond to the optimal concentration of the targeted host volatile which maximize colonization success in attacked trees (Cavaletto et al. 2023).
Vision
Size, shape and color are the key components used by insects to visually discriminate between host and non-host plants (Prokopy and Owens 1978) and these are important for some insects orienting to traps. The spatial distribution of photon flux provides information on shape, size, distance, and motion. Many species of BBWB orient toward objects that stand out against or contrast with their background, such as a tall tree or a dark silhouette. For example, mountain pine beetles, Dendroctonus ponderosae Hopkins, were attracted to dark cards placed against a white background; the larger the card, the greater the attraction (Shepherd 1966). Black multiple-funnel traps and intercept panel traps, designed to resemble dark tree trunks, often catch more BBWB than transparent or white traps (de Groot and Nott 2001; Kerr et al. 2017). However, response to visual stimuli often occurs only when combined with suitable olfactory stimuli, such as host volatiles and pheromones. For example, catches of the southern pine beetle, Dendroctonus frontalis Zimmermann (Strom et al. 1999), and black turpentine beetle, Dendroctonus terebrans (Olivier) (Fatzinger 1985), on pheromone-baited or turpentine-baited traps, respectively, were significantly reduced on white traps compared to black traps. In Buprestidae, there is evidence that visual stimuli drive host selection, although the roles of olfactory cues and their interactions are not as well understood (Imrei et al. 2020a; Santoiemma et al. 2024). Prokopy and Owens (1978) suggested that monophagous and oligophagous insects were more likely to use specific visual stimuli, in concert with olfactory and/or contact chemical stimuli, when foraging for food, mates or oviposition sites than are polyphagous species. Trichromatic color vision is common in insects (Prokopy and Owens 1978; Briscoe and Chittka 2001; van der Kooi et al. 2021) and preferences for different colors have been demonstrated for several BBWB (Campbell and Borden 2006; Crook et al. 2009; Francese et al. 2010a; Kerr et al. 2017; Skvarla and Dowling 2017; Rassati et al. 2019; Cavaletto et al. 2020, 2021; Perkovich et al. 2022; Sukovata et al. 2022) likely because they reflect similar hues as food or brood hosts.
Considering the above-described mechanisms, it is evident that a number of factors can potentially affect trap efficacy. These factors can be both directly linked to the trap (e.g., trap design, trap color, lure placement) but also to the position of the trap in the environment (e.g., trap height) and the characteristics of the environment in which the trap is located (e.g., forest type, disturbance history).
Intrinsic factors
Trap design
Several trap types for BBWB surveys have been developed over the years, but multiple-funnel and intercept panel traps are the most ubiquitous in trapping studies. Multiple-funnel traps, also known as multi-funnel or Lindgren funnel traps, were first developed by Lindgren (1983) for trapping bark and ambrosia beetles. Subsequent studies have demonstrated their efficacy for woodboring beetles as well (de Groot and Nott 2001; McIntosh et al. 2001; Morewood et al. 2002; Dodds et al. 2010b; Miller and Crowe 2011; Rassati et al. 2012). This trap type consists of a series of black vertically aligned funnels (typically twelve total) meant to mimic a tree bole, fitted with a collection cup on the bottom, and topped by a rain cover (Fig. 2A). BBWB are attracted by the shape of the trap and/or the odor plume released from the lures, arrive at the trap, hit the funnels, and then fall into the collecting cup because they cannot regain grip on the slanted slippery surface of the funnels (Lindgren 1983).
Trap types used to survey bark beetles and woodboring beetles for various purposes. Traps include multiple-funnel (A), intercept panel (B), purple multiple-funnel (C), transparent intercept panel (D), fluon coated intercept panel (E), green multiple-funnel with enlarged funnels and collecting cup (F), SLAM aerial malaise (G), bottle (H), glue-coated sticky prism (I), double decker sticky prism (J), fan (K), and Multitrap system (L) traps. Photo credits: KJD—A, B, F, G, H; JAF—C, I; DR—D; Joshua Bruckner—E; Nathan Siegert—J; Emilio Caiti—K; Synergy Semiochemicals—L
Similar to multiple-funnel traps, intercept panel traps were also initially commercially developed for bark beetles (Czokajlo et al. 2001) but then also found to be efficient for woodboring beetles (McIntosh et al. 2001; Miller and Crowe 2011). This trap type consists of two corrugated black plastic panels or reinforced PVC sheets placed perpendicularly to one another, a rain cover on top, and a funnel connected to a collection cup at the bottom (Fig. 2B). Additionally, a hole is cut in the middle of the PVC sheets to allow for lure placement. BBWB attracted by the shape of the trap and/or the odor plume released from the dispenser used to bait it, hit the panels, and fall into the funnel connected to the collection cup. A benefit of intercept panel traps over multiple-funnel traps is they break down into flattened pieces that can be more easily stored when not deployed.
Several modified versions of both multiple-funnel and intercept panel traps have been developed over the years. Examples of variations include number of funnels, ranging from 4 to 16 (e.g., Brar et al. 2012; Francese et al. 2013; Miller et al. 2018; Miller and Crowe 2022), modifications to increase the lower diameter of each funnel (Miller et al. 2013), funnels and panels of colors different than black (Fig. 2C), including transparent ones (Fig. 2D) (de Groot and Nott 2001; Rassati et al. 2012; Kerr et al. 2017; Cavaletto et al. 2021), the addition of glue (Francese et al. 2011) or pure and diluted lubricants on trap surface (Fig. 2E) (Graham et al. 2010; Allison et al. 2011, 2016; Graham and Poland 2012), the addition of liquid to the collection cup (Sweeney et al. 2006; Miller and Duerr 2008; Graham and Poland 2012; Allison et al. 2014), bottom funnel modifications to reduce catches of beneficial insects or non-targets (Ross and Daterman 1998; Martín et al. 2013; Bracalini et al. 2021) and enlargement of funnels connected to the collection cup (Morewood et al. 2002; Allison et al. 2014) (Fig. 2F). Both multiple-funnel and intercept panel traps are durable and can provide five or more years of service, while being moderately priced.
Most of the comparisons between standard or modified versions of multiple-funnel vs. intercept panel traps led to heterogeneous results depending on the target families or species. For example, some studies found that intercept panel traps are more effective than multiple-funnel traps in capturing longhorn beetles (de Groot and Nott 2001, 2003; McIntosh et al. 2001; Morewood et al. 2002; Miller and Crowe 2011; Marchioro et al. 2022a) while others found the opposite trend for species in the same (Rassati et al. 2012; Allison et al. 2014) or other (Dodds et al. 2010b) families. Others found no differences between the two trap types (Haavik et al. 2014). Nonetheless general patterns have been identified. A recent meta-analysis highlighted that (i) intercept panel traps are generally superior to multiple-funnel traps (except for buprestid beetles), (ii) traps treated with a surface treatment to make them slippery perform better than untreated traps and (iii) traps equipped with wet collection cups perform better than traps equipped with dry collection cups (Allison and Redak 2017). Further general patterns also arise when considering morphological traits associated with flight efficiency, maneuverability and eye size, potentially leading to improved predictability of optimal trap design according to species (Staton et al. 2023). A mechanistic understanding of trap functioning will provide insight into why one trap model performs better than another, or why the same trap design differs in its performance among taxa and habitats (Bouwer et al. 2020; Burner et al. 2020), and may lead to improvements in trapping efficacy.
Besides multiple-funnel and intercept panel traps, several other trap types for BBWB are currently in use. SLAM (Sea, Land and Air Malaise) traps represent another albeit less adopted option. Malaise traps are traditionally used to collect flying arthropods, especially Hymenoptera and Diptera (Skvarla et al. 2021), but with a few modifications they can be employed to sample BBWB (Vance et al. 2003; Skvarla and Dowling 2017; Varandi et al. 2018). SLAM retain the structure of a terrestrial Malaise trap, but they are usually equipped with top and bottom collecting cups and are often hung between trees (Fig. 2G). While canopy Malaise traps sometimes catch fewer total specimens of BBWB than do multiple-funnel or intercept panel traps, they often capture a greater number of species, including unique and rare species (Dodds et al. 2010b, 2015). Unfortunately, these traps are much more expensive and less durable than the other traps. Simple traps (e.g., bottle traps) (Fig. 2H) baited with fermenting baits or host volatiles have also been demonstrated to be an efficient tool for monitoring longhorn beetles (Miller et al. 2018; Ruchin et al. 2021) and ambrosia beetle communities (Steininger et al. 2015; Tarno et al. 2021), as well as for citizen science projects to broaden the area surveyed for nonnative ambrosia beetles (Steininger et al. 2015; Ruzzier et al. 2021; Colombari and Battisti 2023). These traps cost very little to make and can be created from recyclables (Frost and Dietrich 1929; Reding et al. 2010), however, they are generally only used for a single season. Theysohn bark beetle traps, commonly employed for monitoring Ips typographus L. population density in Europe (Bakke 1985; Weslien et al. 1989; Galko et al. 2016), consist of a series of horizontal slots across a flat plastic surface. These traps are similar in price to intercept panel traps or multiple-funnel traps and can also provide years of service but are generally recommended only for certain bark beetles. While not broadly implemented, UV light traps capture large communities of insects, including BBWB, and provide a strong tool to broadly survey insects (Wardhaugh and Pawson 2023) or for specific target species (Pawson et al. 2009).
Various traps have been developed over the years driven by survey needs to detect or delimit invasive species. Glue-coated sticky prism traps, for example, (Fig. 2I) have been extensively used for monitoring the invasive buprestid beetle A. planipennis in the USA and Canada (Petrice et al. 2013; Poland et al. 2019). This trap type consists of a three-sided prism made of corrugated plastic, usually green or purple (Crook et al. 2009), covered with insect glue, where each side is 36 cm wide by 60 cm tall (Francese et al. 2008, 2010b) and can be used either baited or unbaited. A more complex but more efficient version of the sticky prism traps developed for monitoring A. planipennis in the USA is represented by the double-decker trap (Poland et al. 2011, 2019; McCullough and Poland 2017). This trap type consists of a 3 m tall PVC pole used as a support of two glue-coated green or purple prism traps built from corrugated plastic which are attached at different heights on the pole (Fig. 2J). The configuration, while equally efficient to a green prism canopy trap, is more effective than a single purple prism trap (Tobin et al. 2021). Canopy-placed sticky panel traps are still used for A. planipennis survey in Canada, but in the USA have now mostly been replaced by multiple-funnel traps. Branch-traps, i.e., green sticky plastic cards fastened to sunlit branches and baited or not with a 3D-printed decoy, have also been employed for monitoring Agrilus spp. (Domingue et al. 2013, 2015), as well as a lighter weight light-green non-sticky multiple-funnel trap developed for jewel beetles (Imrei et al. 2020b) in Europe.
For ambrosia beetles, a recent study showed that a single white panel trap that is sticky on both sides is generally superior to 8-unit multiple-funnel and intercept panel traps (Kendra et al. 2020). Commercially available Japanese beetle traps have also been used successfully to trap ambrosia beetles (Burbano et al. 2012), as have artificially stressed trees (e.g., Ranger et al. 2010) and trap logs (e.g., Reding and Ranger 2020). Trees or logs may also have the benefit of providing substrate for ambrosia beetle fungal mutualists, potentially enhancing attraction to the traps through the addition of fungal volatiles caused by inoculated fungi (Hulcr et al. 2011; Kuhns et al. 2014; Gugliuzzo et al. 2023; but see Tobin et al. 2024).
There are also several new traps available that have mostly been untested against existing trap types. Foldable fan traps (Grégoire et al. 2022) (Fig. 2K) are laser cut from a sheet of polypropylene that can be rapidly produced in large numbers in a lab or by a commercial company and easily transported and deployed in the field with very little effort. They offer a cheaper alternative to more expensive traps, with the potential for multi-year use. The Multitrap system (Synergy Semiochemicals, Canada), consisting of a set of modular parts from which a variety of trap configurations can be constructed, including standard multi-funnel traps, intercept traps, but also a combination of the two (Fig. 2L) is also a new trap design that awaits further testing. Camera-integrated traps have also been developed for BBWB surveillance (Rassati et al. 2016). These traps allow the user to focus the on-site checking only on those traps showing the presence of target insects, avoiding checking of empty traps and reducing the time and costs of the surveillance. To date, camera-integrated traps are commercially available and used operationally for monitoring specific insect pests in traps baited with species-specific sex pheromone, e.g., codling moth in apple orchards, but with improvements in machine learning and image recognition technology (Preti et al. 2021), may eventually be useful for generic BBWB surveys as well.
Lure placement on trap
The placement of lures on traps can influence trapping results. For example, early wind tunnel tests of multiple-funnel traps suggested that two lures, one each placed inside a trap at the center of the middle and bottom funnels would optimize pheromone plumes, thereby increasing catch (Lindgren 1983). Similar results were found using other trap types (Lindgren 1983). When lures were placed inside of modified multiple-funnel traps where lower funnel holes had been enlarged, several BBWB responded positively to modified traps over standard multiple funnel traps with lures outside of traps, while none responded negatively (Miller et al. 2013). It was suggested that this pattern was due to either the ability of the lures to create a large plume emitting from the middle of the trap and expanding outward or to the larger funnel holes that may have facilitated higher capture rates. There are many unknowns about how pheromones on traps interact (i.e., separate point source or single point source) and how this may influence BBWB orientation to traps. Antagonistic effects of multiple-component, generic lures for cerambycids can reduce catches of some beetles (e.g., Miller et al. 2017) but generally do not interfere with the majority of species (Hanks et al. 2012). However, new questions may arise about separating the release devices on different funnels to avoid interference among plumes. Resulting plume structure generated by lure placement is one way trap catches may be influenced. The other way is by blocking of incoming insects, although there is no evidence that even large lures on side of traps reduce BBWB catches (Dodds et al. 2010b). Similar difficulties may be found with intercept panel traps, when multiple lures are placed tightly together at the same hole, and these questions may warrant future research.
Lubricant treatments on trap surface
Initially, multiple-funnel and intercept panel traps were designed for bark beetles, but as their use expanded to include larger taxa (cerambycids, buprestids), it was observed that some of these insects could land on and escape from trap surfaces (McIntosh et al. 2001; de Groot and Nott 2003). These initial observations led to treatments of traps with substances that make the surface more slippery, thereby increasing the chances of capturing BBWB that land on the trap and reducing chances of their escape. When multiple-funnel traps were treated with the lubricant Rain-X (active ingredient polydimethylsiloxane), they generally caught more cerambycids as well as three large buprestid species [(Chalcophora virginiensis (Drury), Buprestis maculativentris (Say), Dicerca tenebrosa (Kirby)] than did untreated traps (de Groot and Nott 2003). The active ingredient, usually used as a lubricant for car windshields temporarily smooths trap surfaces, making them more slippery and preventing beetles from being able to stand or perch on it. Rain-X soon became a standard treatment for multiple-funnel and intercept panel traps (Sweeney et al. 2004; Hanks et al. 2007; Allison et al. 2011; Francese et al. 2011).
With insight gained from Rain-X treatments, other lubricants were tested. Graham et al. (2010) found that intercept panel traps treated with the fluoropolymer, fluon (active ingredient polytetrafluoroethylene), originally used to keep insects from escaping containers during laboratory behavioral assays, caught ~ 14 times more cerambycids than Rain-X or untreated traps. Additionally, fluon-treated multiple-funnel traps caught more longhorned beetles than untreated traps, and treatments lasted for at least two seasons (Graham and Poland 2012). However, a significant drop-off in catch happens 3 years post-application (Dong et al. 2023). Further testing of fluon treatments supported its importance for increasing trap catches while also not interfering with visual cues, such as trap color, on target insects (Lyons et al. 2012; Francese et al. 2013). In addition, fluon can be potentially diluted in water, decreasing the total amount needed and the overall costs. For some cerambycid species, traps coated with fluon concentrations of 100%, 50%, and 10% captured similar numbers of insects (Allison et al. 2016). However, A. planipennis traps treated with a 10% fluon concentration captured fewer insects than 50% fluon concentration (Francese et al. 2013).
The cost of fluon, difficulties applying it uniformly to trap surfaces, and safety concerns for those treating traps (i.e., the need for personal protective equipment) has led to other alternatives being tested. Aerosol Teflon- (also polytetrafluoroethylene) and silicone-based treatments of traps have resulted in increased catches of some BBWB over untreated traps (Allison et al. 2011), however silicone treatments did eventually form a “tacky” film on the trap as the season progressed. When compared with fluon-treated versions, aerosol Teflon-treated multiple-funnel and intercept panel traps were equally successful in capturing some BBWB (Allison et al. 2014), suggesting an effective alternative to fluon.
