Abstract
The spotted-wing drosophila (SWD), Drosophila suzukii (Matsumura), is an invasive species native to East Asia and now widespread worldwide. Major economic damage is caused by the larvae developing within ripening soft-skin fruit. Currently, larval detection in fruit is limited to destructive methods and post-harvest control strategies heavily rely on the use of chemicals or cold to inhibit egg eclosion and larval growth. Feeding larvae are likely to induce substrate-borne vibrations in the berry that could be exploited as cues by predators or to develop a non-invasive pest detection method, an approach previously applied on leaves and wooden structures, but never on fresh fruit. We used a laser vibrometer to detect and characterize the incidental vibrations produced by D. suzukii larvae within fresh blueberries at five different pest age (48, 96, 168, 216 and 264 h). An innovative statistical analysis was performed to assess if infestation level (number of pupae) and pest age (hours after exposure) affect the spectrum and the amplitude of vibrations. The recordings of infested berries were characterized by the presence of a series of broad-band pulses (frequency range 0.1–2 kHz) without a regular temporal pattern, in an amplitude range between 12.1 and 946 µm/s. Furthermore, the analysis revealed the possibility to distinguish between different pest ages and infestation levels. By a spectral analysis of the recordings, the pest ages can be distinguished among each other, but for the age groups at 168 and 216 h after infestation. The vibration amplitude trend gradually increased up to 168–216 h after infestation, and then decreased until fly emergence. Low-infested blueberries showed a faster D. suzukii development time compared to high-infested blueberries. This was reflected into vibrational recordings, as low-infested blueberries exhibited peak amplitude at earlier stage compared to high-infested ones. Results suggest that D. suzukii larvae induce detectable vibrations by feeding within berries that are dependent on infestation level and pest age. We discuss the possible ecological role of such vibrations as cues for unintended receivers, such as predators and parasitoids, and their potential for innovative infestation detection methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apart from being important economical commodities and relevant component of human and animal’s diet, fruits provide microhabitats to a vast group of insects (Sallabanks and Courtney 1992). This interaction can be mutualistic and have neutral or even beneficial effects for some host plants (Wilson 2008), but from an agriculture perspective, infested fruits are a major economical concern. Among frugivore insects residing in fruit pulp there is an invasive pest causing extensive agricultural damage worldwide, the spotted-wing drosophila (SWD), Drosophila suzukii (Matsumura) (Diptera: Drosophilidae). Native to Southeast Asia, since 2008 D. suzukii has invaded North America (Hauser 2011), Europe (Cini et al. 2012), South America (Andreazza et al. 2017) and Northern Africa (Ouantar et al. 2020) thanks its high fecundity (Emiljanowicz et al. 2014; Hamby et al. 2016), wide host range (Lee et al. 2015; Kenis et al. 2016) and thermal adaptability (Ryan et al. 2016). Drosophila suzukii is among the very few Drosophila species able to lay eggs in ripening fruit (Atallah et al. 2014). Development parameters are temperature and humidity-dependent (Tochen et al. 2014, 2016). Under optimal laboratory condition at 22 °C, eggs take 12 to 14 days to reach adulthood, passing through three larval instar stages (5.8 days) and a pupal stage (6 days) (Emiljanowicz et al. 2014; Tochen et al. 2014). While larvae reside most of their life in fruit pulp, pupation can also occur on fruit surface, or detached outside (Kanzawa 1939; Hamby et al. 2016; Woltz and Lee 2017; Bezerra Da Silva et al. 2019). Crop damage is caused by larvae feeding in ripening fruit and serious economic impacts have been reported to raspberry (Farnsworth et al. 2017), blueberry (Yeh et al. 2020), strawberry and blackberry (De Ros et al. 2015), Swiss cherry, plum and grape (Knapp et al. 2021) cultivation.
Pest detection methods rely on monitoring adults utilizing traps and different lures (Cloonan et al. 2018). A less common monitoring method is larval count. that has the advantage of providing immediate information to growers to adjust the most appropriate control strategies depending on the real-time infestation status (Tait et al. 2021). However, at the moment this technique is time-consuming as it requires several steps such as fruit crushing, salt or vacuum extraction and filter collection (Van Timmeren et al. 2017; Babu et al. 2023). A successful non-contact detection method of D. suzukii not only could favor early pest detection, but also post harvesting sector that now heavily relies on chemical application and cold storage to suppress the pest (Walse et al. 2012; Kraft et al. 2020; Mostafa et al. 2021). Insects are known to produce substrate-born vibrations while residing in or on their substrate (Hill et al. 2019; Virant-Doberlet et al. 2023). These vibrations can either be produced by specialized organs, such in the case of mating signals of Hemiptera (Laumann et al. 2011; Virant-Doberlet et al. 2011), or they can be incidental, such in the case of vibrations produced by movement (Devetak 2014; Oberst et al. 2017) or feeding (Kollasch et al. 2020; Turchen et al. 2022). These vibrations have been exploited to detect invertebrates ‘presence within different substrates (Mankin et al. 2011), initially with accelerometers, piezoelectric sensors and microphones (Mankin et al. 1997, 2004; Castellanos and Barbosa 2006; Pearson et al. 2007; Devetak 2014), and more recently utilizing more accurate laser Doppler vibrometers (Zorović and Čokl 2015; Liu et al. 2017; De Luca and Vallejo-Marin 2022). This methodology has been already applied to assess incidental vibrations produced by insects within wood (Zorović and Čokl 2015), and extensively on plant’s leaves (Kollasch et al. 2020; Turchen et al. 2022), but to the authors’ knowledge, never on fruits. Even if most of juvenile insect stages, such as larvae, nymphs and pupae, are substrate-bound and likely depend on perception of vibratory signals, their vibroscape, described as their natural vibrational environment (Šturm et al. 2021), has been rarely studied and it remains poorly understood (Yack and Yadav 2022). While Drosophila adults are known to utilize substrate-born vibrations during courtship, where male tremulations result into female immobilization (Fabre et al. 2012; Mazzoni et al. 2013; McKelvey et al. 2021), little is known about their larval stage. Moreover, the identification and characterization of these incidental vibrational signals are necessary steps to unravel their ecological role. In particular to assess if they function as cues in host-parasitoid interactions, as previously assumed (Vet and Bakker 1985; Girod et al. 2018a), but never assessed thoroughly. Eavesdropping is a common scenario in the animal realm, where numerous and diverse signals (chemical, visual, acoustic, electrical, tactile) are exploited by unintended receivers (Peake 2005; Hughes et al. 2012). This technique is adopted by predators and parasitoids to locate preys and hosts (Broad and Quicke 2000; Takanashi et al. 2016; Yack and Yadav 2022). As biological control (BC) is becoming a relevant practice adopted to suppress this pest population (Lee et al. 2019; Tait et al. 2021), it is fundamental to deepen our knowledge on BC agents host searching, which might not rely solely on chemical, but also on physical cues such as substrate-born vibrations (Higham and Hebets 2013; Nieri et al. 2022).
