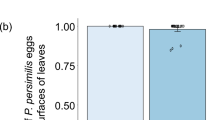
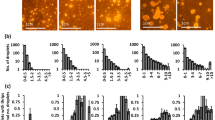
Abstract
Grapevine moths are severe pest insects in European viticulture. Oviposition by grapevine moths is largely influenced by several physical and chemical cues located on the surface of their host plant’s fruits. The contribution of waxy bloom layer on the berry surface for oviposition decision of two European grapevine moth species, Eupoecilia ambiguella and Lobesia botrana, was investigated. An experimental setup was developed to prove oviposition preferences of both species for certain grape varieties and developmental stages based on epicuticular wax extracts. Chemical analysis of epicuticular wax patterns of four different Vitis vinifera varieties revealed differences. However, oleanolic acid was the main component on berry surface waxes and its relative amount decreased between early and late phenological stages. Furthermore, oleanolic acid was responsible for the preference of earlier phenological stages for E. ambiguella oviposition. However, ovipositional variety preferences were triggered by minor components on the wax berry layer. While the oviposition decision of L. botrana was mainly triggered by oleanolic acid, additional cues like olfactory and haptic ones were also important. The ovipositional preferences were discussed in accordance with the results of the chemical analysis in order to elucidate the role of wax compounds for oviposition stimulation.






Similar content being viewed by others
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253. https://doi.org/10.1111/j.1541-0420.2005.00440.x
Anfora G, Tasin M, Bäckmann AC, Leonardelli E, De Cristofaro A, Lucchi A, Ioriatti C (2008) Olfactory responses of Eupoecilia ambiguella (Hübner) (Lepidoptera Tortricidae) females to volatiles from grapevine. IOBC/wprs Bull 36:233–236
Baker EA, Hunt GM, Stevens PJG (1983) Studies of plant cuticle and spray droplet interactions: a fresh approach. Pestic Sci 14:645–658. https://doi.org/10.1002/ps.2780140613
Bianchi G, Murelli C, Vlahov G (1992) Surface waxes from olive fruits. Phytochemistry 31:3503–3506. https://doi.org/10.1016/0031-9422(92)83716-C
Bovey P (1966) Superfamille des Tortricoidea. L’Eudémis de la vigne, vol 2. Entomologie appliquée à l’agriculture. Masson et Cie, Paris
Breiman L (2001) Random forests. Mach Learn 45:5–32. https://doi.org/10.1023/a:1010933404324
Brooks JS, Williams EH, Feeny P (1996) Quantification of contact oviposition stimulants for black swallowtail butterfly, Papilio polyxenes, on the leaf surfaces of wild carrot, Daucus carota. J Chem Ecol 22:2341–2357. https://doi.org/10.1007/BF02029551
Brückner A, Heethoff M (2017) A chemo-ecologists’ practical guide to compositional data analysis. Chemoecology 27:33–46. https://doi.org/10.1007/s00049-016-0227-8
Casado CG, Heredia A (2001) Ultrastructure of the cuticle during growth of the grape berry (Vitis vinifera). Physiol Plant 111:220–224. https://doi.org/10.1034/j.1399-3054.2001.1110213.x
Comménil P, Belingheri L, Audran JC, Collas A, Dehorter B (1996) Anti-botrytis activity in epicuticular waxes of young grape berries of Vitis vinifera (Pinot noir). J Int Sci Vigne Vin 30:7. https://doi.org/10.20870/oeno-one.1996.30.1.1113
Comménil P, Brunet L, Audran JC (1997) The development of the grape berry cuticle in relation to susceptibility to bunch rot disease. J Exp Bot 48:1599–1607. https://doi.org/10.1093/jxb/48.8.1599