Trap cleaning
Trap surfaces can become coated in dust and pollen while other debris such as spider webs and plant material can clog or reduce trap openings to collection cups potentially reducing BBWB catches (Wilkening et al. 1981; Lindgren 1983). When tested, cleaning traps in the field or replacing field deployed traps with clean traps every maintenance period provided mixed results (Dodds and DiGirolomo 2020). Teflon-treated traps caught more cerambycids than traps that were not treated, but replacing dirty traps with clean traps every 2 weeks did not affect overall catch. However, when traps were left in the field and cleaned every other week, overall mean catch of bark and ambrosia beetles (including individuals captured from three species) and one cerambycid species were significantly higher than in traps that were not cleaned. This cleaning probably provided a more slippery surface reducing the beetles’ ability to land on and then take flight from the trap. Regardless of how often traps are cleaned during the season, an argument can be made for cleaning traps between field seasons, as this process should increase the lifespan and efficacy of the traps. Glue-coated surfaces are more susceptible to the detrimental effects of saturation with dust and debris that can reduce trap efficacy. In the USA, A. planipennis guidelines (USDA APHIS PPQ 2023) for prism traps promote the removal of the debris-filled glue followed by a subsequent re-coating of the surface with glue in order to maintain the “sticky” glue surface. While these references are mostly anecdotal and promote preventative maintenance to avoid problems, there has been a paucity of research to compare the effects of cleaning traps on the traps’ abilities to capture and retain beetles.
Trap killing agent
Early traps for BBWB used a variety of killing agents in non-glue-coated flight intercept traps, from isopropanol-filled bottles or cups (Wilkening et al. 1981) to adding a wetting agent or detergent to a water-filled collection trough or cup (Chapman and Kinghorn 1955; Lindgren 1983; Lindgren et al. 1983). As multiple-funnel trap use expanded, a “dry” trap option that relied on a plastic strip with the insecticide dichlorvos (2,2-dichlorovinyl dimethyl phosphate) to kill trapped insects became available. However, as target taxa broadened, a concern grew that dry traps may be allowing escape of larger more agile insects (e.g., cerambycids and siricids) before the pesticide could act, and that wet traps filled with liquid killing agent would catch more BBWB (e.g., Morewood et al. 2002; de Groot and Nott 2003). Subsequent studies confirmed wet cups were superior to dry cups for many BBWB species (Sweeney et al. 2006; Miller and Duerr 2008). Various collection liquids have been used, including saturated salt solution (Graham and Poland 2012; Lyons et al. 2012; Webster et al. 2012a; Petrice and Haack 2015; Wong et al. 2017; Marchioro et al. 2020) or ethylene glycol (Rassati et al. 2019; Cavaletto et al. 2020, 2021; Marchioro et al. 2020). Propylene glycol (generally RV-style antifreeze with 25–30% propylene glycol) is a common trapping agent and preservative used in North America (Miller and Duerr 2008; Francese et al. 2011; Miller and Crowe 2011; Dodds et al. 2015; among others) and has the added benefit of low toxicity to vertebrates compared to ethylene glycol that is deadly. At times, soap or other additives may be added to the collection liquid to reduce surface tension and/or increase bitterness of the solution, thereby warding off vertebrates that might find the solution appealing to drink or insects palatable to eat.
Most trapping surveys are conducted with insect mortality being a goal. However, there are times when capturing live insects is important. Hanks et al. (2007) used a modified intercept panel trap to collect live cerambycid beetles for volatile collection and identification of sex-aggregation pheromones. Francese et al. (2019) suggested that following the addition of an internal, fluon-coated funnel with a long stem (6.9 cm) that decreased the diameter of the bottom funnel of a multiple-funnel trap from 5.0 to 3.9 cm, the multiple-funnel trap had the potential for use as a live trap. This method was utilized by Gould et al. (2018) to collect native non-target cerambycids for host-specificity testing with potential parasitoids of Anoplophora glabripennis (Motschulsky).
Trap color
For some BBWB, trap color is an important consideration when establishing surveys. In early behavioral Y-tube assays, several bark beetle species displayed an affinity for wavelengths in the visible section of the electromagnetic spectrum relating to ultraviolet (426 nm), blue (476 nm), blue/green (500–525 nm), and red (600–625 nm) (Groberman and Borden 1981). However, when Lindgren et al. (1983) incorporated some of these colors (blue, green, red, and clear) into their multiple-funnel trap, black traps caught more beetles. This pattern was further reinforced in subsequent studies (Dubbel et al. 1985; de Groot and Zylstra 1995; Strom and Goyer 2001; Campbell and Borden 2005). Dubbel et al. (1985) suggested that black traps should be used as a standard for the group, and this has been the case for the past 40 years. Interestingly, trapping of European and Canadian scolytines (Marchioro et al. 2020) using purple and green multiple-funnel traps revealed several species that were attracted to traps of these colors, however, no black trap comparison was included in the study. Cavaletto et al. (2020) compared several trap colors against black and found attraction to the novel colors in the species Hylesinus oleiperda (blue and purple) and Scolytus multistriatus (blue and gray). However, these beetles may be outliers and several other species tested were attracted more to black traps. With the exception of yellow and white sticky panel traps catching lower numbers of ambrosia beetles, trap color was mostly unimportant for general surveys of these communities (Werle et al. 2016).
Studies to determine optimal traps for cerambycids relied primarily on variations of black multiple-funnel and intercept panel traps (de Groot and Nott 2001, 2003; McIntosh et al. 2001; Morewood et al. 2002). During these studies, traps would occasionally capture larger buprestid genera (e.g., Chalcophora, Buprestis, Dicerca), but in general this family was much rarer in traps than cerambycids or bark beetles. Studies conducted by Oliver et al. (2002) and later published in Perkovich et al. (2022) showed that purple traps were attractive to Chrysobothris spp., an observation that led to the development of colored traps for A. planipennis (Francese et al. 2005, 2008). Electroretinogram assays demonstrated the ability of A. planipennis to see color in four regions—ultraviolet, blue, green, and red, and when tested in the field, green and purple sticky prism traps outperformed all other colors tested, and green were more attractive than purple (Crook et al. 2009). Colors were later refined based on tests comparing variations in the wavelength and reflectance of the green as well as the red and blue peaks (Francese et al. 2010a, 2010b) and incorporated into commercially available green and purple sticky prism traps and later into double decker traps (Poland et al. 2011) and non-sticky multiple-funnel traps (Francese et al. 2011, 2013). Green multiple-funnel traps and prism traps placed in the canopy caught more beetles (Francese et al. 2011), and with the addition of fluon had higher detection rates in low density areas, than purple prism traps (Crook et al. 2014; Tobin et al. 2021). Additionally, green multiple-funnels, green prism traps, and purple and green and purple double-decker traps exhibited higher catch and detection rates than single purple, canopy prism traps (Tobin et al. 2021). Green traps of varying shades (multiple-funnels, intercept panels and other trap types) also catch high numbers of other Agrilus species (Domingue et al. 2013; Petrice et al. 2013; Petrice and Haack 2015; Rassati et al. 2019; Cavaletto et al. 2020), with the exception of the goldspotted oak borer, Agrilus auroguttatus Schaeffer (Coleman et al. 2014) and Agrilus viridis (L.) (Rhainds et al. 2017) which preferred purple over green traps. For Chrysobothris and Dicerca species, which do not tend to visit flowers or the tree canopy, the preferred trapping color is purple (Petrice and Haack 2015; Cavaletto et al. 2020; Perkovich et al. 2022, 2023). Within the Cerambycidae, trends tend to follow a similar pattern, that is attraction to yellow, green and blue traps by flower-visiting beetles (usually in the subfamily Lepturinae) and to darker colors (red, brown, black) for beetles that do not visit flowers, usually in the Cerambycinae and Lamiinae (de Groot and Nott 2001; Campbell and Borden 2009; Rassati et al. 2019; Cavaletto et al. 2021; Marchioro et al. 2020). With the incorporation of these colors into traps based on the targets sought, there is a greater chance for successful surveys.
Extrinsic factors
Type of habitat
Site and habitat selection is often predicated by survey objectives. For nonnative BBWB survey traps, the proximity to high-risk environs is the most important consideration. These include coastal ports of entry, inland ports, high density warehouse concentrations, and anywhere large concentrations of freight or solid wood packing material and pallets may be stored (Brockerhoff et al. 2006; Rassati et al. 2015a, 2015b; Meurisse et al. 2019). It also includes forests, parks, or arboreta adjacent to these areas (Rassati et al. 2015a; Rabaglia et al. 2019; DiGirolomo et al. 2022; Mas et al. 2023). The goal of this trap placement is to detect any BBWB that may have arrived in products or packing material subsequently discarded within or adjacent to ports, either as the insects disperse from brood material or early in the establishment phase in the adjacent forests. Selection of which site to trap should be adjusted based on risk of BBWB introduction or known threat of a specific species introduction. Previous studies indicated that ports importing large amounts of commodities and surrounded by broadleaf-dominated forest patches should be prioritized over small ports or even big ports surrounded by conifer-dominated forests when generally surveying for nonnative BBWB (Rassati et al. 2015b).
Besides proximity to high-risk environs, other factors can affect site selection when targeting native and nonnative BBWB. Many lessons learned from studies estimating arthropod species diversity in forests may be applied to the design of surveys for detection of nonnative species. For example, biodiversity studies using insecticide fogging of tree canopies have shown that beetle species composition can vary significantly among forest habitats (Guilbert 1997), among different tree species (Davies et al. 1997), among trees of the same tree species (Allison et al. 1997), and even between north and south aspects of the same tree (Richardson et al. 1997). Thus, the selection of survey sites and the tree species in or near where traps are placed may significantly affect the number and composition of species detected. Depending on habitat preference and life history traits, stands in poor condition (i.e., overstocked, declining trees) are often targeted for some species that typically colonize stressed trees and behave as secondary species. North American detection efforts for S. noctilio focused on surveying stands that contained host trees under stress (Dodds and de Groot 2012). Alternatively, healthy stands may also be implemented as survey sites, especially when the target insect is a primary tree killer or associated with living hosts, such as A. glabripennis, Xyleborus glabratus (Eichhoff), and Pityophthorus juglandis Blackman (Formby et al. 2012; Nehme et al. 2014; Wiggins et al. 2014; Fraedrich et al. 2015; Marchioro and Faccoli 2021).
Trap proximity to host trees
Since the foraging behavior of BBWB may be influenced by olfactory and visual stimuli from both host- and non-host trees (Bruce et al. 2005), it follows that efficacy of survey traps may be influenced by the tree species in which they are placed, especially for canopy traps. For example, Ulyshen and Hanula (2007) collected more beetles (but not more beetle species) in unbaited flight intercept traps that were placed in sycamore trees, Platanus occidentalis L., compared to traps placed in the canopies of Quercus phellos L., Liquidambar styriciflua L., or Pinus taeda L., and mean catch of the citrus longhorn beetle, Anoplophora chinensis (Forster), was greater in traps that were placed in the crowns of host trees than in traps hung from wooden poles in openings (Marchioro et al. 2022a).
Host volatile lures that attempt to simulate the blend of volatiles emitted from host trees have proven effective for surveys of A. planipennis (Crook et al. 2008) and Tetropium fuscum (F.) (Sweeney et al. 2004). However, for BBWB species for which we currently have no effective long distance semiochemical attractants (e.g., most species of Buprestidae) it may be worth considering the tree in which traps are placed as the “host volatile lure”. Because the BBWB species assemblages attracted to the vicinity of trees should vary among tree species, it follows that species richness of BBWB in traps should increase when traps are placed in a greater diversity of tree species. However, this is largely hypothetical and is a topic for further research.
Presence of disturbances
Dispersal and host selection in BBWB is understudied for most species. Some species respond positively to disturbance and host presence (Wermelinger et al. 2002; Park and Reid 2007), while others have not shown strong responses (Sanchez-Martinez and Wagner 2002; Gossner et al. 2019). In general, forest disturbances influence wood-inhabiting BBWB in two ways. First, BBWB often respond to damaged, stressed, downed, or dead trees in an area after a disturbance such as tornadoes, straight-line winds, fire, drought, defoliation, pathogens, or silvicultural treatments (Dunbar and Stephens 1975; Muzika et al. 2000; Gandhi et al. 2009; Dodds 2011; Furniss 2014; Kelsey et al. 2014; Sierota and Grodzki 2020; Cours et al. 2022; Miller et al. 2023). Stressed trees and damaged or downed tree material release host volatiles like terpenes and ethanol (Kelsey 2001; Böröczky et al. 2012) that attract insects dispersing and seeking ephemeral reproductive habitat. Sites that have been repeatedly disturbed may have a diversity of downed wood species and decay classes, providing habitat for a larger BBWB community (Økland et al. 1996). The resulting structurally diverse forests also often contain higher species richness of insects than more uniform forests (Storch et al. 2023).
The second way disturbances influence BBWB occurs after colonization of host material when habitat becomes a source of insects that may be captured in detection traps as they disperse away from the habitat (Wichmann and Ravn 2001; Feldhaar and Schauer 2018; Potterf et al. 2019). A potential downside of trapping within disturbed habitats and one cited at times by surveyors is a concern for reduced captures because of competition with reproductive habitat that likely is a stronger source of attraction than a trap. While not studied specifically, this concern does not seem to be warranted. More or equal numbers of insects and species are commonly captured in disturbed habitats compared to controls (Gandhi et al. 2009; Morin et al. 2015; Dodds et al. 2023; Miller et al. 2023).
Crown cover/understory light
While stand disturbance influences local beetle populations, localized crown disturbances resulting in changes to lighting conditions may also influence BBWB at smaller spatial scales. Less is known about this factor as it directly relates to the use of survey traps. However, there is evidence that canopy openness and microhabitat light conditions can influence the broader saproxylic insect community (Bouget and Duelli 2004; Lindhe et al. 2005; Koch Widerberg et al. 2012; Bouget et al. 2013; Seibold et al. 2016; Lettenmaier et al. 2022), suggesting more open conditions could yield better trapping results. Insects are more active in warmer conditions, increasing chances to capture them in traps (Taylor 1963), especially in early spring and late fall. Nonetheless, increased sunlight is not always associated with increased species richness and abundance. For example, ambrosia beetle abundance and species richness has been positively associated with higher canopy coverage estimates (Holuša et al. 2021; Menocal et al. 2022). It is also likely that higher temperatures on traps under more direct sunlight results in increased semiochemical release rates (Nielsen et al. 2019) and potentially a stronger attractive source compared to cooler shaded conditions.
Horizontal and vertical placement
The position of a survey trap relative to its surroundings can significantly affect the community of BBWB captured. Proximity to a forest edge, along either a horizontal- (forest opening–edge–interior) or vertical gradient (understory–mid canopy–upper canopy) can influence climatic variables (temperature, humidity, sunlight, windspeed), plant species diversity and composition, and ultimately resource availability (food, mates, oviposition sites) for herbivorous insects (Murcia 1995; Basset et al. 2003; Lindhe et al. 2005; Vodka et al. 2009; Domingue et al. 2011; Lelito et al. 2011; Ulyshen 2011; Vodka and Cizek 2013; Normann et al. 2016). In a meta-analysis, De Carvalho Guimarães et al. (2014) reported that species richness of insect herbivores was 65% greater along forest edges compared to forest interiors and that the effect was strongest for generalist herbivores in the Lepidoptera and Orthoptera. Significant variation in abundance, species richness, or species composition of beetles (Ulyshen et al. 2004; Wermelinger et al. 2007; Ewers and Didham 2008; Normann et al. 2016) and more specifically BBWB (Igeta et al. 2004; Francese et al. 2008, 2010b; Dodds 2011; Allison et al. 2019; Sweeney et al. 2020) has been observed among traps placed in openings vs. forest edges vs. forest interiors. Similarly, vertical stratification (understory vs. mid- or upper canopy) has been observed for beetles (Sutton et al. 1983; Kato et al. 1995; Preisser et al. 1998; Su and Woods 2001; Hirao et al. 2009; Schroeder et al. 2009; Bouget et al. 2011; Normann et al. 2016; Stork et al. 2016; Weiss et al. 2016) and BBWB in particular (Vance et al. 2003; Igeta et al. 2004; Leksono et al. 2006; Ulyshen and Hanula 2007; Wermelinger et al. 2007; Francese et al. 2008, 2010b; Vodka et al. 2009; Reding et al. 2010; Hanula et al. 2011; Graham et al. 2012; Vodka and Cizek 2013; Dodds 2014; Hardersen et al. 2014; Maguire et al. 2014; Schmeelk et al. 2016; Wong and Hanks 2016; Li et al. 2017; Procházka et al. 2018; Flaherty et al. 2019; Foit et al. 2019; Rassati et al. 2019; Sheehan et al. 2019; Ulyshen and Sheehan 2019; Miller et al. 2020; Sweeney et al. 2020; Meng et al. 2022).