In this study we used a laser vibrometer to detect and characterize incidental vibrations produced by larvae of D. suzukii feeding within fresh fruit pulp. We hypothesized that infested blueberries are characterized by the presence of larval feeding vibrations. We first evaluated the presence of vibrations by comparing the recordings of fruit infested with living larvae and un-infested fruit. We, then, characterized the vibrations and applied innovative statistical methods to test the influence that infestation level and pest age had on the amplitude and spectral parameters of recorded vibrations.
Material and methods
Insect rearing and infestation
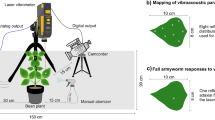
Drosophila suzukii individuals used for fruit infestation were mass reared according to the protocol specified in Rossi Stacconi et al. (2022) and derived from multiple field collections of live adults at different locations in the Trento Province (Italy), during 2020 and 2021 seasons. Considered that traits of recorded substrate play an important role into defining spectra we ensured that every blueberry had similar weight and shape. Fresh blueberries from the same batch were selected and weighted to ensure a standardized weight of 1.5 ± 0.1 g using a precision scale (D-72336, Kern and Sohn, Balingen, Germany). Rotten, soft, deformed or damaged fruits were discarded. Un-infested blueberries were used as control, while the others were placed in D. suzukii mass rearing cages (30 × 30 × 70 cm, Bugdorm BD4F3074, MegaView Science Co., Ltd, Taichung, Taiwan) to allow oviposition. Every 15 min, infested blueberries were removed from the cages and checked under stereoscope (M80, Leica Microsystem, Germany) to count the number of laid eggs and ensure the absence of other living organisms such as mites. Blueberries with 5 to 30 fly eggs were selected for recordings and maintained individually in a plastic cylinder container (diameter 6 cm, height 8 cm) with absorbent cotton at the bottom and a lateral hole (diameter 2 cm) covered with fine mesh for aeration. A small fragment of reflective tape (2 × 2 mm2) was attached to the surface of each blueberry in the same position, near the calyx, to minimize differences in vibrational recordings. For the duration of the experiment, distilled water (3 ml) was poured weekly on the cotton in each container to avoid sample desiccation. Containers were maintained in controlled conditions at 21 ± 2 °C, 70% RH, and 16:8 L:D.
Drosophila suzukii development parameters
The containers holding the infested blueberries were monitored for adults’ eclosion every 48 h. Emerging D. suzukii adults were counted and stored in 70% ethanol solution. One week after last adult emergence, the samples were hydrated and pupae were isolated and counted. The total number of pupae was used as a proxy to estimate blueberries’ infestation level. Blueberries were grouped into two categories: low infestation (L) containing 1 to 6 pupae (n = 14), and high infestation (H) containing 7 to 13 pupae (n = 16). To evaluate D. suzukii development the following parameters were used: the adult survival rate, expressed as the percentage of eggs laid that reached adulthood, the average emersion time (AEM), the first emersion time (FET) and the last emersion time (LET), as the number of days from the infestation date to adult emergence.
Vibrational recording and analysis
Vibrational recordings were taken in a soundproof room between February and April 2022. The blueberries were placed in calyx-up position and individually tested on an anti-vibration table (Astel s.a.s, Ivrea, Italy). Vibrations were recorded using a laser Doppler vibrometer (PDV 100, Polytec, Germany) focused on the reflective tape previously positioned on the blueberry. Recordings were digitized using the software BK Connect (Brüel and Kjær Sound and Vibration A/S, Nærum, Denmark) at 6.4 kHz sample rate and 24-bit depth resolution through a data acquisition device (LAN XI type 3050-B-040, Brüel and Kjær Sound and Vibration A/S, Nærum, Denmark), and stored directly onto a computer hard drive. For each blueberry, vibrations were recorded for 3 min at five different time intervals: at 48, 96, 168, 216 and 264 h after D. suzukii infestation. Control un-infested blueberries were recorded at the same time. Recordings were analyzed using the software BK Connect (Brüel and Kjær Sound and Vibration A/S, Nærum, Denmark) with a Fast Fourier Transformation (FFT), Hanning type, window size of 400 Hz and 66.7% overlap for the frequency range 100–2000 Hz. The amplitude of vibrations was measured as velocity of substrate displacement. To characterize the single pulses induced by the feeding larvae, the peak frequency and the corresponding amplitude were measured for three pulses per recording at three infestation time (28 recordings at 96 h after infestation, 30 at 168 h, and 29 recordings at 216 h). A pulse was defined as a physically unitary or homogeneous sound, composed of a brief succession of sine waves (Alexander 1967). To evaluate the influence of infestation, its level, and pest age, on the entire length of the recording, the spectra averaged for the three-minutes recordings were compared.
Statistical analysis
All statistical analyzes have been carried out in R (4.2) (R Core Team 2020) with the following packages: fda.usc (Febrero-Bande and Oviedo De La Fuente 2012), fdANOVA (Gorecki and Smaga 2018), permuco (Frossard and Renaud 2021), together with custom code developed by the authors and available upon request. To compare D. suzukii development parameters a Shapiro–Wilk normality test followed by non-parametric Mann–Whitney was performed. To compare vibrational recording, following a functional data analysis (Ramsay and Silverman 2005) approach, we analyzed the average spectra for each three minute recording and considered, for each blueberry and time interval, a functional observation of the amplitude of vibrations as a function of the frequency. These curves have then been compared by means of point-wise and global non-parametric two-way functional ANOVA (Zhang and Liang 2014), testing, in particular, the effect of infestation, pest age and infestation level. For the point-wise analysis, we compared the between- and within-groups variances at each frequency, producing a sequence of statistical tests and corresponding p values. The globalizing test statistic (GPF) (Zhang and Liang 2014), was then obtained integrating the point-wise statistic. The corresponding p value was computed through a permutation test (Good 2000). All the statistical analysis described henceforth have been performed for the single frequencies (point-wise tests) and integrating over the frequencies (global tests). To avoid inconsistencies between the results of the two families of tests, p values were always computed through permutation tests, with 100,000 permutations. To test the effect of pest age on recording intensity in infested blueberries, we performed a post-hoc analysis by means of Quade’s test (Conover 1999). Quade’s test is a non-parametric test based on ranks for multiple treatments (represented here by pest age) in randomized complete block designs. To test the effect of the level of infestation on recorded vibrations, always controlling for the effect of pest age, we added different covariates, numeric variables, to the categorical factors in our functional ANOVA model. This resulted in an Analysis of Covariance (ANCOVA) with the following covariates: number of eggs at the infestation time, number of developed pupae, and pupae/eggs rate as a measure of the survival rate of the pest’s larvae. We also examined the interaction between the amounts of eggs and pupae, both by allowing for the interaction in the model and by adding explicitly to the model the ratio among the two variables. For more details about tests and models, see the Supplementary file.
Results
Drosophila suzukii development rate
On average, high infested blueberries had more than twice the number of pupae compared to low infested blueberries (Table 1). Adult survival rate and FET did not differ significantly between the two groups (Table 1). On the contrary, the LET was significantly delayed in high infested samples (3.8 days on average, Table 1), indicating a lower development rate of the larvae in the high infested berries.
Vibrations induced by feeding larvae
Infested samples’ recordings were characterized by the presence of a series of different sized broad-band pulses that displayed no regular temporal patterns (Fig. 1). The amplitude of single pulses ranged from 33.2 to 312.6 µm/s at 96 h (n = 28), from 23.7 to 672.6 µm/s at 168 h (n = 30), and from 12.1 to 946 µm/s at 216 h after infestation (n = 29). The dominant frequency of pulses was on average 1096, 662 and 651 Hz at 96, 168 and 216 h, respectively. No pulses were ever detected in uninfested blueberries. Moreover, the three minutes average spectra had significant higher intensities for all the frequencies (100–2000 Hz) in infested blueberries compared to control ones (analysis of variance, α = 0.05) (Figs. 2, 3).
Visual representation of the three-minutes spectra for control uninfested blueberries (left) and infested blueberries (right). The infested samples displayed higher recorded intensities. Bold lines represent the average of the samples (n = 30) recorded at different pest age time. Shades represent Standard Deviation values
Influence of pest age on vibration amplitude
Among infested blueberries, the pest age had a significant effect on the vibration amplitude for almost all frequencies (α = 0.05). Only at around 1800 Hz the effect was not significant (p > 0.05) (Fig. 3). The Globalising Pointwise Quade test revealed that over all frequencies, all pest ages could be discriminated among each other (p < 0.001), except for two groups, 168 and 216 h (T-statistic: 0.84, p value = 0.12) (Table 2). The pairwise pointwise Quade test (Fig. 4) revealed that the amplitude of vibrations recorded at 48 h was significantly different from the one recorded at other pest ages (α = 0.05) for almost all frequencies, except at very low frequencies (100–125 Hz), and few more frequencies (upper limit 600 Hz) in comparison with the recordings performed at 96 h. The vibration amplitude at 96 h was significantly different on average from the one recorded at 168 and 216 h for frequencies under 1200 Hz (α = 0.05). The pairwise comparison of the vibration amplitude between the 96 h and 264 h groups was not significantly different for most of the frequencies (p > 0.05) (Fig. 4).
Influence of infestation level on vibration amplitude
The analysis of variance confirmed that different infestation levels (Control, Low and High) had a significant effect on the vibration amplitude for all the frequency range (α = 0.05) (Fig. 5). Overall, we observed an increase of the vibration amplitude at increasing infestation levels: the higher the infestation level the higher the vibration amplitude (Fig. 6). The vibration amplitude trend over time was characterized by a gradual increase (up to 168–216 h after the infestation) followed by a gradual decrease until flies’ emergence. The average maximum amplitude was recorded at 0.104 µm/s after 168 h for samples with low infestation, whereas samples with high infestation recorded the average maximum amplitude at 0.189 µm/s after 216 h. The functional analysis of covariance (ANCOVA) performed on infested berries confirmed that, among infestation level factors considered into the analysis, the number of pupae better explained the observed vibration amplitude recorded for all frequencies (α = 0.05) (Fig. 7). The interaction between egg and pupae resulting from the model was unreliable at low frequency range (100–400 Hz) and above 1500 Hz (α = 0.05). Both the number of laid eggs and the ratio between pupae and eggs, did not explain the vibration amplitude for any frequency (p > 0.05) (Fig. 7).
Visual representation of vibrations amplitude recorded at different pest age and infestation level (C Control; L Low infestation; H High infestation) previously tested with ANOVA (Fig. 5). For each box, the central line represents the median, the limits of the box are the 25th and 75th percentiles. The cross symbol (x) represents the mean. Whiskers extending outside the box illustrate data range, while dots are considered outliers
p value resulting from the functional Analysis of covariance (ANCOVA) obtained by a permutation method. Pupae represent the number of pupae that developed into the sample, eggs represent the number of counted eggs at infestation time, pupae/eggs represent the ratio of the variables, and eggs-pupae interaction represent their interaction resulting from the model
Discussion
Infested samples were characterized by the presence of broad-band pulses that are within the sensitivity range of the species (Mamiya et al. 2018). The spectral analysis revealed the possibility to distinguish infested from un-infested samples. Additionally, in our standardized context we were able to distinguish different pest ages and infestation levels. Vibrations produced by hymenopteran and lepidopteran larvae feeding on leaves exhibit similar wave forms to those we described (Kollasch et al. 2020), supporting the hypothesis that the recorded pulses are D. suzukii larvae’s mandibles chewing the fruit pulp. Kollasch et al. (2020) described peak amplitude of pulses ranging between 1 and 3 mm/s, whereas our recorded amplitudes ranged from 12.1 to 946 µm/s. Such lower values could be explained by several factors, as the difference in size of the emitters used in the two studies, the location of the emitters with respect to recording point on the substrate and the shape and type of the substrate (Oberst et al. 2019) In fact, the insect size is directly related to the amplitude of produced vibrations (Vick et al. 1988; Mankin et al. 2010); and while D. suzukii larval length ranges from 1 mm (1st instar) to 4 mm (3rd instar) (Van Timmeren et al. 2017), the lepidopteran and hymenopteran larvae tested by Kollasch et al. (2020) were all late instars (3rd to 5th) ranging from 10 to 30 mm in length. Furthermore, amplitude of vibrations is inversely related to the distance of the emitters with respect to the recording point. In our study the larvae were embedded in a spherical substrate (blueberries), whereas recordings by Kollasch et