Trends along these gradients vary among and within BBWB families (Ulyshen et al. 2004; Wermelinger et al. 2007; Normann et al. 2016; Allison et al. 2019; Sweeney et al. 2020) and feeding guilds (Sheehan et al. 2019; Ulyshen and Sheehan 2019). Buprestids were often more abundant and/or species rich in the tree canopy than the understory (Francese et al. 2008, 2010b; Rassati et al. 2019; Sheehan et al. 2019; Sweeney et al. 2020) whereas in Scolytinae there was the opposite trend (Ulyshen and Hanula 2007; Hanula et al. 2011; Dodds 2014; Hardersen et al. 2014; Flaherty et al. 2019; Sheehan et al. 2019; Ulyshen and Sheehan 2019; Meng et al. 2022) or no trend (Wermelinger et al. 2007; Maguire et al. 2014; Meng et al. 2022). Inconsistency in vertical distribution of Scolytinae is partly because the subfamily consists of more than one feeding guild. Species richness of ambrosia beetles tends to decrease with trap height, due to greater humidity and other conditions favoring fungal growth in the understory (Ulyshen 2011) whereas the opposite trend has been observed for phloem/wood feeders (Procházka et al. 2018; Sheehan et al. 2019; Ulyshen and Sheehan 2019). Similar differences in feeding patterns may partially explain why species richness of longhorn beetles is sometimes greater (Ulyshen and Hanula 2007; Maguire et al. 2014; Flaherty et al. 2019; Rassati et al. 2019; Ulyshen and Sheehan 2019; Sweeney et al. 2020; Meng et al. 2022), similar (Vance et al. 2003; Graham et al. 2012; Hardersen et al. 2014; Schmeelk et al. 2016; Wong and Hanks 2016; Flaherty et al. 2019; Meng et al. 2022), or lower (Wermelinger et al. 2007; Dodds 2014) in the canopy than the understory. Species that preferentially breed in large diameter stems, stumps or downed trees are usually more active in the understory (Brar et al. 2012; Procházka et al. 2018; Flaherty et al. 2019; Miller et al. 2020) whereas species that breed in small branches and twigs are more active in the canopy (Vance et al. 2003; Ulyshen and Hanula 2007).
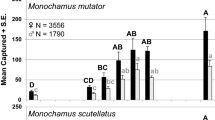
Abundance of all three BBWB families tended to be greater along forest edges or in openings than inside the forest but there were exceptions for individual species of scolytines and cerambycids. For example, catch of Acmaeops proteus proteus (Kirby) was greatest along the forest edge whereas catches of Euderces pini (Fabr.), Neoclytus acuminatus (F.), and Anelaphus pumilus (Newman) were greatest in the forest interior, and catches of Monochamus mutator LeConte and M. scutellatus (Say) were greatest in a clearing (Allison et al. 2019). In studies that simultaneously tested effects of horizontal and vertical trap position, Scolytinae were more abundant in the canopy than the understory in traps placed inside the forest but not along the forest edge (Sweeney et al. 2020) and BBWB species composition differed between mid- and upper canopy traps inside the forest but not at the forest edge (Sheehan et al. 2019). Effects of trap height on catch of buprestids (Wermelinger et al. 2007) and other saproxylic beetles (Vodka and Cizek 2013) were less pronounced or absent when traps were placed along a forest edge vs. the forest interior, suggesting that degree of sun exposure is associated with vertical stratification of some species.
The effects of horizontal or vertical trap placement is often context dependent, varying among forest types (Procházka et al. 2018; Meng et al. 2022), silvicultural treatments (Su and Woods 2001), size and age of forest clearings (Ulyshen et al. 2004; Allison et al. 2019; Sweeney et al. 2020), seasons (Hardersen et al. 2014), degree of slope (Sutton et al. 1983), dominant tree species (Vance et al. 2003), and the semiochemical lure used to bait traps (Flaherty et al. 2019). Depending on the attraction range of an attractant, baited traps may reduce the effects of horizontal and vertical placement on catch of certain species (Ulyshen and Sheehan 2019). In spite of inconsistency in trends of species richness or abundance of BBWB along horizontal or vertical gradients, most studies have shown that species composition differs between strata, and that many species are captured solely or predominantly in the canopy or understory, edge or interior (Vance et al. 2003; Leksono et al. 2006; Wermelinger et al. 2007; Vodka et al. 2009; Graham et al. 2012; Dodds 2014; Hardersen et al. 2014; Schmeelk et al. 2016; Webster et al. 2016; Wong and Hanks 2016; Procházka et al. 2018; Flaherty et al. 2019; Ulyshen and Sheehan 2019; Miller et al. 2020; Sweeney et al. 2020; Meng et al. 2022). Therefore, when the objective is to detect as many species as possible of BBWB present at a site, traps should ideally be deployed in both the canopy and understory, and along forest edges as well as inside the forest.
BBWB species composition in mid-canopy traps has reflected that in both canopy and understory traps suggesting it may be a good compromise when budgets or logistical limitations make it impractical to sample at more than one trap height (Weiss et al. 2016; Procházka et al. 2018; Ulyshen and Sheehan 2019). However, Sheehan et al. (2019) suggested that traps deployed at an intermediate height of 5 m would fail to detect certain species active in the upper canopy.
Using knowledge on factors affecting trap efficacy to improve surveillance programs
As described above, many factors influence BBWB behavior and response to semiochemical-baited traps. It is often difficult to address all factors when deploying traps due to budget constraints and logistical realities. However, there are several considerations to be mindful of when developing surveys that can help improve results. First, there is rarely a perfect survey, but a sub-optimal trap deployed in only one vertical or horizontal strata is better than no survey at a location. Placing traps in almost any location is informative but placing traps in more than one vertical or horizontal strata (e.g., canopy and understory, forest edge and interior) often increases the number of BBWB species detected and is recommended if budgets allow. Second, baiting traps with multiple pheromones can increase the number of species detected with little or no negative effect on catch of other BBWB, an approach that is especially useful for generic surveillance. Third, using more than one trap color, e.g., green or purple traps, as well as black traps, can increase detection efficacy of longhorn beetles and jewel beetles. Treating these traps with a lubricant and conducting regular maintenance and cleaning can help improve trap efficacy. Finally, simply increasing the number of traps per site and using multiple trap types usually increases the numbers of BBWB species detected. In general, our review has found that, for generic surveys, the greater the diversity in trapping methods used (e.g., more than one type of lure blend, trap height, trap color) the more BBWB species are detected and the greater the chances of detecting nonnative species that may be present. However, with limited budgets these considerations must be weighed against the number of sites that can be surveyed per year. One way in which increased costs of diversifying trapping methods may be offset is by using a different suite of lure blends, trap colors, etc. in alternating years, as done by the Canadian Food Inspection Agency for nonnative BBWB surveillance (Thurston et al. 2022). For surveillance of individual target species during a specific survey, trap type, lure, color, height, etc. should follow established protocols that have proven most efficacious in terms of mean catch or detecting the species at sites with low population densities. If no protocols exist, using methodology developed for closely related insects in the same genus or family is an appropriate starting point.
A practical example of how to use these considerations and the available knowledge can be illustrated by two case studies (Fig. 3). For a generic survey for nonnative BBWB (Fig. 3A), efforts should concentrate on coastal or inland ports and surrounding forests with traps deployed across available sites (Rassati et al. 2015a, b). The number of traps strongly depends on the budget, but even a low number of traps can lead to important results (Mas et al. 2023). In forested areas adjacent to ports, black intercept panel traps set up in the understory should be coupled with green multiple funnel traps set up in the canopy to increase likelihood of intercepting jewel beetles as well as other BBWB species active in the latter forest stratum (Rassati et al. 2019; Marchioro et al. 2020). Ideally, traps should be placed in the interior of the forested area and along the edge to account for different BBWB foraging preferences of species that may be present (Ulyshen et al. 2004; Wermelinger et al. 2007; Allison et al. 2019; Sweeney et al. 2020). The same trap combination (i.e., black intercept and green multi-funnel traps) should be attached to available supports at a height of 1.5–2 m in areas of the active port where timber is stored before being sent to the destination, or where containers are opened for visual inspections (Rassati et al. 2014). Traps within the port environs and in adjacent forested areas should be baited with a multi-lure blend composed of pheromones and kairomones that are known to attract a wide array of taxa without affecting catches of jewel beetles (e.g., Roques et al. 2023). When budget permits, black intercept traps should be coupled with traps of different colors, for example yellow or blue, to also attract longhorned beetle species that are not attracted by semiochemicals used in the survey (e.g., Lepturinae). All traps should be treated with fluon or other lubricants (Allison et al. 2011; Graham and Poland 2012) and the collector cup should be filled with propylene glycol or other liquids rather than left dry with an insecticide (Allison and Redak 2017).
Example of how to exploit available knowledge on intrinsic and extrinsic factors affecting trap efficacy in surveillance programs. Here we use as case studies a generic surveillance targeting bark and ambrosia beetles, longhorn beetles and jewel beetles (A) and a specific surveillance targeting longhorn beetles of the genus Monochamus (B) carried out in a port area and its surrounding natural area (e.g., a forest). For each of them, information on the best trap type, trap color, lure (multi-lure blend vs. specific lure), trap height, and horizontal position of the traps are provided. Black or green dots on the black and white landscapes represent the traps
As an example of a specific surveillance program, native and non-native Monochamus spp. pose a serious risk in some areas because they vector the destructive pine wood nematode [Bursaphelenchus xylophilus (Steiner & Bührer) Nickle] (Mamiya 1988). A surveillance program targeting Monochamus spp. should also be concentrated in ports and surrounding forested areas (Fig. 3B). Black intercept panel traps are optimal for Monochamus spp. and should be baited with a blend of pheromones and kairomones that are efficient for several species in the genus (Allison et al. 2012; Miller et al. 2016; Boone et al. 2019; Foit et al. 2019). There may be some benefit of having traps in tree canopies when available (Foit et al. 2019), but Monochamus spp. have also been frequently captured in understory traps (de Groot and Nott 2004; Dodds 2014; Allison et al. 2019). Traps should be treated with a lubricant, such as fluon (Jaworski et al. 2022; Dong et al. 2023), and a wet collection cup should be used with propylene glycol as the liquid preservative (Allison and Redak 2017). Possibly, traps should be set up both on the forest edge and in the forest interior as different Monochamus spp. prefer either the former or latter habitat (Allison et al. 2019), and any areas of recent disturbance may also help improve trapping results (Dodds 2011; Dodds et al. 2019).
Concluding remarks and future research
Despite the large amount of existing research, our understanding of factors affecting the efficacy of BBWB surveys is still incomplete. Additional studies are necessary to further understand species-specific responses and to elucidate whether general patterns can be described. In addition, while there has been much research on the effects of trap types, lures, colors, etc., on the relative abundance of BBWB captured in traps, there are very few papers that describe the relationship between trap catch and actual population or attack density of the target BBWB species (but see Hanula et al. 2011). Greater understanding of these relationships will improve our interpretation of trap catches in determining risk and monitoring spread of invasive BBWB, especially during specific surveys. Furthermore, a rapid increase in cerambycid pheromone chemistry knowledge over the last 2 decades has greatly increased our ability to detect many of these species in surveys (Hanks and Millar 2016; Roques et al. 2023). New information on the role of fungal volatiles (Hulcr et al. 2011; Kuhns et al. 2014) for attracting some ambrosia beetles offers opportunities to narrow survey efforts for these species. Unfortunately, knowledge of buprestid pheromones and attractive semiochemicals is much more limited at this time (Silk et al. 2019). Further research in this area may lead to improved lures and greater detection efficacy of Buprestidae. Lastly, innovative technologies to reproduce the most attractive visual cues on trap panels [e.g., the microstructures present on elytra of jewel beetles which are responsible for the light scattering effect used to locate mates (Domingue et al. 2014)] can strongly improve trap attractiveness, especially toward species for which pheromones are not yet known.
References
Allison A, Samuelson GA, Miller SE (1997) Patterns of beetle species diversity in Castanopsis acuminatissima (Fagaceae) trees studied with canopy fogging in mid-montane New Guinea rainforest. In: Stork NE, Adis J, Didham RK (eds) Canopy arthropods. Chapman & Hall, London, pp 224–236
Allison JD, Borden JH (2001) Observations on the behavior of Monochamus scutellatus (Coleoptera: Cerambycidae) in northern British Columbia. J Entomol Soc BC 98:195–200
Allison JD, Redak RA (2017) The impact of trap type and design features on survey and detection of bark and woodboring beetles and their associates: a review and meta-analysis. Annu Rev Entomol 62:127–146. https://doi.org/10.1146/annurev-ento-010715-023516
Allison JD, Borden JH, Seybold SJ (2004) A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 14:123–150. https://doi.org/10.1007/s00049-004-0277-1
Allison JD, Johnson CW, Meeker JR, Strom BL, Butler SM (2011) Effect of aerosol surface lubricants on the abundance and richness of selected forest insects captured in multiple-funnel and panel traps. J Econ Entomol 104:1258–1264. https://doi.org/10.1603/EC11044
Allison JD, McKenney JL, Millar JG, McElfresh JS, Mitchell RF et al (2012) Response of the woodborers Monochamus carolinensis and Monochamus titillator (Coleoptera: Cerambycidae) to known cerambycid pheromones in the presence and absence of the host plant volatile alpha-pinene. Environ Entomol 41:1587–1596. https://doi.org/10.1603/EN12185
Allison JD, McKenney JL, Miller DR, Gimmel ML (2013) Kairomonal responses of natural enemies and associates of the southern Ips (Coleoptera: Curculionidae: Scolytinae) to ipsdienol, ipsenol and cis-verbenol. J Insect Behav 26:321–335. https://doi.org/10.1007/s10905-012-9349-1
Allison JD, Bhandari BD, McKenney JL, Millar JG (2014) Design factors that influence the performance of flight intercept traps for the capture of longhorned beetles (Coleoptera: Cerambycidae) from the subfamilies Lamiinae and Cerambycinae. PLoS ONE 9:e93203. https://doi.org/10.1371/journal.pone.0093203