al. (2020) were performed on larvae feeding on the surface of a flat substrate (leaves); thus, in our case it was impossible to know the exact distance between the emitting larva and the recording point on the blueberry surface. Probably this is the reason behind the variability of amplitude of the recorded pulses. Although D. suzukii infestation was carried out at the same time to reduce biases, different larvae could have displayed different development time (therefore size) and were simultaneously active within the substrate, at different distance from the recording point. Interestingly, the amplitude range we observed is very similar to that of the vibrations produced by the larvae of the Asian longhorned beetle (Anoplophora glabripennis Motschulsky) feeding within wood logs (10–960 µm/s) (Zorović and Čokl 2015), despite such larvae can measure up to 50 mm in length (CABI 2020). This may suggest a major influence of the location of the emitter with respect to the substrate (on or within) compared to the effect of the insect. In modern berries processing, quality check traditionally performed by human operators is leaving place to automatized imaging systems that allow to effectively sort and grade fruit based on its appearance (Wang et al. 2021). These detection technologies mainly rely on physical aspects of the fruit surface (Patel et al. 2012; Blasco et al. 2017), and cannot detect the presence of pests located within fruit pulp. Our results demonstrate that vibrational detection is possible and its integration into fruit processing systems can potentially give a more comprehensive picture of fruit status. One constrains of vibrational detection is the need to isolate the recorded sample to reduce the interference of background noises. This issue could be tackled coupling the recording with other detection systems that are generally placed in controlled area, such as computer vision systems and odor detection (E-Nose) (Wang et al. 2021), or filtering the noise digitally (Bentler and Chiou 2006). Considering that currently post-harvest suppression of D. suzukii requires costly long exposure to CO2, not always effective to completely kill the immature stages (Mostafa et al. 2021), a preliminary pest detection could limit its application, reducing both environmental and economical associated costs.
Besides detection for monitoring or control purposes, vibrations recorded in this study are potentially relevant in the Drosophila ecology. In fact, feeding vibrations could have both an intraspecific and interspecific function. Intraspecific communication in Drosophila relies also on vibrational stimuli (Fabre et al. 2012; Mazzoni et al. 2013; McKelvey et al. 2021). In particular, Drosophila femoral chordotonal organs neurons (FeCO) are sensitive to low amplitude vibrations in the 200–2000 Hz range. In particular, it has been shown that frequency that corresponds to the highest sensitivity is 400 Hz at 0.9 µm/s, and 800 Hz at the smaller amplitude of 0.054 µm/s (Mamiya et al. 2018). The pulses we recorded are above this threshold and the peak frequency is within the sensitive range, suggesting that D. suzukii could exploit the vibrations induced by feeding larvae as a cue to identify infested berries. Host searching behavior and oviposition site selection in D. suzukii is regulated by visual, mechanical and chemical cues (Cloonan et al. 2018; Kidera and Takahashi 2020) but vibrations could represent an additional modality to acquire insights on the suitability of host patches. Specific odors emitted by plants or microorganisms and the color of ripening berries are important for long-range location of suitable hosts, indeed these cues are used for traps’ design (Cloonan et al. 2018; Alawamleh et al. 2021). At short-range, surface secretions produced after oviposition have an aggregative function and stimulate conspecific females to lay eggs shortly after infestation (Tait et al. 2020). However, this effect rapidly decreases (a few hours) as the chemical cues left by the ovipositing female start degrading (Tait et al. 2020). We hypothesize that vibrational cues could be involved in oviposition site selection at advanced stages (> 12 h after infestation), when eggs hatch and larvae start producing incidental vibrations. At this stage, the chemical cues deriving from previous oviposition events could be outcompeted by the vibrational cues induced by larvae developing within the fruit. This would be consistent with the fact that multiple concurrent ovipositions on a single fruit increase offspring survival through different cooperation mechanisms (Tait et al. 2020), whereas ovipositing on fruit where older larvae are already present could be disadvantageous due to intraspecific competition (i.e., cannibalism of eggs and younger larvae) (Bezerra Da Silva et al. 2019). Moreover, if flies perceive the differences in the spectra that we identified in our analysis, vibrations induced by larvae could provide adult flies with information about both the infestation level and the larval stage. Future studies on D. suzukii perception of mechanical stimuli and the behavioral response to vibrational playbacks are needed to test this hypothesis. Incidental vibrations produced by developing D. suzukii larvae could also serve as a cue for parasitoids searching for their host (Broad and Quicke 2000). Several parasitoid wasps have documented to attack D. suzukii both in its native and invaded area (Girod et al. 2018a, b; Lee et al. 2019; Daane et al. 2021), some of which are currently being reared and released as biological control agents (Rossi Stacconi et al. 2022; Fellin et al. 2023). Larval parasitoids in particular could benefit from vibrational detection, for these species both the host location within the substrate and the development stage are crucial for successful parasitization (Wang et al. 2018). For instance, G. brasiliensis have a preference toward young larvae of 1–2 days old (Wang et al. 2018) Larval parasitoids’ exploitation of vibrotaxis for host detection has been previously assumed (Vet and Bakker 1985; Girod et al. 2018a), but never assessed thoroughly. In several hymenopteran groups (bees, wasps and ants), evaluation on responses of chordotonal organs to vibrational stimuli revealed that the sensitivities range between 1.8 and 80 µm/s (Strauß et al. 2021), suggesting that the signals we recorded in infested samples could be detected by parasitoid wasps, such as larval parasitoids of D. suzukii.