Allison JD, Graham EE, Poland TM, Strom BL (2016) Dilution of fluon before trap surface treatment has no effect on longhorned beetle (Coleoptera: Cerambycidae) captures. J Econ Entomol 109:1215–1219. https://doi.org/10.1093/jee/tow081
Allison J, Strom B, Sweeney J, Mayo P (2019) Trap deployment along linear transects perpendicular to forest edges: impact on capture of longhorned beetles (Coleoptera: Cerambycidae). J Pest Sci 92:299–308. https://doi.org/10.1007/s10340-018-1008-7
Aukema JE, Leung B, Kovacs K, Chivers C, Britton KO et al (2011) Economic impacts of non-native forest insects in the continental United States. PLoS ONE 6(9):e24587. https://doi.org/10.1371/journal.pone.0024587
Baker TC, Roelofs WL (1981) Initiation and termination of Oriental fruit moth male response to pheromone concentrations in the field. Environ Entomol 10:211–218. https://doi.org/10.1093/ee/10.2.211
Baker TC, Meyer W, Roelofs WL (1981) Sex pheromone dosage and blend specificity of response by Oriental fruit moth males. Entomol Exp Appl 30:269–279. https://doi.org/10.1111/j.1570-7458.1981.tb03110.x
Baker TC, Willis MA, Phelan PL (1984) Optomotor anemotaxis polarizes self-steered zigzagging in flying moths. Physiol Entomol 9:365–376. https://doi.org/10.1111/j.1365-3032.1984.tb00777.x
Bakke A (1985) Deploying pheromone-baited traps for monitoring Ips typographus populations. J Appl Entomol 99:33–39. https://doi.org/10.1111/j.1439-0418.1985.tb01956.x
Barnouin T, Soldati F, Roques A, Faccoli M, Kirkendall LR et al (2020) Bark beetles and pinhole borers recently or newly introduced to France (Coleoptera: Curculionidae, Scolytinae, Platypodinae). Zootaxa 4877:051–074. https://doi.org/10.11646/zootaxa.4877.1.2
Bartelt RJ, Cossé AA, Zilkowski BW, Fraser I (2007) Antennally active macrolide from the emerald ash borer Agrilus planipennis emitted predominantly by females. J Chem Ecol 33:1299–1302. https://doi.org/10.1007/s10886-007-9316-z
Bashford R (2012) The development of a port surrounds trapping system for the detection of exotic forest insect pests in Australia. In: Oteng-Amoako EA (ed) New advances and contributions to forestry research. InTech Open, Rijeka, pp 85–100. https://doi.org/10.5772/35068
Basset Y, Novotny V, Miller SE, Kitching RL (2003) Conclusion: arthropods, canopies and interpretable patterns. In: Basset Y, Novotny V, Miller SE, Kitching RL (eds) Arthropods of tropical forests: spatio-temporal dynamics and resource use in the canopy. Cambridge University Press, Cambridge, pp 394–405
Bauer J, Vité JP (1975) Host selection by Trypodendron lineatum. Naturwissenschaften 62:539–539. https://doi.org/10.1007/BF00609080
Billings RF, Upton WW (2010) A methodology for assessing annual risk of southern pine beetle outbreaks across the southern region using pheromone traps. In: Pye JM, Rauscher HM, Sands Y, Lee DC, Beatty JS (eds) Advances in threat assessment and their application to forest and rangeland managment. USDA Forest Service, Pacific Northwest and Southern Research Stations, PNW-GTR-802, Portland, pp 73–86
Boone CK, Sweeney J, Silk P, Hughes C, Webster RP et al (2019) Monochamus species from different continents can be effectively detected with the same trapping protocol. J Pest Sci 92:3–11. https://doi.org/10.1007/s10340-018-0954-4
Borden JH, Miller DR (2000) Dose-dependent and species-specific responses of pine bark beetles (Coleoptera: Scolytidae) to monoterpenes in association with pheromones. Can Entomol 132:183–195. https://doi.org/10.4039/Ent132183-2
Böröczky K, Crook DJ, Jones TH, Kenny JC, Zylstra KE et al (2009) Monoalkenes as contact sex pheromone components of the woodwasp Sirex noctilio. J Chem Ecol 35:1202–1211. https://doi.org/10.1007/s10886-009-9693-6
Böröczky K, Zylstra K, McCartney N, Mastro V, Tumlinson J (2012) Volatile profile differences and the associated Sirex noctilio activity in two host tree species in the northeastern United States. J Chem Ecol 38:213–221. https://doi.org/10.1007/s10886-012-0077-y
Bouget C, Duelli P (2004) The effects of windthrow on forest insect communities: a literature review. Biol Conserv 118:281–299. https://doi.org/10.1016/j.biocon.2003.09.009
Bouget C, Brin A, Brustel H (2011) Exploring the “last biotic frontier”: Are temperate forest canopies special for saproxylic beetles? For Ecol Manag 261:211–220. https://doi.org/10.1016/j.foreco.2010.10.007
Bouget C, Larrieu L, Nusillard B, Parmain G (2013) In search of the best local habitat drivers for saproxylic beetle diversity in temperate deciduous forests. Biodivers Conserv 22:2111–2130. https://doi.org/10.1007/s10531-013-0531-3
Bouwer MC, MacQuarrie CJK, Aguirre-Gil OJ, Slippers B, Allison JD (2020) Impact of intercept trap type on plume structure: a potential mechanism for differential performance of intercept trap designs for species. J Pest Sci 93:993–1005. https://doi.org/10.1007/s10340-020-01204-y
Bracalini M, Croci F, Ciardi E, Mannucci G, Papucci E et al (2021) Ips sexdentatus mass-trapping: mitigation of its negative effects on saproxylic beetles larger than the target. Forests 12:175. https://doi.org/10.3390/f12020175
Brar GS, Capinera JL, Mclean S, Kendra PE, Ploetz RC et al (2012) Effect of trap size, trap height and age of lure on sampling Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), and its flight periodicity and seasonality. Fla Entomol 95:1003–1011. https://doi.org/10.1653/024.095.0428
Brattli JG, Andersen J, Nilssen AC (1998) Primary attraction and host tree selection in deciduous and conifer living Coleoptera: Scolytidae, Curculionidae, Cerambycidae and Lymexylidae. J Appl Entomol 122:345–352. https://doi.org/10.1111/j.1439-0418.1998.tb01511.x
Briscoe AD, Chittka L (2001) The evolution of color vision in insects. Annu Rev Entomol 46:471–510. https://doi.org/10.1146/annurev.ento.46.1.471
Brockerhoff EG, Jones DC, Kimberley MO, Suckling DM, Donaldson T (2006) Nationwide survey for invasive wood-boring and bark beetles (Coleoptera) using traps baited with pheromones and kairomones. For Ecol Manag 228:234–240. https://doi.org/10.1016/j.foreco.2006.02.046
Bruce TJA, Pickett JA (2011) Perception of plant volatile blends by herbivorous insects—finding the right mix. Phytochemistry 72:1605–1611. https://doi.org/10.1016/j.phytochem.2011.04.011
Bruce TJA, Wadhams LJ, Woodcock CM (2005) Insect host location: a volatile situation. Trends Plant Sci 10:269–274. https://doi.org/10.1016/j.tplants.2005.04.003
Burbano EG, Wright MG, Gillette NE, Mori S, Dudley N et al (2012) Efficacy of traps, lures, and repellents for Xylosandrus compactus (Coleoptera: Curculionidae) and other ambrosia beetles on Coffea arabica plantations and Acacia koa nurseries in hawaii. Environ Entomol 41:133–140. https://doi.org/10.1603/en11112
Burner RC, Birkemoe T, Olsen SL, Sverdrup-Thygeson A (2020) Sampling beetle communities: trap design interacts with weather and species traits to bias capture rates. Ecol Evol 10:14300–14308. https://doi.org/10.1002/ece3.7029
Byers JA, Zhang QH, Birgersson G (2004) Avoidance of nonhost plants by a bark beetle, Pityogenes bidentatus, in a forest of odors. Naturwissenschaften 91:215–219. https://doi.org/10.1007/s00114-004-0520-1
Campbell SA, Borden JH (2005) Bark reflectance spectra of conifers and angiosperms: implications for host discrimination by coniferophagous bark and timber beetles. Can Entomol 137:719–722. https://doi.org/10.4039/n04-082
Campbell SA, Borden JH (2006) Integration of visual and olfactory cues of hosts and non-hosts by three bark beetles (Coleoptera: Scolytidae). Ecol Entomol 31:437–449. https://doi.org/10.1111/j.1365-2311.2006.00809.x
Campbell SA, Borden JH (2009) Additive and synergistic integration of multimodal cues of both hosts and non-hosts during host selection by woodboring insects. Oikos 118:553–563. https://doi.org/10.1111/j.1600-0706.2009.16761.x
Cardé RT (1984) Chemo-orientation in flying insects. In: Bell WJ, Cardé RT (eds) Chemical ecology of insects. Springer, Boston, pp 111–124. https://doi.org/10.1007/978-1-4899-3368-3_5
Cardé RT, Willis MA (2008) Navigational strategies used by insects to find distant, wind-borne sources of odor. J Chem Ecol 34:854–866. https://doi.org/10.1007/s10886-008-9484-5
Carrasco D, Larsson MC, Anderson P (2015) Insect host plant selection in complex environments. Curr Opin Insect Sci 8:1–7. https://doi.org/10.1016/j.cois.2015.01.014
Cavaletto G, Faccoli M, Marini L, Spaethe J, Magnani G et al (2020) Effect of trap color on captures of bark- and wood-boring beetles (Coleoptera; Buprestidae and Scolytinae) and associated predators. InSects 11:749. https://doi.org/10.3390/insects11110749
Cavaletto G, Faccoli M, Marini L, Spaethe J, Giannone F et al (2021) Exploiting trap color to improve surveys of longhorn beetles. J Pest Sci 94:871–883. https://doi.org/10.1007/s10340-020-01303-w
Cavaletto G, Ranger CM, Reding ME, Montecchio L, Rassati D (2023) Species-specific effects of ethanol concentration on host colonization by four common species of ambrosia beetles. J Pest Sci 96:833–843. https://doi.org/10.1007/s10340-022-01537-w
CFIA (2017) Plant protection survey report 2016–2017. Canadian Food Inspection Agency. https://publications.gc.ca/site/eng/9.831610/publication.html. Accessed 27 Nov 2023
Chapman JA, Kinghorn JM (1955) Window flight traps for insects. Can Entomol 87:46–47. https://doi.org/10.4039/Ent8746-1
Chenier JVR, Philogene BJR (1989) Field responses of certain forest Coleoptera to conifer monoterpenes and ethanol. J Chem Ecol 15:1729–1746. https://doi.org/10.1007/BF01012261
Coleman TW, Chen YG, Graves AD, Hishinuma SM, Grulke NE et al (2014) Developing monitoring techniques for the invasive goldspotted oak borer (Coleoptera: Buprestidae) in California. Environ Entomol 43:729–743. https://doi.org/10.1603/En13162
Colombari F, Battisti A (2023) Citizen science at school increases awareness of biological invasions and contributes to the detection of exotic ambrosia beetles. NeoBiota 84:211–229. https://doi.org/10.3897/neobiota.84.95177
Cooperband MF, Cardé RT (2006) Comparison of plume structures of carbon dioxide emitted from different mosquito traps. Med Vet Entomol 20:1–10. https://doi.org/10.1111/j.1365-2915.2006.00614.x
Cooperband MF, Boroczky K, Hartness A, Jones TH, Zylstra KE et al (2012) Male-produced pheromone in the European woodwasp, Sirex noctilio. J Chem Ecol 38:52–62. https://doi.org/10.1007/s10886-012-0060-7
Cours J, Sire L, Ladet S, Martin H, Parmain G et al (2022) Drought-induced forest dieback increases taxonomic, functional, and phylogenetic diversity of saproxylic beetles at both local and landscape scales. Landscape Ecol 37:2025–2043. https://doi.org/10.1007/s10980-022-01453-5
Crook DJ, Khrimian A, Francese JA, Fraser I, Poland TM et al (2008) Development of a host-based semiochemical lure for trapping emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae). Environ Entomol 37:356–365. https://doi.org/10.1093/ee/37.2.356
Crook DJ, Francese JA, Zylstra KE, Fraser I, Sawyer AJ et al (2009) Laboratory and field response of the emerald ash borer (Coleoptera: Buprestidae), to selected regions of the electromagnetic spectrum. J Econ Entomol 102:2160–2169. https://doi.org/10.1603/029.102.0620
Crook DJ, Böröczky K, Zylstra KE, Mastro VC, Tumlinson JH (2012) The chemical ecology of Sirex noctilio. In: Slippers B, de Groot P, Wingfield MJ (eds) The sirex woodwasp and its fungal symbiont: research and management of a worldwide invasive pest. Springer, New York, pp 148–158. https://doi.org/10.1007/978-94-007-1960-6_11
Crook DJ, Francese JA, Rietz ML, Lance DR, Hull-Sanders HM et al (2014) Improving detection tools for emerald ash borer (Coleoptera: Buprestidae): comparison of multifunnel traps, prism traps, and lure types at varying population densities. J Econ Entomol 107:1496–1501. https://doi.org/10.1603/EC14041
Czokajlo D, Ross D, Kirsch P (2001) Intercept panel trap, a novel trap for monitoring forest Coleoptera. J for Sci 47:63–65
Davies JG, Stork NE, Brendell MJD, Hine SJ (1997) Beetle species diversity and faunal similarity in Venezuelan rainforest tree canopies. In: Stork NE, Adis J, Didham RK (eds) Canopy Arthropods. Chapman & Hall, London, pp 85–103
De Carvalho Guimarães CD, Viana JPR, Cornelissen T (2014) A meta-analysis of the effects of fragmentation on herbivorous insects. Environ Entomol 43:537–545. https://doi.org/10.1603/EN13190
de Groot P, Nott R (2001) Evaluation of traps of six different designs to capture pine sawyer beetles (Coleoptera: Scolytidae). Agric for Entomol 3:107–111. https://doi.org/10.1046/j.1461-9563.2001.00087.x
de Groot P, Nott RW (2003) Response of Monochamus (Col., Cerambycidae) and some Buprestidae to flight intercept traps. J Appl Entomol 127:548–552. https://doi.org/10.1046/j.1439-0418.2003.00799.x
de Groot P, Nott RW (2004) Response of the whitespotted sawyer beetle, Monochamus s. scutellatus, and associated woodborers to pheromones of some Ips and Dendroctonus bark beetles. J Appl Entomol 128:483–487. https://doi.org/10.1111/j.1439-0418.2004.00877.x
de Groot P, Zylstra BF (1995) Factors affecting capture of male red pine cone beetles, Conophthorus resinosae Hopkins (Coleoptera: Scolytidae), in pheromone traps. Can Entomol 127:851–858. https://doi.org/10.4039/Ent127851-6
Dicke M, van Loon JJA (2000) Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol Exp Appl 97:237–249. https://doi.org/10.1046/j.1570-7458.2000.00736.x
DiGirolomo MF, Dodds KJ (2014) Cerambycidae bycatch from Asian longhorned beetle survey traps placed in forested environs. Northeast Nat 21:28–34. https://doi.org/10.1656/045.021.0310
DiGirolomo MF, Bohne MJ, Dodds KJ, Gapinski AT, DelRosso JS et al (2022) Utilizing urban arboreta for detection of native and non-native wood-inhabiting beetles. Agric for Entomol 24:72–96. https://doi.org/10.1111/afe.12470
Dodds KJ (2011) Effects of habitat type and trap placement on captures of bark (Coleoptera: Scolytidae) and longhorned (Coleoptera: Cerambycidae) beetles in semiochemical-baited traps. J Econ Entomol 104:879–888. https://doi.org/10.1603/EC10358
Dodds KJ (2014) Effects of trap height on captures of arboreal insects in pine stands of northeastern United States of America. Can Entomol 146:80–89. https://doi.org/10.4039/tce.2013.57
Dodds KJ, de Groot P (2012) Sirex, surveys, and management: challenges of having Sirex noctilio in North America. In: Slippers B, Wingfield MJ, de Groot P (eds) The sirex woodwasp and its fungal symbiont: research and management of a worldwide invasive pest. Springer, Dordrecht, pp 265–286. https://doi.org/10.1007/978-94-007-1960-6_19
Dodds KJ, DiGirolomo MF (2020) Effect of cleaning multiple-funnel traps on captures of bark and woodboring beetles in northeastern United States. InSects 11:702. https://doi.org/10.3390/insects11100702
Dodds KJ, Orwig DA (2011) An invasive urban forest pest invades natural environments—Asian longhorned beetle in northeastern US hardwood forests. Can J for Res 41:1729–1742. https://doi.org/10.1139/x11-097
Dodds KJ, Ross DW (2002a) Sampling range and range of attraction of Dendroctonus pseudotsugae pheromone-baited traps. Can Entomol 134:343–355. https://doi.org/10.4039/Ent134343-3
Dodds KJ, Ross DW (2002b) Relative and seasonal abundance of wood borers (Buprestidae, Cerambycidae) and Cucujidae trapped in Douglas-fir beetle pheromone-baited traps in northern Idaho. Pan-Pac Entomol 78:120–131
Dodds KJ, de Groot P, Orwig DA (2010a) The impact of Sirex noctilio in Pinus resinosa and Pinus sylvestris stands in New York and Ontario. Can J for Res 40:212–223. https://doi.org/10.1139/X09-181
Dodds KJ, Dubois GD, Hoebeke ER (2010b) Trap type, lure placement, and habitat effects on Cerambycidae and Scolytinae (Coleoptera) catches in the northeastern United States. J Econ Entomol 103:698–707. https://doi.org/10.1603/EC09395