Our analysis revealed the possibility to discriminate larvae age based on recordings amplitude. As spectral parameters are influenced by the recorded substrate (Oberst et al. 2019), this was possible because in our experiment blueberries had similar weight and shape. Different fruit traits should be evaluated in future studies to assess how they influence the spectra. These data could lead to the creation of a model that accounts for both pest age and fruit characteristics. Our recordings suggests that vibrations not only carry binary information regarding host presence or absence, but potentially also relevant information regarding their developmental stage. If proven, the eavesdropping performed by parasitoids could contribute to explain their selectivity toward the different host stages of D. suzukii (Rossi Stacconi et al. 2015; Wang et al. 2018). The trend of the amplitude of vibrations over time after infestation can be explained by D. suzukii life cycle, and in particular by pupation. Once pupal stage is reached, D. suzukii, as all insects, does not feed anymore and it is almost immobile, thus the gradual decrease of vibration amplitude observed in our experiment can be explained by the reduction in individual actively feeding. In fact, the highest amplitude values have been observed at 168 h after infestation, which is when larvae probably were close to pupation according to the observed emersion rates, which are in agreement with those of similar experiments conducted on blueberries in comparable climatic conditions (Tochen et al. 2014, 2016; Winkler et al. 2020). In previous studies, the development time from egg to adult took on average 14 ± 0.1 days and larvae pupated approximately eight days after infestation (192 h) (Tochen et al. 2014, 2016), a time at which we observed signal amplitude plateau and its gradual decrease. The increase in amplitude from 24 to 168 h can be explained by the increase in size of the larvae, for the previously mentioned effect on size over vibrations amplitude. Among the two infestation level groups, L blueberries better represented a natural scenario, in which low numbers of larvae develop within a single berry (Burrack et al. 2013). The later occurrence of the average maximum amplitude observed in H blueberries and its slower decrease compared with L blueberries reflect an overall lower development rate observed in blueberries where larval density was higher. Also emersion times, AET and LET, in highly infested samples was significantly delayed, most likely due to the occurrence of higher intraspecific competition pressure and relative limited availability of food source, both factors that have been proven to extend development time (Hardin et al. 2015; Bezerra Da Silva et al. 2019). The ANCOVA revealed also an important aspect about the development rate and success of D. suzukii infestations. Contrary to the number of pupae, the eggs number was not a good indicator to explain changes in the recorded spectra. This evidence supports the fact that egg count is an unreliable proxy to preliminary quantify infestation level. Egg count can be easily performed, even automatized (Waithe et al. 2015) and still a valid method to assess insect fecundity and oviposition preference (Tochen et al. 2014; Ng’oma et al. 2018; Kidera and Takahashi 2020; Fowler et al. 2022), but it poorly represents the number of larvae developing in the infested fruit. Many factors affect D. suzukii development from egg to adulthood, such as temperature and humidity (Winkler et al. 2021), interspecific competition (Bezerra Da Silva et al. 2019) and host plant (Olazcuaga et al. 2019). This makes it difficult to estimate accurately infestation level from egg count, especially in samples taken from field and uncontrolled environment. Vibrational recording on the other hand could be a more practical alternative that does not rely on sample history.
Laser vibrometer recordings have been proven a successful non-destructive methodology for early detection of incidental vibrations induced by D. suzukii larvae feeding in fresh fruit pulp. Furthermore, the applied analysis has been proven useful to compare vibrational spectral parameters and discriminate both pest ages and infestation level at standardized berry weight. This analysis could be useful in further studies aiming to investigate the effect of broad-band vibrations on other organisms. Moreover, this study paves the way for development of automated monitoring systems that could use a similar comparison analysis to not only detect the occurrence of vibrations but also quantify or identify the infestation level. Additionally, we provided new insights on the ecology of Drosophila species, showing that not only adults, but also larvae are emitters of vibrational cues and signals. Which could play a role in both intra and interspecific communication.
Author contributions
All authors contributed to study conception and design. LF performed material preparation and data collection. Statistical analysis was performed by GB with the supervision of CA. LF and GB led the writing of the manuscript. Supervision by RN, MVRS, VM and GA. All authors read and approved the manuscript.
References
Alawamleh A, ðurović G, Maddalena G et al (2021) Selection of lactic acid bacteria species and strains for efficient trapping of Drosophila suzukii. InSects 12:1–13. https://doi.org/10.3390/insects12020153
Alexander RD (1967) Acoustical communication in arthropods. Annu Rev Entomol 12:495–526. https://doi.org/10.1146/annurev.en.12.010167.002431
Andreazza F, Bernardi D, dos Santos RSS et al (2017) Drosophila suzukii in Southern neotropical region: current status and future perspectives. Neotrop Entomol 46:591–605. https://doi.org/10.1007/s13744-017-0554-7
Atallah J, Teixeira L, Salazar R et al (2014) The making of a pest: the evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. In: Proceedings of the Royal Society 281. Royal Society. https://doi.org/10.1098/rspb.2013.2840
Babu A, Adhikari R, Sial AA (2023) Vacuum extraction: an effective larval sampling method for spotted-wing drosophila in small fruit crops. J Econ Entomol 116(5):1750–1759. https://doi.org/10.1093/jee/toad160
Bentler R, Chiou LK (2006) Digital noise reduction: an overview. Trends Amplif 10:67–82. https://doi.org/10.1177/1084713806289514