Dodds KJ, Allison JD, Miller DR, Hanavan RP, Sweeney J (2015) Considering species richness and rarity when selecting optimal survey traps: comparisons of semiochemical baited flight intercept traps for Cerambycidae in eastern North America. Agric for Entomol 17:36–47. https://doi.org/10.1111/afe.12078
Dodds KJ, DiGirolomo MF, Fraver S (2019) Response of bark beetles and woodborers to tornado damage and subsequent salvage logging in northern coniferous forests of Maine, USA. For Ecol Manag 450:117489. https://doi.org/10.1016/j.foreco.2019.117489
Dodds KJ, Cancelliere J, DiGirolomo MF (2023) Bark beetle and woodborer responses to stand thinning and prescribed fire in northeastern US coastal and inland pitch pine barrens. Agric for Entomol 25:522–535. https://doi.org/10.1111/afe.12572
Domingue MJ, Csóka G, Tóth M, Vétek G, Pénzes B et al (2011) Field observations of visual attraction of three European oak buprestid beetles toward conspecific and heterospecific models. Entomol Exp Appl 140:112–121. https://doi.org/10.1111/j.1570-7458.2011.01139.x
Domingue MJ, Imrei Z, Lelito JP, Muskovits J, Janik G et al (2013) Trapping of European buprestid beetles in oak forests using visual and olfactory cues. Entomol Exp Appl 148:116–129. https://doi.org/10.1111/eea.12083
Domingue MJ, Lakhtakia A, Pulsifer DP, Hall LP, Badding JV et al (2014) Bioreplicated visual features of nanofabricated buprestid beetle decoys evoke stereotypical male mating flights. Proc Natl Acad Sci USA 111:14106–14111. https://doi.org/10.1073/pnas.141281011
Domingue MJ, Pulsifer DP, Lakhtakia A, Berkebile J, Steiner KC et al (2015) Detecting emerald ash borers (Agrilus planipennis) using branch traps baited with 3D-printed beetle decoys. J Pest Sci 88:267–279. https://doi.org/10.1007/s10340-014-0598-y
Dong YF, Xie P, Zheng KW, Gu YT, Fan JT (2023) Teflon coating and anti-escape ring improve trapping efficiency of the longhorn beetle Monochamus alternatus. Appl Sci Basel. https://doi.org/10.3390/app13031664
Dubbel V, Kerck K, Sohrt M, Mangold S (1985) Influence of trap color on the efficiency of bark beetle pheromone traps. J Appl Entomol 99:59–64. https://doi.org/10.1111/j.1439-0418.1985.tb01960.x
Dunbar DM, Stephens GR (1975) Association of twolined chestnut borer and shoestring fungus with mortality of defoliated oak in Connecticut. For Sci 21:169–174. https://doi.org/10.1093/forestscience/21.2.169
Elkinton JS, Cardé RT (1984) Odor dispersion. In: Bell WJ, Cardé RT (eds) Chemical ecology of insects. Springer, Boston, pp 73–91. https://doi.org/10.1007/978-1-4899-3368-3_3
Embrey S, Remais JV, Hess J (2012) Climate change and ecosystem disruption: the health impacts of the North American Rocky Mountain pine beetle infestation. Am J Public Health 102:818–827. https://doi.org/10.2105/AJPH.2011.300520
Erbilgin N, Powell JS, Raffa KF (2003) Effect of varying monoterpene concentrations on the response of Ips pini (Coleoptera: Scolytidae) to its aggregation pheromone: implications for pest management and ecology of bark beetles. Agric for Entomol 5:269–274. https://doi.org/10.1046/j.1461-9563.2003.00186.x
Evans WG (1964) Infra-red receptors in Melanophila acuminata DeGeer. Nature 202:211. https://doi.org/10.1038/202211a0
Ewers RM, Didham RK (2008) Pervasive impact of large-scale edge effects on a beetle community. Proc Natl Acad Sci USA 105:5426–5429. https://doi.org/10.1073/pnas.0800460105
Fadamiro HY, Wyatt TD, Birch MC (1998) Flying beetles respond as moths predict: optomotor anemotaxis to pheromone plumes at different heights. J Insect Behav 11:549–557. https://doi.org/10.1023/A:1022367430354
Fan J-T, Denux O, Courtin C, Bernard A, Javal M et al (2019) Multi-component blends for trapping native and exotic longhorn beetles at potential points-of-entry and in forests. J Pest Sci 92:281–297. https://doi.org/10.1007/s10340-018-0997-6
Fatzinger CW (1985) Attraction of the black turpentine beetle (Coleoptera, Scolytidae) and other forest Coleoptera to turpentine-baited traps. Environ Entomol 14:768–775. https://doi.org/10.1093/ee/14.6.768
Fayt P, Machmer MM, Steeger C (2005) Regulation of spruce bark beetles by woodpeckers–a literature review. For Ecol Manag 206:1–14. https://doi.org/10.1016/j.foreco.2004.10.054
Feldhaar H, Schauer B (2018) Dispersal of Saproxylic Insects. In: Ulyshen MD (ed) Saproxylic insects: diversity, ecology and conservation. Springer International Publishing, Cham, pp 515–546. https://doi.org/10.1007/978-3-319-75937-1_15
Feller IC, McKee KL (1999) Small gap creation in Belizean mangrove forests by a wood–boring insect. Biotropica 31:607–617. https://doi.org/10.1111/j.1744-7429.1999.tb00408.x
Fiala T, Holuša J (2023) A monitoring network for the detection of invasive ambrosia and bark beetles in the Czech Republic: principles and proposed design. Front for Glob Change 6:1239748. https://doi.org/10.3389/ffgc.2023.1239748
Flaherty L, Quiring D, Pureswaran D, Sweeney J (2013) Preference of an exotic wood borer for stressed trees is more attributable to pre-alighting than post-alighting behaviour. Ecol Entomol 38:546–552. https://doi.org/10.1111/een.12045
Flaherty L, Gutowski JMG, Hughes C, Mayo P, Mokrzycki T et al (2019) Pheromone-enhanced lure blends and multiple trap heights improve detection of bark and wood-boring beetles potentially moved in solid wood packaging. J Pest Sci 92:309–325. https://doi.org/10.1007/s10340-018-1019-4
Foit J, Cermák V, Gaar V, Hradil K, Novy V et al (2019) New insights into the life history of Monochamus galloprovincialis can enhance surveillance strategies for the pinewood nematode. J Pest Sci 92:1203–1215. https://doi.org/10.1007/s10340-019-01110-y
Formby JP, Schiefer TL, Riggins JJ (2012) First records of Xyleborus glabratus (Coleoptera: Curculionidae) in Alabama and in Harrison County, Mississippi. Fla Entomol 95:192–193. https://doi.org/10.1653/024.095.0129
Fraedrich SW, Johnson CW, Menard RD, Harrington TC, Olatinwo R et al (2015) First report of Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae) and laurel wilt in Louisiana, USA: the disease continues westward on sassafras. Fla Entomol 98:1266–1268. https://doi.org/10.1653/024.098.0445
Francese JA, Mastro V, Oliver JB, Lance DR, Youssef N et al (2005) Evaluation of colors for trapping Agrilus planipennis (Coleoptera: Buprestidae). J Entomol Sci 40:93–95. https://doi.org/10.18474/0749-8004-40.1.93
Francese JA, Oliver JB, Fraser I, Lance DR, Youssef N et al (2008) Influence of trap placement and design on capture of the emerald ash borer (Coleoptera: Buprestidae). J Econ Entomol 101:1831–1837. https://doi.org/10.1603/0022-0493-101.6.1831
Francese JA, Crook DJ, Fraser I, Lance DR, Sawyer AJ et al (2010a) Optimization of trap color for emerald ash borer (Coleoptera: Buprestidae). J Econ Entomol 103:1235–1241. https://doi.org/10.1603/EC10088
Francese JA, Fraser I, Rietz ML, Crook DJ, Lance DR et al (2010b) Relation of color, size, and canopy placement of prism traps in determining capture of emerald ash borer (Coleoptera: Buprestidae). Can Entomol 142:596–600. https://doi.org/10.4039/n10-041
Francese JA, Fraser I, Lance DR, Mastro VC (2011) Efficacy of multifunnel traps for capturing emerald ash borer (Coleoptera: Buprestidae): effect of color, glue, and other trap coatings. J Econ Entomol 104:901–908. https://doi.org/10.1603/EC11038
Francese JA, Rietz ML, Mastro VC (2013) Optimization of multifunnel traps for emerald ash borer (Coleoptera: Buprestidae): influence of size, trap coating, and color. J Econ Entomol 106:2415–2423. https://doi.org/10.1603/EC13014
Francese JA, Booth EG, Lopez VM, Sorensen B (2019) Alternative survey methods for the emerald ash borer. Fla Entomol 102:243–245. https://doi.org/10.1653/024.102.0142
Franklin AJ, Grégoire J-C (2001) Dose-dependent response and preliminary observations on attraction range of Ips typographus to pheromones at low release rates. J Chem Ecol 27:2425–2435. https://doi.org/10.1023/A:1013619313415
Frost SW, Dietrich H (1929) Coleoptera taken from bait-traps. Ann Entomol Soc Am 22:427–437. https://doi.org/10.1093/aesa/22.3.427
Furniss MM (2014) The Douglas-fir beetle in western forests: a historical perspective—part 1. Am Entomol 60:84–96. https://doi.org/10.1093/ae/60.2.84
Galko J, Nikolov C, Kimoto T, Kunca A, Gubka A et al (2014) Attraction of ambrosia beetles to ethanol baited traps in a Slovakian oak forest. Biologia 69:1376–1383. https://doi.org/10.2478/s11756-014-0443-z
Galko J, Nikolov C, Kunca A, Vakula J, Gubka A et al (2016) Effectiveness of pheromone traps for the European spruce bark beetle: a comparative study of four commercial products and two new models. Lesnicky Casopis 62:207–215. https://doi.org/10.1515/forj-2016-0027
Gallego D, Galián J, Diez JJ, Pajares JA (2008) Kairomonal responses of Tomicus destruens (Col., Scolytidae) to host volatiles α-pinene and ethanol. J Appl Entomol 132:654–662. https://doi.org/10.1111/j.1439-0418.2008.01304.x
Gandhi KJK, Gilmore DW, Haack RA, Katovich SA, Krauth SJ et al (2009) Application of semiochemicals to assess the biodiversity of subcortical insects following an ecosystem disturbance in a sub-boreal forest. J Chem Ecol 35:1384–1410. https://doi.org/10.1007/s10886-009-9724-3
Ginzel MD, Hanks LM (2003) Contact pheromones as mate recognition cues of four species of longhorned beetles (Coleoptera: Cerambycidae). J Insect Behav 16:181–187. https://doi.org/10.1023/A:1023911701159
Gossner MM, Falck K, Weisser WW (2019) Effects of management on ambrosia beetles and their antagonists in European beech forests. For Ecol Manag 437:126–133. https://doi.org/10.1016/j.foreco.2019.01.034
Gould JR, Aflague B, Murphy TC, McCartin L, Elkinton JS et al (2018) Collecting nontarget wood-boring insects for host-specificity testing of natural enemies of cerambycids: a case study of (Coleoptera: Bothrideridae), a parasitoid of the Asian longhorned beetle (Coleoptera: Cerambycidae). Environ Entomol 47:1440–1450. https://doi.org/10.1093/ee/nvy121
Graham SA (1925) The felled tree trunk as an ecological unit. Ecology 6:397–411. https://doi.org/10.2307/1929106
Graham EE, Poland TM (2012) Efficacy of fluon conditioning for capturing cerambycid beetles in different trap designs and persistence on panel traps over time. J Econ Entomol 105:395–401. https://doi.org/10.1603/EC11432
Graham EE, Mitchell RF, Reagel PF, Barbour JD, Millar JG et al (2010) Treating panel traps with a fluoropolymer enhances their efficiency in capturing cerambycid beetles. J Econ Entomol 103:641–647. https://doi.org/10.1603/ec10013
Graham EE, Poland TM, McCullough DG, Millar JG (2012) A comparison of trap type and height for capturing cerambycid beetles (Coleoptera). J Econ Entomol 105:837–846. https://doi.org/10.1603/EC12053
Grégoire J-C, Caiti E, Hasbroucq S, Molenberg J-M, Willenz S (2022) When the beetles hit the fan: the fan-trap, an inexpensive, light and scalable insect trap under a creative commons license, for monitoring and experimental use. InSects 13:1122. https://doi.org/10.3390/insects13121122
Groberman LJ, Borden JH (1981) Behavioral response of Dendroctonus pseudotsugae and Trypodendron lineatum (Coleoptera: Scolytidae) to selected wavelenght regions of the visible spectrum. Can J Zool 59:2159–2165. https://doi.org/10.1139/z81-292
Grommes AC, Millar JG, Hanks LM (2023) Dose–responses of seven species of cerambycid beetles to synthesized pheromones in field experiments. J Econ Entomol 116:2035–2042. https://doi.org/10.1093/jee/toad193
Gugliuzzo A, Kreuzwieser J, Ranger CM, Tropea Garzia G, Biondi A et al (2023) Volatiles of fungal cultivars act as cues for host-selection in the fungus-farming ambrosia beetle Xylosandrus germanus. Front Microbiol. https://doi.org/10.3389/fmicb.2023.1151078
Guilbert E (1997) Arthropod biodiversity in New Caledonian forest canopies: a global approach by fogging. In: Stork NE, Adis J, Didham RK (eds) Canopy arthropods. Chapman & Hall, London, pp 263–275
Haack RA (2001) Intercepted Scolytidae (Coleoptera) at U.S. ports of entry: 1985–2000. Integr Pest Manage Rev 6:253–282. https://doi.org/10.1023/A:1025715200538
Haack RA, Poland TM (2001) Evolving management strategies for a recently discovered exotic forest pest: the pine shoot beetle, Tomicus piniperda (Coleoptera). Biol Invasions 3:307–322. https://doi.org/10.1023/A:1015298114837
Haack RA, Hardin JA, Caton BP, Petrice TR (2022) Wood borer detection rates on wood packaging materials entering the United States during different phases of ISPM 15 implementation and regulatory changes. Front Glob Change 5:1069117. https://doi.org/10.3389/ffgc.2022.1069117
Haavik LJ, Batista E, Dodds KJ, Johnson W, Meeker JR et al (2014) Type of intercept trap not important for capturing female Sirex noctilio and S. nigricornis (Hymenoptera: Siricidae) in North America. J Econ Entomol 107:1295–1298. https://doi.org/10.1603/EC14094
Haavik LJ, Dodds KJ, Allison JD (2018) Sirex noctilio (Hymenoptera: Siricidae) in Ontario (Canada) pine forests: observations over five years. Can Entomol. https://doi.org/10.4039/tce.2018.18
Hanks LM, Millar JG (2013) Field bioassays of cerambycid pheromones reveal widespread parsimony of pheromone structures, enhancement by host plant volatiles, and antagonism by components from heterospecifics. Chemoecology 23:21–44. https://doi.org/10.1007/s00049-012-0116-8
Hanks LM, Millar JG (2016) Sex and aggregation-sex pheromones of cerambycid beetles: basic science and practical applications. J Chem Ecol 42:631–654. https://doi.org/10.1007/s10886-016-0733-8
Hanks LM, Millar JG, Moreira JA, Barbour JD, Lacey ES et al (2007) Using generic pheromone lures to expedite identification of aggregation pheromones for the cerambycid beetles Xylotrechus nauticus, Phymatodes lecontei, and Neoclytus modestus modestus. J Chem Ecol 33:889–907. https://doi.org/10.1007/s10886-007-9275-4
Hanks LM, Millar JG, Mongold-Diers JA, Wong JCH, Meier LR et al (2012) Using blends of cerambycid beetle pheromones and host plant volatiles to simultaneously attract a diversity of cerambycid species. Can J for Res 42:1050–1059. https://doi.org/10.1139/x2012-06
Hanula JL, Ulyshen MD, Horn S (2011) Effect of trap type, trap position, time of year, and beetle density on captures of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae). J Econ Entomol 104:501–508. https://doi.org/10.1603/EC10263
Hardersen S, Curletti G, Leseigneur L, Platia G, Liberti G et al (2014) Spatio-temporal analysis of beetles from the canopy and ground layer in an Italian lowland forest. Bull Insectol 67:87–97
Hirao T, Murakami M, Kashizaki A (2009) Importance of the understory stratum to entomofaunal diversity in a temperate deciduous forest. Ecol Res 24:263–272. https://doi.org/10.1007/s11284-008-0502-4
Hoch G, Connell J, Roques A (2020) Testing multi-lure traps for surveillance of native and alien longhorn beetles (Coleoptera, Cerambycidae) at ports of entry and in forests in Austria. Manag Biol Invasions 11:677–688. https://doi.org/10.3391/mbi.2020.11.4.04
Holuša J, Fiala T, Foit J (2021) Ambrosia beetles prefer closed canopies: a case study in oak forests in central Europe. Forests 12:1223. https://doi.org/10.3390/f12091223
Hulcr J, Mann R, Stelinski LL (2011) The scent of a partner: ambrosia beetles are attracted to volatiles from their fungal symbionts. J Chem Ecol 37:1374–1377. https://doi.org/10.1007/s10886-011-0046-x
Hurley BP, Garnas J, Cooperband MF (2015) Assessing trap and lure effectiveness for the monitoring of Sirex noctilio. Agric for Entomol 17:64–70. https://doi.org/10.1111/afe.12081
Igeta Y, Esaki K, Kato K, Kamata N (2004) Spatial distribution of a flying ambrosia beetle Platypus quercivorus (Coleoptera: Platypodidae) at the stand level. Appl Entomol Zool 39:583–589. https://doi.org/10.1303/aez.2004.583