Bezerra Da Silva CS, Park KR, Blood RA, Walton VM (2019) Intraspecific competition affects the pupation behavior of spotted-wing Drosophila (Drosophila suzukii). Sci Rep. https://doi.org/10.1038/s41598-019-44248-6
Blasco J, Munera S, Aleixos N et al (2017) Machine vision-based measurement systems for fruit and vegetable quality control in postharvest. In: Hitzmann B (ed) Measurement, modeling and automation in advanced food processing. Springer, Cham, pp 71–91. https://doi.org/10.1007/10_2016_51
Broad GR, Quicke DLJ (2000) The adaptive significance of host location by vibrational sounding in parasitoid wasps. Proc R Soc B: Biol Sci 267:2403–2409. https://doi.org/10.1098/rspb.2000.1298
Burrack HJ, Fernandez GE, Spivey T, Kraus DA (2013) Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumara (Diptera: Drosophilidae), an invasive frugivore. Pest Manag Sci 69:1173–1180. https://doi.org/10.1002/ps.3489
CABI (2020) Anoplophora glabripennis (Asian longhorned beetle). In: Invasive species compendium. https://www.cabi.org/isc/datasheet/5557. Accessed 4 May 2023. https://doi.org/10.1079/cabicompendium.5557
Castellanos I, Barbosa P (2006) Evaluation of predation risk by a caterpillar using substrate-borne vibrations. Anim Behav 72:461–469. https://doi.org/10.1016/j.anbehav.2006.02.005
Cini A, Ioriatti C, Anfora G (2012) A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull Insectol 65:149–160
Cloonan KR, Abraham J, Angeli S et al (2018) Advances in the chemical ecology of the spotted wing Drosophila (Drosophila suzukii) and its applications. J Chem Ecol 44:922–939. https://doi.org/10.1007/s10886-018-1000-y
Conover WJ (1999) Practical nonparametric statistics. Wiley, New York. https://doi.org/10.4236/ojs.2014.47047
Daane KM, Wang X, Hogg BN, Biondi A (2021) Potential host ranges of three Asian larval parasitoids of Drosophila suzukii. J Pest Sci 94:1171–1182. https://doi.org/10.1007/s10340-021-01368-1
De Luca PA, Vallejo-Marín M (2022) Blooms and buzzing bees: bridging buzz pollination and biotremology. In: Hill PSM, Mazzoni V, Stritih-Peljhan N, Virant-Doberlet M, Wessel A (eds) Biotremology: physiology, ecology, and evolution, vol 8. Springer, Cham, pp 261–292. https://doi.org/10.1007/978-3-030-97419-0_11
De Ros G, Conci S, Pantezzi T, Savini G (2015) The economic impact of invasive pest Drosophila suzukii on berry production in the province of Trento, Italy. J Berry Res 5:89–96. https://doi.org/10.3233/JBR-150092
Devetak D (2014) Sand-borne vibrations in prey detection and orientation of antlions. In: Cocroft RB, Gogala M, Hill PSM, Wessel A (eds) Studying vibrational communication. Springer, Berlin, pp 319–330. https://doi.org/10.1007/978-3-662-43607-3_16
Emiljanowicz LM, Ryan GD, Langille A, Newman J (2014) Development, reproductive output and population growth of the fruit fly pest Drosophila suzukii (Diptera: Drosophilidae) on artificial diet. J Econ Entomol 107:1392–1398. https://doi.org/10.1603/EC13504
Fabre CCG, Hedwig B, Conduit G et al (2012) Substrate-borne vibratory communication during courtship in Drosophila melanogaster. Curr Biol 22:2180–2185. https://doi.org/10.1016/j.cub.2012.09.042
Farnsworth D, Hamby KA, Bolda M et al (2017) Economic analysis of revenue losses and control costs associated with the spotted wing drosophila, Drosophila suzukii (Matsumura), in the California raspberry industry. Pest Manag Sci 73:1083–1090. https://doi.org/10.1002/ps.4497
Febrero-Bande M, Oviedo De La Fuente M (2012) Statistical computing in functional data analysis: the R package fda. usc. JSS J Stat Softw 51:1–28. https://doi.org/10.18637/jss.v051.i04
Fellin L, Grassi A, Puppato S et al (2023) First report on classical biological control releases of the larval parasitoid Ganaspis brasiliensis against Drosophila suzukii in northern Italy. Biocontrol 68:1–12. https://doi.org/10.1007/s10526-022-10174-2
Fowler EK, Leigh S, Rostant WG et al (2022) Memory of social experience affects female fecundity via perception of fly deposits. BMC Biol. https://doi.org/10.1186/s12915-022-01438-5
Frossard J, Renaud O (2021) Permutation tests for regression, ANOVA, and comparison of signals: the permuco package. J Stat Softw 99:1–32. https://doi.org/10.18637/JSS.V099.I15
Girod P, Lierhmann O, Urvois T et al (2018a) Host specificity of Asian parasitoids for potential classical biological control of Drosophila suzukii. J Pest Sci 91(4):1241–1250. https://doi.org/10.1007/s10340-018-1003-z
Girod P, Borowiec N, Buffington M et al (2018b) The parasitoid complex of D. suzukii and other fruit feeding Drosophila species in Asia. Sci Rep 8:1–8. https://doi.org/10.1038/s41598-018-29555-8
Good P (2000) Permutation tests: a practical guide to resampling methods for testing hypotheses, 2nd edn. Springer, New York. https://doi.org/10.1007/978-1-4757-3235-1
Gorecki T, Smaga L (2018) fdANOVA: analysis of variance for univariate and multivariate functional data. https://doi.org/10.1007/s00180-018-0842-7
Hamby KA, Bellamy DE, Chiu JC et al (2016) Biotic and abiotic factors impacting development, behavior, phenology, and reproductive biology of Drosophila suzukii. J Pest Sci 89:605–619. https://doi.org/10.1007/s10340-016-0756-5
Hardin JA, Kraus DA, Burrack HJ (2015) Diet quality mitigates intraspecific larval competition in Drosophila suzukii. Entomol Exp Appl 156:59–65. https://doi.org/10.1111/eea.12311
Hauser M (2011) A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag Sci 67:1352–1357. https://doi.org/10.1002/ps.2265
Higham JP, Hebets EA (2013) An introduction to multimodal communication. Behav Ecol Sociobiol 67:1381–1388. https://doi.org/10.1007/s00265-013-1590-x
Hill PSM, Mazzoni V, Narins P et al (2019) Quo vadis, biotremology? In: Hill PSM, Lakes-Harlan R, Mazzoni V et al (eds) Biotremology: studying vibrational behavior. Springer, Cham. https://doi.org/10.1007/978-3-030-22293-2_1
Hughes NK, Kelley JL, Banks PB (2012) Dangerous liaisons: the predation risks of receiving social signals. Ecol Lett 15:1326–1339. https://doi.org/10.1111/j.1461-0248.2012.01856.x
Kanzawa T (1939) Studies on Drosophila suzukii mats. Yamanashi Agricultural Experimental Station, Kofu
Kenis M, Tonina L, Eschen R et al (2016) Non-crop plants used as hosts by Drosophila suzukii in Europe. J Pest Sci 89:735–748. https://doi.org/10.1007/s10340-016-0755-6