Imrei Z, Lohonyai Z, Csóka G, Muskovits J, Szanyi S et al (2020a) Improving trapping methods for buprestid beetles to enhance monitoring of native and invasive species. For Int J for Res. https://doi.org/10.1093/forestry/cpz071
Imrei Z, Lohonyai Z, Muskovits J, Matula E, Vuts J et al (2020b) Developing a non-sticky trap design for monitoring jewel beetles. J Appl Entomol 144:224–231. https://doi.org/10.1111/jen.12727
Inward DJG (2019) Three new species of ambrosia beetles established in Great Britain illustrate unresolved risks from imported wood. J Pest Sci 93:117–126. https://doi.org/10.1007/s10340-019-01137-1
Jactel H, Bonifacio L, van Halder I, Vétillard F, Robinet C et al (2019) A novel, easy method for estimating pheromone trap attraction range: application to the pine sawyer beetle. Agric for Entomol 21:8–14. https://doi.org/10.1111/afe.12298
Jantz OK, Rudinsky JA (1966) Studies of the olfactory behavior of the Douglas-fir beetle, Dendroctonus pseudotsugae Hopkins. Agricultural Experiment Station OSU, Corvallis, pp 1–38
Jaworski T, Plewa R, Dziuk A, Sukovata L (2022) Different responses of Monochamus galloprovincialis and three non−target species to trap type, colour, and lubricant treatment. Ann For Res 65:31–46. https://doi.org/10.15287/afr.2022.2359
Johnson CB, Kazantzis A, Skoula M, Mitteregger U, Novak J (2004) Seasonal, populational and ontogenic variation in the volatile oil content and composition of individuals of Origanum vulgare subsp. Hirtum, assessed by GC headspace analysis and by SPME sampling of individual oil glands. Phytochem Anal 15:286–292. https://doi.org/10.1002/pca.780
Kato M, Inoue T, Hamid AA, Nagamitsu T, Merdek MB et al (1995) Seasonality and vertical structure of light-attracted insect communities in a dipterocarp forest in Sarawak. Res Popul Ecol 37:59–79. https://doi.org/10.1007/BF02515762
Kelsey RG (2001) Chemical indicators of stress in trees: their ecological significance and implication for forestry in eastern Oregon and Washington. Northwest Sci 75:70–76
Kelsey RG, Joseph G (2003) Ethanol in ponderosa pine as an indicator of physiological injury from fire and its relationship to secondary beetles. Can J for Res 33:870–884. https://doi.org/10.1139/x03-007
Kelsey RG, Gallego D, Sánchez-García FJ, Pajares JA (2014) Ethanol accumulation during severe drought may signal tree vulnerability to detection and attack by bark beetles. Can J for Res 44:554–561. https://doi.org/10.1139/cjfr-2013-0428
Kendra PE, Montgomery WS, Narvaez TI, Carrillo D (2020) Comparison of trap designs for detection of Euwallacea nr. fornicatus and other Scolytinae (Coleoptera: Curculionidae) that vector fungal pathogens of avocado trees in Florida. J Econ Entomol 113:980–987. https://doi.org/10.1093/jee/toz311
Kennedy JS, Ludlow AR, Sanders CJ (1980) Guidance-system used in moth sex attraction. Nature 288:475–477. https://doi.org/10.1038/288475a0
Kerr JL, Kelly D, Bader MKF, Brockerhoff EG (2017) Olfactory cues, visual cues, and semiochemical diversity interact during host location by invasive forest beetles. J Chem Ecol 43:17–25. https://doi.org/10.1007/s10886-016-0792-x
Klimetzek D, Köhler J, Vité JP, Kohnle U (1986) Dosage response to ethanol mediates host selection by “secondary” bark beetles. Naturwissenschaften 73:270–272. https://doi.org/10.1007/BF00367783
Klooster WS, Herms DA, Knight KS, Herms CP, McCullough DG et al (2014) Ash (Fraxinus spp.) mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer (Agrilus planipennis). Biol Invasions 16:859–873. https://doi.org/10.1007/s10530-013-0543-7
Knopf JAE, Pitman GB (1972) Aggregation pheromone for manipulation of the Douglas-fir beetle. J Econ Entomol 65:723–726. https://doi.org/10.1093/jee/65.3.723
Koch Widerberg M, Ranius T, Drobyshev I, Nilsson U, Lindbladh M (2012) Increased openness around retained oaks increases species richness of saproxylic beetles. Biodivers Conserv 21:3035–3059. https://doi.org/10.1007/s10531-012-0353-8
Kondo MC, Han S, Donovan GH, MacDonald JM (2017) The association between urban trees and crime: evidence from the spread of the emerald ash borer in Cincinnati. Landscape Urban Plann 157:193–199. https://doi.org/10.1016/j.landurbplan.2016.07.003
Kovacs KF, Haight RG, McCullough DG, Mercader RJ, Siegert NW et al (2010) Cost of potential emerald ash borer damage in U.S. communities, 2009–2019. Ecol Econ 69:569–578. https://doi.org/10.1016/j.ecolecon.2009.09.004
Kuenen LPS, Baker TC (1982) Optomotor regulation of ground velocity in moths during flight to sex-pheromone at different heights. Physiol Entomol 7:193–202. https://doi.org/10.1111/j.1365-3032.1982.tb00289.x
Kuhns EH, Tribuiani Y, Martini X, Meyer WL, Peña J et al (2014) Volatiles from the symbiotic fungus Raffaelea lauricola are synergistic with Manuka lures for increased capture of the Redbay ambrosia beetle Xyleborus glabratus. Agric for Entomol 16:87–94. https://doi.org/10.1111/afe.12037
Lacey ES, Ginzel MD, Millar JG, Hanks LM (2004) Male-produced aggregation pheromone of the cerambycid beetle Neoclytus acuminatus acuminatus. J Chem Ecol 30:1493–1507. https://doi.org/10.1023/B:JOEC.0000042064.25363.42
Larsson MC (2016) Pheromones and other semiochemicals for monitoring rare and endangered species. J Chem Ecol 42:853–868. https://doi.org/10.1007/s10886-016-0753-4
Leksono AS, Takada K, Nakagoshi N, Nakamura K (2006) Species composition of Modellidae and Cerambycidae (Coleoptera) in a coppice woodland. J for Res 11:61–64. https://doi.org/10.1007/s10310-005-0181-8
Lelito JP, Domingue MJ, Fraser I, Mastro VC, Tumlinson JH et al (2011) Field investigation of mating behaviour of Agrilus cyanescens and Agrilus subcinctus. Can Entomol 143:370–379. https://doi.org/10.4039/n11-011
Lettenmaier L, Seibold S, Bässler C, Brandl R, Gruppe A et al (2022) Beetle diversity is higher in sunny forests due to higher microclimatic heterogeneity in deadwood. Oecologia 198:825–834. https://doi.org/10.1007/s00442-022-05141-8
Li Y, Meng QF, Silk P, Gao WT, Mayo P et al (2017) Effect of semiochemicals and trap height on catch of Neocerambyx raddei in Jilin province, China. Entomol Exp Appl 164:94–101. https://doi.org/10.1111/eea.12600
Lindgren BS (1983) A multiple funnel trap for scolytid beetles (Coleoptera). Can Entomol 115:299–302. https://doi.org/10.4039/Ent115299-3
Lindgren BS, Borden JH, Chong L, Friskie LM, Orr DB (1983) Factors influencing the efficiency of pheromone-baited traps for three species of ambrosia beetles (Coleoptera: Scolytidae). Can Entomol 115:303–313. https://doi.org/10.4039/Ent115303-3
Lindhe A, Lindelöw Å, Åsenblad N (2005) Saproxylic beetles in standing dead wood density in relation to substrate sun-exposure and diameter. Biodivers Conserv 14:3033–3053
Linsley EG (1959) Ecology of Cerambycidae. Annu Rev Entomol 4:99–138. https://doi.org/10.1007/s10531-004-0314-y
Lu W, Wang Q, Tian MY, He XZ, Zeng XL et al (2007) Mate location and recognition in Glenea cantor (Fabr.) (Coleoptera: Cerambycidae: Lamiinae): roles of host plant health, female sex pheromone, and vision. Environ Entomol 36:864–870. https://doi.org/10.1093/ee/36.4.864
Lyons DB, Lavallée R, Kyei-Poku G, Van Frankenhuyzen K, Johny S et al (2012) Towards the development of an autocontamination trap system to manage populations of emerald ash borer (Coleoptera: Buprestidae) with the native entomopathogenic fungus, Beauveria bassiana. J Econ Entomol 105:1929–1939. https://doi.org/10.1603/EC12325
Magarey RD, Colunga-Garcia M, Fieselmann DA (2009) Plant biosecurity in the United States: roles, responsibilities, and information needs. Bioscience 59:875–884. https://doi.org/10.1525/bio.2009.59.10.9
Maguire DY, Robert K, Brochu K, Larrivée M, Buddle CM et al (2014) Vertical stratification of beetles (Coleoptera) and flies (Diptera) in temperate forest canopies. Environ Entomol 43:9–17. https://doi.org/10.1603/EN13056
Maki EC, Millar JG, Rodstein J, Hanks LM, Barbour JD (2011) Evaluation of mass trapping and mating disruption for managing Prionus californicus (Coleoptera: Cerambycidae) in hop production yards. J Econ Entomol 104:933–938. https://doi.org/10.1603/EC10454
Mamiya Y (1988) History of pine wilt disease in Japan. J Nematol 20:219–226
Marchioro M, Faccoli M (2021) Successful eradication of the Asian longhorn beetle, Anoplophora glabripennis, from north-eastern Italy: protocol, techniques and results. InSects 12:877. https://doi.org/10.3390/insects12100877
Marchioro M, Rassati D, Faccoli M, Van Rooyen K, Kostanowicz C et al (2020) Maximizing bark and ambrosia beetle (Coleoptera: Curculionidae) catches in trapping surveys for longhorn and jewel beetles. J Econ Entomol 113:2745–2757. https://doi.org/10.1093/jee/toaa181
Marchioro M, Bianchi A, Ciampitti M, Faccoli M (2022a) Testing trapping protocols for detecting the Citrus Longhorn Beetle, Anoplophora chinensis (Coleoptera: Cerambycidae). J Appl Entomol 146:607–614. https://doi.org/10.1111/jen.12978
Marchioro M, Faccoli M, Dal Cortivo M, Branco M, Roques A et al (2022b) New species and new records of exotic Scolytinae (Coleoptera, Curculionidae) in Europe. Biodivers Data J 10:e93995. https://doi.org/10.3897/BDJ.10.e93995
Martin DM, Gershenzon J, Bohlmann J (2003) Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol 132:1586–1599. https://doi.org/10.1104/pp.103.021196
Martín A, Etxebeste I, Pérez G, Álvarez G, Sánchez E et al (2013) Modified pheromone traps help reduce bycatch of bark-beetle natural enemies. Agric for Entomol 15:86–97. https://doi.org/10.1111/j.1461-9563.2012.00594.x
Mas H, Santoiemma G, Lencina JL, Gallego D, Pérez-Laorga E et al (2023) Investigating beetle communities in and around entry points can improve surveillance at national and international scale. NeoBiota 85:145–165. https://doi.org/10.3897/neobiota.85.103904
McCullough DG, Poland TM (2017) Building double-decker traps for early detection of emerald ash borer. J vis Exp 128:e55252. https://doi.org/10.3791/55252
McCullough DG, Poland TM, Anulewicz AC, Cappaert D (2009) Emerald ash borer (Coleoptera: Buprestidae) attraction to stressed or baited ash trees. Environ Entomol 38:1668–1679. https://doi.org/10.1603/022.038.0620
McIntosh RL, Katinic PJ, Allison JD, Borden JH, Downey DL (2001) Comparative efficacy of five types of trap for woodborers in the Cerambycidae, Buprestidae and Siricidae. Agric for Entomol 3:113–120. https://doi.org/10.1046/j.1461-9563.2001.00095.x
McPherson EG, Xiao Q, van Doorn NS, de Goede J, Bjorkman J et al (2017) The structure, function and value of urban forests in California communities. Urban Urban Gree 28:43–53. https://doi.org/10.1016/j.ufug.2017.09.013
Melin M, Vihervuori L, Koivula M, Velmala S (2023) Pheromone-based monitoring of invasive alien insects along the border of Finland and Russia—methods and unintentionally caught species: brief report. Baltic for 28:1–6. https://doi.org/10.46490/BF639
Meng LZ, Wang J, Liu YH (2022) Impact of vegetation and climate types on vertical stratification of wood-boring longhorn and bark beetles (Coleoptera: Cerambycidae and Scolytinae) along altitude gradients in Yunnan, Southwest China. Agric for Entomol 24:320–331. https://doi.org/10.1111/afe.12496
Menocal O, Kendra PE, Padilla A, Chagas PC, Chagas EA et al (2022) Influence of canopy cover and meteorological factors on the abundance of bark and ambrosia beetles (Coleoptera: Curculionidae) in avocado orchards affected by laurel wilt. Agronomy 12:547. https://doi.org/10.3390/agronomy12030547
Metcalf RL, Kogan M (1987) Plant volatiles as insect attractants. Crit Rev Plant Sci 5:251–301. https://doi.org/10.1080/07352688709382242
Meurisse N, Rassati D, Hurley BP, Brockerhoff EG, Haack RA (2019) Common pathways by which non-native forest insects move internationally and domestically. J Pest Sci 92:13–27. https://doi.org/10.1007/s10340-018-0990-0
Millar JG, Hanks LM (2017) Chemical ecology of cerambycids. In: Wang Q (ed) Cerambycidae of the world: biology and pest management. CRC Press, New York, pp 161–208
Miller DR, Crowe CM (2011) Relative performance of Lindgren multiple-funnel, intercept panel, and colossus pipe traps in catching Cerambycidae and associated species in the southeastern United States. J Econ Entomol 104:1934–1941. https://doi.org/10.1603/ec11166
Miller DR, Crowe CM (2022) Trap type affects catches of bark and woodboring beetles in a southern pine stand. J Entomol Sci 57:145–155. https://doi.org/10.1093/jee/toz271
Miller DR, Duerr DA (2008) Comparison of arboreal beetle catches in wet and dry collection cups with Lindgren multiple funnel traps. J Econ Entomol 101:107–113. https://doi.org/10.1093/jee/101.1.107
Miller DR, Rabaglia RJ (2009) Ethanol and (-)-alpha-pinene: attractant kairomones for bark and ambrosia beetles in the southeastern US. J Chem Ecol 35:435–448. https://doi.org/10.1007/s10886-009-9613-9
Miller DR, Asaro C, Crowe CM, Duerr DA (2011) Bark beetle pheromones and pine volatiles: attractant kairomone lure blend for longhorn beetles (Cerambycidae) in pine stands of the southeastern United States. J Econ Entomol 104:1245–1257. https://doi.org/10.1603/EC11051
Miller DR, Crowe CM, Barnes BF, Gandhi KJK, Duerr DA (2013) Attaching lures to multiple-funnel traps targeting saproxylic beetles (Coleoptera) in pine stands: inside or outside funnels? J Econ Entomol 106:206–214. https://doi.org/10.1603/EC12254
Miller DR, Crowe CM, Mayo PD, Silk PJ, Sweeney JD (2015) Responses of Cerambycidae and other insects to traps baited with ethanol, 2,3-Hexanediol, and 3,2-Hydroxyketone lures in North-Central Georgia. J Econ Entomol 108:2354–2365. https://doi.org/10.1093/jee/tov220
Miller DR, Allison JD, Crowe CM, Dickinson DM, Eglitis A et al (2016) Pine sawyers (Coleoptera: Cerambycidae) attracted to α-pinene, monochamol, and ipsenol in North America. J Econ Entomol 109:1205–1214. https://doi.org/10.1093/jee/tow071
Miller DR, Crowe CM, Mayo PD, Reid LS, Silk PJ et al (2017) Interactions between ethanol, syn-2,3-hexanediol, 3-hydroxyhexan-2-one, and 3-hydroxyoctan-2-one lures on trap catches of hardwood longhorn beetles in southeastern United States. J Econ Entomol 110:2119–2128. https://doi.org/10.1093/jee/tox188
Miller DR, Crowe CM, Ginzel MD, Ranger CM, Schultz PB (2018) Comparison of baited bottle and multiple-funnel traps for ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) in eastern United States. J Entomol Sci 53:347–360. https://doi.org/10.18474/JES17-107.1
Miller DR, Crowe CM, Sweeney JD (2020) Trap height affects catches of bark and woodboring beetles (Coleoptera: Curculionidae, Cerambycidae) in baited multiple-funnel traps in southeastern United States. J Econ Entomol 113:273–280. https://doi.org/10.1093/jee/toz271
Miller CN, Barnes BF, Kinz S, Spinner SC, Vogt JT et al (2023) Woodboring beetle (Buprestidae, Cerambycidae) responses to Hurricane Michael in variously damaged southeastern US pine plantations. For Sci 69:272–285. https://doi.org/10.1093/forsci/fxac058
Moeck HA (1970) Ethanol as the primary attractant for the ambrosia beetle Trypodendron lineatum (Coleoptera: Scolytidae). Can Entomol 102:985–995. https://doi.org/10.4039/Ent102985-8
Moeck HA (1971) Field test of ethanol as a scolytid attractant. Bi-Mon Res Notes Can for Serv 27:11–12
Montgomery ME, Wargo PM (1983) Ethanol and other host-derived volatiles as attractants to beetles that bore into hardwoods. J Chem Ecol 9:181–190. https://doi.org/10.1007/BF00988035
Morewood WD, Hein KE, Katinic PJ, Borden JH (2002) An improved trap for large wood-boring insects, with special reference to Monochamus scutellatus (Coleoptera: Cerambycidae). Can J for Res 32:519–525. https://doi.org/10.1139/x01-224
Morin MB, Hébert C, Berthiaume R, Bauce É, Brodeur J (2015) Short-term effect of selection cutting in boreal balsam fir forest on cerambycid and scolytid beetles. J Appl Entomol 139:553–566. https://doi.org/10.1111/jen.12175
Müller MM, Varama M, Heinonen J, Hallaksela A-M (2002) Influence of insects on the diversity of fungi in decaying spruce wood in managed and natural forests. For Ecol Manag 166:165–181. https://doi.org/10.1016/S0378-1127(01)00671-5
Murcia C (1995) Edge effects in fragmented forests—implications for conservation. Trends Ecol Evol 10:58–62. https://doi.org/10.1016/S0169-5347(00)88977-6
Murlis J, Elkinton JS, Carde RT (1992) Odor plumes and how insects use them. Annu Rev Entomol 37:505–532. https://doi.org/10.1146/annurev.en.37.010192.002445
Muzika RM, Liebhold AM, Twery MJ (2000) Dynamics of twolined chestnut borer Agrilus bilineatus as influenced by defoliation and selection thinning. Agric for Entomol 2:283–289. https://doi.org/10.1046/j.1461-9563.2000.00077.x
Nahrung HF, Liebhold AM, Brockerhoff EG, Rassati D (2023) Forest insect biosecurity: processes, patterns, predictions, pitfalls. Annu Rev Entomol 68:211–229. https://doi.org/10.1146/annurev-ento-120220-010854
Nehme ME, Trotter RT, Keena MA, McFarland C, Coop J et al (2014) Development and evaluation of a trapping system for Anoplophora glabripennis (Coleoptera: Cerambycidae) in the United States. Environ Entomol 43:1034–1044. https://doi.org/10.1603/EN14049
Nielsen M-C, Sansom CE, Larsen L, Worner SP, Rostás M et al (2019) Volatile compounds as insect lures: factors affecting release from passive dispenser systems. N Z J Crop Hortic Sci 47:208–223. https://doi.org/10.1080/01140671.2019.1604554
Noldt U, Fettkother R, Dettner K (1995) Structure of the sex pheromone-producing prothoracic glands of the male old house borer, Hylotrupes bajulus (L.) (Coleoptera, Cerambycidae). Int J Insect Morphol Embryol 24:223–234. https://doi.org/10.1016/0020-7322(95)93345-D
Normann C, Tscharntke T, Scherber C (2016) Interacting effects of forest stratum, edge and tree diversity on beetles. For Ecol Manag 361:421–431. https://doi.org/10.1016/j.foreco.2015.11.002
Økland B, Bakke A, Hågvar S, Kvamme T (1996) What factors influence the diversity of saproxylic beetles? A multiscaled study from a spruce forest in southern Norway. Biodivers Conserv 5:75–100. https://doi.org/10.1007/BF00056293
Oliver JB, Youssef N, Fare D, Halcomb M, Scholl S et al. (2002) Monitoring buprestid borers in production nursery areas. In: Paper presented at the Proceedings of the 29th Annual Meeting of the Tennessee Entomological Society, Nasheville
Park J, Reid ML (2007) Distribution of a bark beetle, Trypodendron lineatum, in a harvested landscape. For Ecol Manag 242:236–242. https://doi.org/10.1016/j.foreco.2007.01.042
Parker K, Ryall K, Aukema BH, Silk P (2020) Early detection of Agrilus planipennis: investigations into the attractive range of the sex pheromone (3Z)-lactone. Entomol Exp Appl 168:166–173. https://doi.org/10.1111/eea.12872
Pawson SM, Watt MS, Brockerhoff EG (2009) Using differential responses to light spectra as a monitoring and control tool for Arhopalus ferus (Coleoptera: Cerambycidae) and other exotic wood-boring pests. J Econ Entomol 102:79–85. https://doi.org/10.1603/029.102.0112
Pearce DW (2001) The economic value of forest ecosystems. Ecosyst Health 7:284–296. https://doi.org/10.1046/j.1526-0992.2001.01037.x
Peck RW, Martinez AE, Ross DW (1997) Seasonal flight patterns of bark and ambrosia beetles (Coleoptera: Scolytidae) in northeastern Oregon. Pan-Pac Entomol 73:204–212
Perkovich CL, Addesso KM, Basham JP, Fare DC, Youssef NN et al (2022) Effects of color attributes on trap capture rates of Chrysobothris femorata (Coleoptera: Buprestidae) and related species. Environ Entomol 51:737–746. https://doi.org/10.1093/ee/nvac038
Perkovich CL, Oliver JB, Addesso KM, Basham JP, Youssef NN (2023) Effects of trap shape, size and color variations on capture rates of Chrysobothris (Coleoptera: Beuprestidae) and related buprestids. Fla Entomol 106:63–66. https://doi.org/10.1653/024.106.0111
Person HL (1931) Theory in explanation of the selection of certain trees by the western pine beetle. J for 29:696–699. https://doi.org/10.1093/jof/29.5.696
Petrice TR, Haack RA (2015) Comparison of different trap colors and types for capturing Agrilus (Coleoptera: Buprestidae) and other buprestids. Great Lakes Entomol 48:45–66. https://doi.org/10.22543/0090-0222.2310
Petrice TR, Haack RA, Poland TM (2013) Attraction of Agrilus planipennis (Coleoptera: Buprestidae) and other buprestids to sticky traps of various colors and shapes. Great Lakes Entomol 46:13–30. https://doi.org/10.22543/0090-0222.2260
Poland TM, Rassati D (2019) Improved biosecurity surveillance of non-native forest insects: a review of current methods. J Pest Sci 92:37–49. https://doi.org/10.1007/s10340-018-1004-y
Poland TM, McCullough DG, Anulewicz AC (2011) Evaluation of double-decker traps for emerald ash borer (Coleoptera: Buprestidae). J Econ Entomol 104:517–531. https://doi.org/10.1603/ec10254
Poland TM, Petrice TR, Ciaramitaro TM (2019) Trap designs, colors, and lures for emerald ash borer detection. Front for Glob Change 2:80. https://doi.org/10.3389/ffgc.2019.00080
Potterf M, Nikolov C, Kočická E, Ferenčík J, Mezei P et al (2019) Landscape-level spread of beetle infestations from windthrown- and beetle-killed trees in the non-intervention zone of the Tatra National Park, Slovakia (Central Europe). For Ecol Manag 432:489–500. https://doi.org/10.1016/j.foreco.2018.09.050
Preisser E, Smith DC, Lowman MD (1998) Canopy and ground level insect distribution in a temperate forest. Selbyana 19:141–146
Preti M, Verheggen F, Angeli S (2021) Insect pest monitoring with camera-equipped traps: strengths and limitations. J Pest Sci 94:203–217. https://doi.org/10.1007/s10340-020-01309-4
Procházka J, Cizek L, Schlaghamersky J (2018) Vertical stratification of scolytine beetles in temperate forests. Insect Conserv Diver 11:534–544. https://doi.org/10.1111/icad.12301
Prokopy RJ, Owens ED (1978) Visual generalist with visual specialist phytophagous insects—host selection behavior and application to management. Entomol Exp Appl 24:609–620. https://doi.org/10.1111/j.1570-7458.1978.tb02824.x
Pureswaran DS, Borden JH (2005) Primary attraction and kairomonal host discrimination in three species of Dendroctonus (Coleoptera: Scolytidae). Agric for Entomol 7:219–230. https://doi.org/10.1111/j.1461-9555.2005.00264.x
Pureswaran DS, Gries R, Borden JH (2004) Quantitative variation in monoterpenes in four species of conifers. Biochem Syst Ecol 32:1109–1136. https://doi.org/10.1016/j.bse.2004.04.006
Rabaglia RJ, Cognato AI, Hoebeke ER, Johnson CW, LaBonte JR et al (2019) Early detection and rapid response: a 10-year summary of the USDA Forest Service program of surveillance for non-native bark and ambrosia beetles. Am Entomol 65:29–42. https://doi.org/10.1093/ae/tmz015
Ranger CM, Reding M, Persad AB, Herms DA (2010) Ability of stress-related volatiles to attract and induce attacks by Xylosandrus germanus and other ambrosia beetles. Agric for Entomol 12:177–185. https://doi.org/10.1111/j.1461-9563.2009.00469.x
Rassati D, Toffolo EP, Battisti A, Faccoli M (2012) Monitoring of the pine sawyer beetle Monochamus galloprovincialis by pheromone traps in Italy. Phytoparasitica 40:329–336. https://doi.org/10.1007/s12600-012-0233-5
Rassati D, Toffolo EP, Roques A, Battisti A, Faccoli M (2014) Trapping wood boring beetles in Italian ports: a pilot study. J Pest Sci 87:61–69. https://doi.org/10.1007/s10340-013-0499-5
Rassati D, Faccoli M, Marini L, Haack RA, Battisti A et al (2015a) Exploring the role of wood waste landfills in early detection of non-native wood-boring beetles. J Pest Sci 88:563–572. https://doi.org/10.1007/s10340-014-0639-6
Rassati D, Faccoli M, Toffolo EP, Battisti A, Marini L (2015b) Improving the early detection of alien wood-boring beetles in ports and surrounding forests. J Appl Ecol 52:50–58. https://doi.org/10.1111/1365-2664.12347
Rassati D, Faccoli M, Chinellato F, Hardwick S, Suckling DM et al (2016) Web-based automatic traps for early detection of alien wood-boring beetles. Entomol Exp Appl 160:91–95. https://doi.org/10.1111/eea.12453
Rassati D, Marini L, Marchioro M, Rapuzzi P, Magnani G et al (2019) Developing trapping protocols for wood-boring beetles associated with broadleaf trees. J Pest Sci 92:267–279. https://doi.org/10.1007/s10340-018-0984-y
Rassati D, Marchioro M, Flaherty L, Poloni R, Edwards S et al (2021) Response of native and exotic longhorn beetles to common pheromone components provides partial support for the pheromone-free space hypothesis. Insect Sci 28:793–810. https://doi.org/10.1111/1744-7917.12790
Reding ME, Ranger CM (2020) Attraction of invasive ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) to ethanol-treated tree bolts. J Econ Entomol 113:321–329. https://doi.org/10.1093/jee/toz282
Reding M, Oliver J, Schultz P, Ranger C (2010) Monitoring flight activity of ambrosia beetles in ornamental nurseries with ethanol-baited traps: influence of trap height on captures. J Environ Hortic 28:85–90. https://doi.org/10.24266/0738-2898-28.2.85
Reding ME, Schultz PB, Ranger CM, Oliver JB (2011) Optimizing ethanol-baited traps for monitoring damaging ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) in ornamental nurseries. J Econ Entomol 104:2017–2024. https://doi.org/10.1603/EC11119
Rhainds M, Kimoto T, Galko J, Nikolov C, Ryall K et al (2017) Survey tools and demographic parameters of Slovakian Agrilus associated with beech and poplar. Entomol Exp Appl 162:328–335. https://doi.org/10.1111/eea.12546
Richardson B, Burgin S, Azarbayjani F, Lutubula S (1997) Distinguishing the woods from the trees. In: Stork NE, Adis J, Didham RK (eds) Canopy arthropods. Chapman & Hall, London, pp 497–509
Rodman KC, Andrus RA, Butkiewicz CL, Chapman TB, Gill NS et al (2021) Effects of bark beetle outbreaks on forest landscape pattern in the southern Rocky Mountains, USA. Remote Sens 13:1089. https://doi.org/10.3390/rs13061089
Roling MP, Kearby WH (1975) Seasonal flight and vertical distribution of Scolytidae attracted to ethanol in an oak-hickory forest in Missouri. Can Entomol 107:1315–1320. https://doi.org/10.4039/Ent1071315-12
Roques A, Ren L, Rassati D, Shi J, Akulov E et al (2023) Worldwide tests of generic attractants, a promising tool for early detection of non-native cerambycid species. NeoBiota 84:169–209. https://doi.org/10.3897/neobiota.84.91096
Ross DW, Daterman GE (1998) Pheromone-baited traps for Dendroctonus pseudotsugae (Coleoptera: Scolytidae): influence of selected release rates and trap designs. J Econ Entomol 91:500–506. https://doi.org/10.1093/jee/91.2.500
Ruchin AB, Egorov LV, Khapugin AA (2021) Usage of fermental traps for the study of the species diversity of Coleoptera. InSects 12:407. https://doi.org/10.3390/insects14040404
Rudinsky JA, Morgan M, Libbey LM, Michael RR (1973) Sound production in Scolytidae: 3-methyl-2-cyclohexen-l-one released by the female Douglas fir beetle in response to male sonic signal. Environ Entomol 2:505–510. https://doi.org/10.1093/ee/2.4.505
Ruzzier E, Galli A, Bani L (2021) Monitoring exotic beetles with inexpensive attractants: a case study. InSects 12:462. https://doi.org/10.3390/insects12050462
Ruzzier E, Haack RA, Curletti G, Roques A, Volkovitsh MG et al (2023) Jewels on the go: exotic buprestids around the world (Coleoptera, Buprestidae). Neobiota 84:107–135. https://doi.org/10.3897/neobiota.84.90829
Sakai T, Nakagawa Y, Takahashi J, Iwabuchi K, Ishii K (1984) Isolation and identification of the male sex pheromone of the grape borer Xylotrechus pyrrhoderus Bates (Coleoptera: Cerambycidae). Chem Lett 13:263–264. https://doi.org/10.1246/cl.1984.263
Sanchez-Martinez G, Wagner MR (2002) Bark beetle community structure under four ponderosa pine forest stand conditions in northern Arizona. For Ecol Manag 170:145–160. https://doi.org/10.1016/S0378-1127(01)00771-X
Santoiemma G, Williams D, Booth E, Cavaletto G, Connell J et al (2024) Efficacy of trapping protocols for Agrilus jewel beetles (Coleoptera: Buprestidae): a multi-country assessment. J Pest Sci in Press. https://doi.org/10.1007/s10340-023-01728-z
Santos-Silva A, Botero JP, Nascimento FEL, Silva WD (2020) A new synonym and seventeen new distributional records in South American Cerambycidae (Coleoptera), with notes on Chlorethe scabrosa Zajciw, 1963. Pap Avuls Zool 60:e20206010. https://doi.org/10.11606/1807-0205/2020.60.10
Savely HE Jr (1939) Ecological relations of certain animals in dead pine and oak logs. Ecol Monogr 9:321–385. https://doi.org/10.2307/1943233
Schlyter F (1992) Sampling range, attraction range, and effective attraction radius: estimates of trap efficiency and communication distance in coleopteran pheromone and host attractant systems. J Appl Entomol 114:439–454. https://doi.org/10.1111/j.1439-0418.1992.tb01150.x
Schmeelk TC, Millar JG, Hanks LM (2016) Influence of trap height and bait type on abundance and species diversity of cerambycid beetles captured in forests of east-central Illinois. J Econ Entomol 109:1750–1757. https://doi.org/10.1093/jee/tow102
Schroeder LM (1988) Attraction of the bark beetle Tomicus piniperda and some other bark- and wood-living beetles to the host volatiles α-pinene and ethanol. Entomol Exp Appl 46:203–210. https://doi.org/10.1111/j.1570-7458.1988.tb01113.x
Schroeder LM (1992) Olfactory recognition of nonhosts aspen and birch by conifer bark beetles Tomicus piniperda and Hylurgops palliatus. J Chem Ecol 18:1583–1593. https://doi.org/10.1007/BF00993231
Schroeder LM, Lindelöw Å (1989) Attraction of scolytids and associated beetles by different absolute amounts and proportions of α-pinene and ethanol. J Chem Ecol 15:807–817. https://doi.org/10.1007/BF01015179
Schroeder B, Buddle CM, Saint-Germain M (2009) Activity of flying beetles (Coleoptera) at two heights in canopy gaps and intact forests in a hardwood forest in Quebec. Can Entomol 141:515–520. https://doi.org/10.4039/n09-022
Seibold S, Bässler C, Brandl R, Büche B, Szallies A et al (2016) Microclimate and habitat heterogeneity as the major drivers of beetle diversity in dead wood. J Appl Ecol 53:934–943. https://doi.org/10.1111/1365-2664.12607
Seidl R, Klonner G, Rammer W, Essl F, Moreno A et al (2018) Invasive alien pests threaten the carbon stored in Europe’s forests. Nat Commun 9:1626. https://doi.org/10.1038/s41467-018-04096-w
Sheehan TN, Ulyshen MD, Horn S, Hoebeke ER (2019) Vertical and horizontal distribution of bark and woodboring beetles by feeding guild: is there an optimal trap location for detection? J Pest Sci 92:327–341. https://doi.org/10.1007/s10340-018-1026-5
Shepherd RF (1966) Factors influencing the orientation and rates of activity of Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). Can Entomol 98:507–518. https://doi.org/10.4039/Ent98507-5
Sierota Z, Grodzki W (2020) Picea abies–Armillaria–Ips: a strategy or coincidence? Forests 11:1023. https://doi.org/10.3390/f11091023
Silk PJ, Sweeney J, Wu JP, Price J, Gutowski JM et al (2007) Evidence for a male-produced pheromone in Tetropium fuscum (F.) and Tetropium cinnamopterum (Kirby) (Coleoptera : Cerambycidae). Naturwissenschaften 94:697–701. https://doi.org/10.1007/s00114-007-0244-0
Silk PJ, Ryall K, Mayo P, Lemay MA, Grant G et al (2011) Evidence for a volatile pheromone in Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) that increases attraction to a host foliar volatile. Environ Entomol 40:904–916. https://doi.org/10.1603/EN11029
Silk P, Mayo P, Ryall K, Roscoe L (2019) Semiochemical and communication ecology of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). InSects 10:323. https://doi.org/10.3390/insects10100323