Kidera H, Takahashi KH (2020) Chemical cues from competitors change the oviposition preference of Drosophila suzukii. Entomol Exp Appl 168:304–310. https://doi.org/10.1111/eea.12889
Knapp L, Mazzi D, Finger R (2021) The economic impact of Drosophila suzukii: perceived costs and revenue losses of Swiss cherry, plum and grape growers. Pest Manag Sci 77:978–1000. https://doi.org/10.1002/ps.6110
Kollasch AM, Abdul-Kafi AR, Body MJA et al (2020) Leaf vibrations produced by chewing provide a consistent acoustic target for plant recognition of herbivores. Oecologia 194:1–13. https://doi.org/10.1007/s00442-020-04672-2
Kraft LJ, Yeh DA, Gómez MI, Burrack HJ (2020) Determining the effect of postharvest cold storage treatment on the survival of immature Drosophila suzukii (Diptera: Drosophilidae) in small fruits. J Econ Entomol 113:2427–2435. https://doi.org/10.1093/jee/toaa185
Laumann RA, Čokl A, Lopes APS et al (2011) Silent singers are not safe: selective response of a parasitoid to substrate-borne vibratory signals of stink bugs. Anim Behav 82:1175–1183. https://doi.org/10.1016/j.anbehav.2011.08.017
Lee JC, Dreves AJ, Cave AM et al (2015) Infestation of wild and ornamental noncrop fruits by Drosophila suzukii (Diptera: Drosophilidae). Ann Entomol Soc Am 108:117–129. https://doi.org/10.1093/aesa/sau014
Lee JC, Wang X, Daane KM et al (2019) Biological control of spotted-wing Drosophila (Diptera: Drosophilidae)-current and pending tactics. J Integr Pest Manag 10:13. https://doi.org/10.1093/jipm/pmz012
Liu H, Lee SH, Chahl JS (2017) A review of recent sensing technologies to detect invertebrates on crops. Precis Agric 18:635–666. https://doi.org/10.1007/s11119-016-9473-6
Mamiya A, Gurung P, Tuthill JC (2018) Neural coding of leg proprioception in Drosophila. Neuron 100:636–650. https://doi.org/10.1016/j.neuron.2018.09.009
Mankin RW, Shuman D, Coffelt JA (1997) Acoustic counting of adult insects with differing rates and intensities of sound production in stored wheat. J Econ Entomol 90(4):1032–1038. https://doi.org/10.1093/jee/90.4.1032
Mankin RW, Weaver DK, Grieshop M et al (2004) Acoustic system for insect detection in plant stems: comparisons of cephus cinctus in wheat and Metamasius callizona in bromeliads. J Agric Urban Entomol 21:239–248
Mankin RW, Hodges RD, Nagle HT et al (2010) Acoustic indicators for targeted detection of stored product and Urban insect pests by inexpensive infrared, acoustic, and vibrational detection of movement. J Econ Entomol 103:1636–1646. https://doi.org/10.1603/EC10126
Mankin RW, Hagstrum DW, Smith MT et al (2011) Perspective and promise: a century of insect acoustic detection and monitoring. Am Entomol 57:30–44. https://doi.org/10.1093/ae/57.1.30
Mazzoni V, Anfora G, Virant-Doberlet M (2013) Substrate vibrations during courtship in three Drosophila species. PLoS ONE 8:1–8. https://doi.org/10.1371/journal.pone.0080708
McKelvey EGZ, Gyles JP, Michie K et al (2021) Drosophila females receive male substrate-borne signals through specific leg neurons during courtship. Curr Biol 31:3894–3904. https://doi.org/10.1016/j.cub.2021.06.002
Mostafa M, Amor AI, Admane N et al (2021) Reduction of post-harvest injuries caused by Drosophila suzukii in some cultivars of sweet cherries using a high carbon dioxide level and cold storage. InSects 12:1–10. https://doi.org/10.3390/insects12111009
Ng’oma E, King EG, Middleton KM (2018) A model-based high throughput method for fecundity estimation in fruit fly studies. Fly (austin) 12:183–190. https://doi.org/10.1080/19336934.2018.1562267
Nieri R, Anfora G, Mazzoni V, Stacconi MVR (2022) Semiochemicals, semiophysicals and their integration for the development of innovative multi-modal systems for agricultural pests’ monitoring and control. Entomol Generalis 42:167–183. https://doi.org/10.1127/entomologia/2021/1236
Oberst S, Bann G, Lai JCS, Evans TA (2017) Cryptic termites avoid predatory ants by eavesdropping on vibrational cues from their footsteps. Ecol Lett 20:212–221. https://doi.org/10.1111/ele.12727
Oberst S, Lai LCS, Evans TA (2019) Physical basis of vibrational behavior: channel properties, noise and excitation signal extraction. In: Hill PSM, Lakes-Harlan R, Mazzoni V et al (eds) Biotremology: studying vibrational behavior. Springer, Cham. https://doi.org/10.1007/978-3-030-22293-2_5
Olazcuaga L, Rode NO, Foucaud J, Facon B, Ravigné V, Ausset A, Leménager N, Loiseau A, Gautier M, Estoup A, Hufbauer R (2019) Oviposition preference and larval performance of Drosophila suzukii (Diptera: Drosophilidae), spotted-wing Drosophila: effects of fruit identity and composition. Environ Entomol 48:867–881. https://doi.org/10.1093/ee/nvz062
Ouantar M, Anfora G, Bouharoud R, Chebli B (2020) First report of Drosophila suzukii (Diptera: Drosophiladae) in North Africa. Moroccan J Agric Sci 1:277–279
Patel KK, Kar A, Jha SN, Khan MA (2012) Machine vision system: a tool for quality inspection of food and agricultural products. J Food Sci Technol 49:123–141. https://doi.org/10.1007/s13197-011-0321-4
Peake TM (2005) Eavesdropping in communication networks. In: McGregor PK (ed) Animal communication networks. Cambridge University Press, Cambridge, pp 13–37. https://doi.org/10.1017/CBO9780511610363.004
Pearson TC, Cetin AE, Tewfik AH, Haff RP (2007) Feasibility of impact-acoustic emissions for detection of damaged wheat kernels. Digit Signal Process: A Rev J 17:617–633. https://doi.org/10.1016/j.dsp.2005.08.002
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ramsay (James O) JO, Silverman BW (2005) Functional data analysis. Springer, Berlin
Rossi Stacconi MV, Buffington M, Daane KM et al (2015) Host stage preference, efficacy and fecundity of parasitoids attacking Drosophila suzukii in newly invaded areas. Biol Control 84:28–35. https://doi.org/10.1016/j.biocontrol.2015.02.003
Rossi-Stacconi MV, Wang X, Stout A et al (2022) Methods for rearing the parasitoid Ganaspis brasiliensis, a promising biological control agent for the invasive Drosophila suzukii. JoVE. https://doi.org/10.3791/63898
Ryan GD, Emiljanowicz L, Wilkinson F et al (2016) Thermal tolerances of the spotted-wing drosophila Drosophila suzukii (Diptera: Drosophilidae). J Econ Entomol 109:746–752. https://doi.org/10.1093/jee/tow006
Sallabanks R, Courtney SP (1992) Frugivory, seed predation, and insect-vertebrate interactions. Annu Rev Entomol 37:377–400. https://doi.org/10.1146/annurev.en.37.010192.002113
Strauß J, Stritih-Peljhan N, Nieri R et al (2021) Communication by substrate-borne mechanical waves in insects: from basic to applied biotremology. In: Advances in insect physiology. Academic Press Inc., pp 189–307. https://doi.org/10.1016/bs.aiip.2021.08.002
Šturm R, Rexhepi B, López Díez JJ et al (2021) Hay meadow vibroscape and interactions within insect vibrational community. iScience 24:1–17. https://doi.org/10.1016/j.isci.2021.103070
Tait G, Park K, Nieri R et al (2020) Reproductive site selection: evidence of an oviposition cue in a highly adaptive dipteran, Drosophila suzukii (diptera: drosophilidae). Environ Entomol 49:355–363. https://doi.org/10.1093/EE/NVAA005
Tait G, Mermer S, Stockton D et al (2021) Drosophila suzukii (Diptera: Drosophilidae): a decade of research toward a sustainable integrated pest management program. J Econ Entomol 114:1950–1974. https://doi.org/10.1093/jee/toab158
Takanashi T, Fukaya M, Nakamuta K et al (2016) Substrate vibrations mediate behavioral responses via femoral chordotonal organs in a cerambycid beetle. Zool Lett 2:1–7. https://doi.org/10.1186/s40851-016-0053-4
Tochen S, Dalton DT, Wiman N et al (2014) Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ Entomol 43:501–510. https://doi.org/10.1603/EN13200
Tochen S, Woltz JM, Dalton DT et al (2016) Humidity affects populations of Drosophila suzukii (Diptera: Drosophilidae) in blueberry. J Appl Entomol 140:47–57. https://doi.org/10.1111/jen.12247
Turchen LM, Cosme L, Yack JE, Guedes RNC (2022) What’s shaking for caterpillars? Leaf-borne vibratory stimuli and behavioral responses in the fall armyworm, Spodoptera frugiperda. J Pest Sci. https://doi.org/10.1007/s10340-022-01496-2
Van Timmeren S, Diepenbrock LM, Bertone MA et al (2017) A filter method for improved monitoring of Drosophila suzukii (Diptera: Drosophilidae) larvae in fruit. J Integr Pest Manag 8:23. https://doi.org/10.1093/jipm/pmx019
Vet LEM, Bakker K (1985) A comparative functional approach to the host detection behavior of parasitic wasps. 2. A quantitative study on eight eucoilid species. Oikos. https://doi.org/10.2307/3565790
Vick KW, Webb JC, Weaver BA, Litzkow C (1988) Sound detection of stored-product insects that feed inside kernels of grain. J Econ Entomol 81:1489–1493. https://doi.org/10.1093/jee/81.5.1489
Virant-Doberlet M, King RA, Polajnar J, Symondson WOC (2011) Molecular diagnostics reveal spiders that exploit prey vibrational signals used in sexual communication. Mol Ecol 20:2204–2216. https://doi.org/10.1111/j.1365-294X.2011.05038.x
Virant-Doberlet M, Stritih-Peljhan N, Žunič-Kosi A, Polajnar J (2023) Functional diversity of vibrational signaling systems in insects. Annu Rev Entomol 68:191–210. https://doi.org/10.1146/annurev-ento-120220
Waithe D, Rennert P, Brostow G, Piper MDW (2015) QuantiFly: robust trainable software for automated Drosophila egg counting. PLoS ONE 10:1–16. https://doi.org/10.1371/journal.pone.0127659
Walse SS, Krugner R, Tebbets JS (2012) Postharvest treatment of strawberries with methyl bromide to control spotted wing drosophila, Drosophila suzukii. J Asia Pac Entomol 15:451–456. https://doi.org/10.1016/j.aspen.2012.05.003
Wang X, Nance AH, Jones JML et al (2018) Aspects of the biology and reproductive strategy of two Asian larval parasitoids evaluated for classical biological control of Drosophila suzukii. Biol Control 121:58–65. https://doi.org/10.1016/j.biocontrol.2018.02.010
Wang D, Zhang M, Mujumdar AS, Yu D (2021) Advanced detection techniques using artificial intelligence in processing of berries. Food Eng Rev 14:176–199. https://doi.org/10.1007/s12393-021-09298-5
Wilson A (2008) Insect frugivore interactions: the potential for beneficial and neutral effects on host plants. Doctoral dissertation, Queensland University of Technology. https://eprints.qut.edu.au/17023/
Winkler A, Jung J, Kleinhenz B, Racca P (2020) A review on temperature and humidity effects on Drosophila suzukii population dynamics. Agric for Entomol 22:179–192. https://doi.org/10.1111/afe.12381
Winkler A, Jung J, Kleinhenz B, Racca P (2021) Estimating temperature effects on Drosophila suzukii life cycle parameters. Agric for Entomol 23:361–377. https://doi.org/10.1111/afe.12438
Woltz JM, Lee JC (2017) Pupation behavior and larval and pupal biocontrol of Drosophila suzukii in the field. Biol Control 110:62–69. https://doi.org/10.1016/j.biocontrol.2017.04.007
Yack JE, Yadav C (2022) Vibratory sensing and communication in caterpillars. In: Hill PSM, Mazzoni V, Strith-Peljhan N et al (eds) Biotremology: physiology, ecology, and evolution. Springer, Cham, pp 471–491. https://doi.org/10.1007/978-3-030-97419-0_19
Yeh DA, Drummond FA, Gómez MI, Fan X (2020) The Economic impacts and management of spotted wing Drosophila (Drosophila Suzukii): the case of wild blueberries in maine. J Econ Entomol 113:1262–1269. https://doi.org/10.1093/jee/toz360
Zhang JT, Liang X (2014) One-way ANOVA for functional data via globalizing the pointwise F-test. Scand J Stat 41:51–71. https://doi.org/10.1111/sjos.12025
Zorović M, Čokl A (2015) Laser vibrometry as a diagnostic tool for detecting wood-boring beetle larvae. J Pest Sci 88:107–112. https://doi.org/10.1007/s10340-014-0567-5
Funding
Open access funding provided by Fondazione Edmund Mach - Istituto Agrario di San Michele all'Adige within the CRUI-CARE Agreement. The study was supported by the SWAT Project (Fondazione Edmund Mach with the contribution of the Autonomous Province of Trento). LF PhD fellowship is funded by Fondazione Edmund Mach, the University of Trento, Laimburg Research Center and Sant’Orsola s.c.a.. RN is supported by the co-financing of the European Union—FSE-REACT-EU, PON Research and Innovation 2014–2020 DM1062/2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Tim Haye.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fellin, L., Bertagnolli, G., Mazzoni, V. et al. Detection and characterization of incidental vibrations from Drosophila suzukii in infested fruits. J Pest Sci (2024). https://doi.org/10.1007/s10340-023-01711-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10340-023-01711-8