Silk PJ, Ryall KL, Grant G, Roscoe LE, Mayo P et al (2020) Tree girdling and host tree volatiles provides a useful trap for bronze birch borer Agrilus anxius Gory (Coleoptera: Buprestidae). For Int J for Res 93:265–272. https://doi.org/10.1093/forestry/cpz021
Silverstein RM, Rodin JO, Wood DL (1966) Sex attractants in frass produced by male Ips confusus in ponderosa pine. Science 154:509–510. https://doi.org/10.1126/science.154.3748.509
Skvarla MJ, Dowling APG (2017) A comparison of trapping techniques (Coleoptera: Carabidae, Buprestidae, Cerambycidae, and Curculionoidea excluding Scolytinae). J Insect Sci 17:7. https://doi.org/10.1093/jisesa/iew098
Skvarla MJ, Holland JD (2011) Nontarget insects caught on emerald ash borer purple monitoring traps in western Pennsylvania. North J Appl for 28:219–221. https://doi.org/10.1093/njaf/28.4.219
Skvarla MJ, Larson JL, Fisher JR, Dowling APG (2021) A review of terrestrial and canopy malaise traps. Ann Entomol Soc Am 114:27–47. https://doi.org/10.1093/aesa/saaa044
Staton T, Girling RD, Redak RA, Smith SM, Allison JD (2023) Can morphological traits explain species-specific differences in meta-analyses? A case study of forest beetles. Ecol Appl 33:e2838. https://doi.org/10.1002/eap.2838
Steininger MS, Hulcr J, Šigut M, Lucky A (2015) Simple and efficient trap for bark and ambrosia beetles (Coleoptera: Curculionidae) to facilitate invasive species monitoring and citizen involvement. J Econ Entomol 108:1115–1123. https://doi.org/10.1093/jee/tov014
Storch F, Boch S, Gossner MM, Feldhaar H, Ammer C et al (2023) Linking structure and species richness to support forest biodiversity monitoring at large scales. Ann for Sci 80:3. https://doi.org/10.1186/s13595-022-01169-1
Stork NE, Stone M, Sam L (2016) Vertical stratification of beetles in tropical rainforests as sampled by light traps in North Queensland, Australia. Austral Ecol 41:168–178. https://doi.org/10.1111/aec.12286
Strom BL, Goyer RA (2001) Effect of silhouette color on trap catches of Dendroctonus frontalis (Coleoptera: Scolytidae). Ann Entomol Soc Am 94:948–953. https://doi.org/10.1603/0013-8746(2001)094[0948:EOSCOT]2.0.CO;2
Strom BL, Roton LM, Goyer RA, Meeker JR (1999) Visual and semiochemical disruption of host finding in the southern pine beetle. Ecol Appl 9:1028–1038. https://doi.org/10.2307/2641348
Su JC, Woods SA (2001) Importance of sampling along a vertical gradient to compare the insect fauna in managed forests. Environ Entomol 30:400–408. https://doi.org/10.1603/0046-225X-30.2.400
Suckling DM (2015) Can we replace toxicants, achieve biosecurity, and generate market position with semiochemicals? Front Ecol Evol 3:17. https://doi.org/10.3389/fevo.2015.00017
Sukovata L, Dziuk A, Plewa R, Jaworski T (2022) The effect of trap color on catches of Monochamus galloprovincialis and three most numerous non-target insect species. InSects 13:220. https://doi.org/10.3390/insects13030220
Sullivan BT, Fettig CJ, Otrosina WJ, Dalusky MJ, Berisford CW (2003) Association between severity of prescribed burns and subsequent activity of conifer-infesting beetles in stands of longleaf pine. For Ecol Manag 185:327–340. https://doi.org/10.1016/S0378-1127(03)00223-8
Sun JH, Miao ZW, Zhang Z, Zhang ZN, Gillette NE (2004) Red turpentine beetle, Dendroctonus valens LeConte (Coleoptera: Scolytidae), response to host semiochemicals in China. Environ Entomol 33:206–212. https://doi.org/10.1603/0046-225X-33.2.206
Sutton SL, Ash CPJ, Grundy A (1983) The vertical distribution of flying insects in lowland rain-forests of Panama, Papua New Guinea and Brunei. Zool J Linn Soc 78:287–297. https://doi.org/10.1111/j.1096-3642.1983.tb00868.x
Sweeney J, de Groot P, MacDonald L, Smith S, Cocquempot C et al (2004) Host volatile attractants and traps for detection of Tetropium fuscum (F.), Tetropium castaneum L., and other longhorned beetles (Coleoptera: Cerambycidae). Environ Entomol 33:844–854. https://doi.org/10.1603/0046-225X-33.4.844
Sweeney J, Gutowski JM, Price J, de Groot P (2006) Effect of semiochemical release rate, killing agent, and trap design on detection of Tetropium fuscum (F.) and other longhorn beetles (Coleoptera: Cerambycidae). Environ Entomol 35:645–654. https://doi.org/10.1603/0046-225X-35.3.645
Sweeney DJ, Silk JP, Grebennikov V (2014) Efficacy of semiochemical-baited traps for detection of longhorn beetles (Coleoptera: Cerambycidae) in the Russian Far East. Eur J Entomol 111:397–406. https://doi.org/10.14411/eje.2014.049
Sweeney DJ, Silk P, Grebennikov V, Mandelshtam M (2016) Efficacy of semiochemical-baited traps for detection of Scolytinae species (Coleoptera: Curculionidae) in the Russian Far East. Eur J Entomol 113:84–97. https://doi.org/10.14411/eje.2016.010
Sweeney J, Hughes C, Webster V, Kostanowicz C, Webster R et al (2020) Impact of horizontal edge-interior and vertical canopy-understory gradients on the abundance and diversity of bark and woodboring beetles in survey traps. InSects 11:573. https://doi.org/10.3390/insects11090573
Tarno H, Setiawan Y, Kusuma CB, Fitriyah M, Hudan AN et al (2021) Species composition of bark and ambrosia beetles captured using ethanol baited traps on different hosts in East Java. Indonesia Zool Stud 60:55. https://doi.org/10.6620/ZS.2021.60-55
Taylor LR (1963) Analysis of the effect of temperature on insects in flight. J Anim Ecol 32:99–117. https://doi.org/10.2307/2520
Thistle HW, Strom B, Strand T, Peterson HG, Lamb BK et al (2011) Atmospheric dispersion from a point source in four southern pine thinning scenarios: basic relationships and case studies. Trans Am Soc Agric Biol Eng 54:1219–1236. https://doi.org/10.13031/2013.39021
Thurston GS, Slater A, Nei I, Roberts J, McLachlan Hamilton K et al (2022) New Canadian and Provincial records of Coleoptera resulting from annual Canadian Food Inspection Agency surveillance for detection of non-native, potentially invasive forest insects. InSects 13:708. https://doi.org/10.3390/insects13080708
Tluczek AR, McCullough DG, Poland TM (2011) Influence of host stress on emerald ash borer (Coleoptera: Buprestidae) adult density, development, and distribution in Fraxinus pennsylvanica trees. Environ Entomol 40:357–366. https://doi.org/10.1603/EN10219
Tobin PC, Strom BL, Francese JA, Herms DA, McCullough DG et al (2021) Evaluation of trapping schemes to detect emerald ash borer (Coleoptera: Buprestidae). J Econ Entomol 114:1201–1210. https://doi.org/10.1093/jee/toab065
Tobin KN, Ethington MW, Ginzel MD (2024) Volatiles from nutritional fungal symbiont influence the attraction of Anisandrus maiche (Coleoptera: Curculionidae) to ethanol-baited traps. Environ Entomol 53:108–115. https://doi.org/10.1093/ee/nvad121
Torres-Vila LM, Sanchez-Gonzalez A, Merino-Martinez J, Ponce-Escudero F, Conejo-Rodriguez Y et al (2013) Mark-recapture of Cerambyx welensii in dehesa woodlands: dispersal behaviour, population density, and mass trapping efficiency with low trap densities. Entomol Exp Appl 149:273–281. https://doi.org/10.1111/eea.12133
Ulyshen MD (2011) Arthropod vertical stratification in temperate deciduous forests: implications for conservation-oriented management. For Ecol Manag 261:1479–1489. https://doi.org/10.1016/j.foreco.2011.01.033
Ulyshen MD (2016) Wood decomposition as influenced by invertebrates. Biol Rev 91:70–85. https://doi.org/10.1111/brv.12158
Ulyshen MD, Hanula JL (2007) A comparison of the beetle (Coleoptera) fauna captured at two heights above the ground in a North American temperate deciduous forest. Am Midl Nat 158:260–278
Ulyshen MD, Sheehan TN (2019) Trap height considerations for detecting two economically important forest beetle guilds in southeastern US forests. J Pest Sci 92:253–265. https://doi.org/10.1007/s10340-017-0883-7
Ulyshen MD, Hanula JL, Horn S, Kilgo JC, Moorman CE (2004) Spatial and temporal patterns of beetles associated with coarse woody debris in managed bottomland hardwood forests. For Ecol Manag 199:259–272. https://doi.org/10.1016/j.foreco.2004.05.046
USDA APHIS PPQ (2023) Emerald ash borer survey guidelines. https://www.aphis.usda.gov/plant_health/plant_pest_info/emerald_ash_b/downloads/eab-survey-guidelines.pdf. Accessed 31 Oct 2023
van der Kooi CJ, Stavenga DG, Arikawa K, Belusic G, Kelber A (2021) Evolution of insect color vision: from spectral sensitivity to visual ecology. Annu Rev Entomol 66:435–461. https://doi.org/10.1146/annurev-ento-061720-071644
Vance CC, Kirby KR, Malcolm JR, Smith SM (2003) Community composition of longhorned beetles (Coleoptera: Cerambycidae) in the canopy and understorey of sugar maple and white pine stands in south-central Ontario. Environ Entomol 32:1066–1074. https://doi.org/10.1603/0046-225X-32.5.1066
Varandi HB, Kalashian M, Barari H, Rezaei Taleshi SA (2018) The diversity of wood-boring beetles caught by different traps in northern forests of Iran. Tropical Drylands 2:65–74. https://doi.org/10.13057/tropdrylands/t020205
Vodka Š, Cizek L (2013) The effects of edge-interior and understorey-canopy gradients on the distribution of saproxylic beetles in a temperate lowland forest. For Ecol Manag 304:33–41. https://doi.org/10.1016/j.foreco.2013.04.007
Vodka S, Konvicka M, Cizek L (2009) Habitat preferences of oak-feeding xylophagous beetles in a temperate woodland: implications for forest history and management. J Insect Conserv 13:553–562. https://doi.org/10.1007/s10841-008-9202-1
Wallace HR (1953) The ecology of the insect fauna of pine stumps. J Anim Ecol 22:154–171. https://doi.org/10.2307/1698
Wardhaugh CW, Pawson SM (2023) Multi-trap sampling of arthropod communities at ports of first entry informs biosecurity surveillance programs. J Pest Sci. https://doi.org/10.1007/s10340-023-01714-5
Webster RP, Klimaszewski J, Sweeney JD, DeMerchant I (2012a) New Staphylinidae (Coleoptera) records with new collection data from New Brunswick, and an addition to the fauna of Quebec, Canada: Aleocharinae. Zookeys 186:83–118. https://doi.org/10.3897/zookeys.186.2655
Webster RP, Sweeney J, DeMerchant ID, Silk P, Mayo P (2012b) New Coleoptera records from New Brunswick, Canada: Cerambycidae. ZooKeys 179:309–311. https://doi.org/10.3897/zookeys.179.2601
Webster RP, Alderson CA, Webster VL, Hughes CC, Sweeney JD (2016) Further contributions to the longhorn beetle (Coleoptera, Cerambycidae) fauna of New Brunswick and Nova Scotia, Canada. Zookeys 552:109–122. https://doi.org/10.3897/zookeys.552.6039
Weiss M, Prochazka J, Schlaghamersky J, Cizek L (2016) Fine-scale vertical stratification and guild composition of saproxylic beetles in lowland and montane forests: similar patterns despite low faunal overlap. PLoS ONE 11:e0149506. https://doi.org/10.1371/journal.pone.0149506
Werle CT, Bray AM, Oliver JB, Blythe EK, Sampson BJ (2016) Ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) captures using colored traps in southeast Tennessee and south Mississippi. J Entomol Sci 49:373–382. https://doi.org/10.18474/0749-8004-49.4.373
Wermelinger B, Duelli P, Obrist MK (2002) Dynamics of saproxylic beetles (Coleoptera) in windthrow areas in alpine spruce forests. For Snow Landsc Res 77:133–148
Wermelinger B, Fluckiger PF, Obrist MK, Duelli P (2007) Horizontal and vertical distribution of saproxylic beetles (Col., Buprestidae, Cerambycidae, Scolytinae) across sections of forest edges. J Appl Entomol 131:104–114. https://doi.org/10.1111/j.1439-0418.2006.01128.x
Weslien J, Annila E, Bakke A, Bejer B, Eidmann HH et al (1989) Estimating risks for spruce bark beetle (Ips typographus (L.)) damage using pheromone-baited traps and trees. Scand J for Res 4:87–98. https://doi.org/10.1080/02827588909382549
Wichmann L, Ravn HP (2001) The spread of Ips typographus (L.) (Coleoptera: Scolytidae) attacks following heavy windthrow in Denmark, analysed using GIS. For Ecol Manag 148:31–39. https://doi.org/10.1016/S0378-1127(00)00477-1
Wickham JD, Harrison RD, Lu W, Guo ZP, Millar JG et al (2014) Generic lures attract cerambycid beetles in a tropical montane rain forest in southern China. J Econ Entomol 107:259–267. https://doi.org/10.1603/EC13333
Wiggins GJ, Grant JF, Lambdin PL, Merten P, Nix KA et al (2014) Discovery of walnut twig beetle, Pityophthorus juglandis, associated with forested black walnut, Juglans nigra, in the Eastern U.S. Forests 5:1185–1193. https://doi.org/10.3390/f5061185
Wilkening AJ, Foltz JL, Atkinson TH, Connor MD (1981) An omnidirectional flight trap for ascending and descending insects. Can Entomol 113:453–455. https://doi.org/10.4039/ENT113453-5
Wittman JT, Silk P, Parker K, Aukema BH (2021) Optimizing early detection strategies: defining the effective attraction radius of attractants for emerald ash borer Agrilus planipennis Fairmaire. Agric for Entomol 23:527–535. https://doi.org/10.1111/afe.12457
Wong JCH, Hanks LM (2016) Influence of fermenting bait and vertical position of traps on attraction of cerambycid beetles to pheromone lures. J Econ Entomol 109:2145–2150. https://doi.org/10.1093/jee/tow197
Wong JCH, Meier LR, Zou Y, Mongold-Diers JA, Hanks LM (2017) Evaluation of methods used in testing attraction of cerambycid beetles to pheromone-baited traps. J Econ Entomol 110:2269–2274. https://doi.org/10.1093/jee/tox211
Wyatt TD, Phillips ADG, Gregoire JC (1993) Turbulence, trees and semiochemicals: wind-tunnel orientation of the predator, Rhizophagus grandis, to its bark beetle prey, Dendroctonus micans. Physiol Entomol 18:204–210. https://doi.org/10.1111/j.1365-3032.1993.tb00469.x
Xu H, Turlings TCJ (2018) Plant volatiles as mate-finding cues for insects. Trends Plant Sci 23:100–111. https://doi.org/10.1016/j.tplants.2017.11.004
Zeneli G, Tsitsimpikou C, Petrakis PV, Naxakis G, Habili D et al (2001) Foliar and cortex oleoresin variability of silver fir (Abies alba Mill.) in Albania. Z Für Naturforsch C 56:531–539. https://doi.org/10.1515/znc-2001-7-810
Zhang Q-H, Schlyter F (2003) Redundancy, synergism, and active inhibitory range of non-host volatiles in reducing pheromone attraction in European spruce bark beetle Ips typographus. Oikos 101:299–310. https://doi.org/10.1034/j.1600-0706.2003.111595.x
Zhang QH, Schlyter F (2004) Olfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agric for Entomol 6:1–19. https://doi.org/10.1111/j.1461-9555.2004.00202.x
Zhang QH, Birgersson G, Zhu J, Löfstedt C, Schlyter F (1999) Leaf volatiles from nonhost deciduous trees: variation by tree species, season and temperature, and electrophysiological activity in Ips typographus. J Chem Ecol 25:1923–1943. https://doi.org/10.1023/A:1020994119019
Žunič Kosi A, Zou YF, Hoskovec M, Vrezec A, Stritih N et al (2017) Novel, male-produced aggregation pheromone of the cerambycid beetle Rosalia alpina, a priority species of European conservation concern. PLoS ONE 12:e0183279. https://doi.org/10.1371/journal.pone.0183279
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement. DR was supported by the Agritech National Research Center that received funding from the European Union Next-GenerationEU (Piano Nazionale di Ripresa e Resilienza (PNRR)—Missione 4 Componente 2, Investimento 1.4–D.D. 1032 17 June 2022, CN00000022). JS was supported by the Pest Risk management program of Natural Resources Canada, Canadian Forest Service.
Author information
Authors and Affiliations
Contributions
All authors contributed to the planning and implementation of this review. Draft manuscripts were prepared by all authors, and all approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no financial or non-financial conflict of interests.
Additional information
Communicated by Jian Duan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dodds, K.J., Sweeney, J., Francese, J.A. et al. Factors affecting catches of bark beetles and woodboring beetles in traps. J Pest Sci (2024). https://doi.org/10.1007/s10340-024-01774-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10340-024-01774-1