Cozzi G, Pascale M, Perrone G, Visconti A, Logrieco A (2006) Effect of Lobesia botrana damages on black aspergilli rot and ochratoxin A content in grapes. Int J Food Microbiol 111:S88–S92. https://doi.org/10.1016/j.ijfoodmicro.2006.03.012
Fermaud M, Giboulot A (1992) Influence of Lobesia botrana larvae on field severity of Botrytis rot of grape berries. Plant Dis 76:404–409. https://doi.org/10.1094/PD-76-0404
Fermaud M, Le Menn R (1992) Transmission of Botrytis cinerea to grapes by grape berry moth larvae. Phytopathology 82:1393–1398. https://doi.org/10.1094/Phyto-82-1393
Gabel B, Thiéry D (1996) Oviposition response of Lobesia botrana females to long-chain free fatty acids and esters from its eggs. J Chem Ecol 22:161–171. https://doi.org/10.1007/bf02040207
Gripenberg S, Mayhew PJ, Parnell M, Roslin T (2010) A meta-analysis of preference–performance relationships in phytophagous insects. Ecol Lett 13:383–393. https://doi.org/10.1111/j.1461-0248.2009.01433.x
Gross J, Gündermann G (2016) Principles of IPM in cultivated crops and implementation of innovative strategies for sustainable plant protection. In: Horowitz A, Ishaaya I (eds) Advances in insect control and resistance management. Springer, Cham, pp 9–26
Harari AR, Zahavi T, Gordon D, Anshelevich L, Harel M, Ovadia S, Dunkelblum E (2007) Pest management programmes in vineyards using male mating disruption. Pest Manag Sci 63:769–775. https://doi.org/10.1002/ps.1365
Ioriatti C, Anfora G, Tasin M, De Cristofaro A, Witzgall P, Lucchi A (2011) Chemical Ecology and Management of Lobesia botrana (Lepidoptera: Tortricidae). J Econ Entomol 104:1125–1137. https://doi.org/10.1603/ec10443
Jetter R, Kunst L, Samuels AL (2006) Composition of plant cuticular waxes. In: Riederer M, Müller C (eds) Annual plant reviews biology of the plant cuticle, vol 23. Blackwell, Oxford, pp 145–181
Juma G et al (2016) Influence of host-plant surface chemicals on the oviposition of the cereal stemborer busseola fusca. J Chem Ecol 42:394–403. https://doi.org/10.1007/s10886-016-0704-0
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:1–33. https://doi.org/10.18637/jss.v069.i01
Li G, Ishikawa Y (2006) Leaf epicuticular wax chemicals of the Japanese knotweed Fallopia japonica as oviposition stimulants for Ostrinia latipennis. J Chem Ecol 32:595–604. https://doi.org/10.1007/s10886-005-9022-7
Liaw A, Wiener M (2002) Classification and regression by randomForest. R News 2:18–22
Lorenz DH, Eichhorn KW, Blei-Holder H, Klose R, Meier U, Weber E (1994) Phänologische Entwicklungsstadien der Weinrebe (Vitis vinifera L. ssp.). Vitic Enol Sci 49:66–70
Maher N, Thiéry D (2003) Bunch extracts of Vitis vinifera at different development stages stimulate or deter oviposition in Lobesia botrana females. IOBC/wprs Bull 26:135–149
Maher N, Thiéry D (2004a) A bioassay to evaluate the activity of chemical stimuli from grape berries on the oviposition of Lobesia botrana (Lepidoptera: Tortricidae). Bull Entomol Res 94:27–33. https://doi.org/10.1079/ber2003276
Maher N, Thiéry D (2004b) Distribution of chemo- and mechanoreceptors on the tarsi and ovipositor of female European grapevine moth, Lobesia botrana. Entomol Exp Appl 110:135–143. https://doi.org/10.1111/j.0013-8703.2004.00131.x
Maher N, Thiéry D, Städler E (2006) Oviposition by Lobesia botrana is stimulated by sugars detected by contact chemoreceptors. Physiol Entomol 31:14–22. https://doi.org/10.1111/j.1365-3032.2005.00476.x
Marchal P (1912) Rapport sur les travaux acomplis par la mission dÕetudes de la Cochylis et de lÕEudemis. Libr. Polythec. Paris et Liège, Paris
Markheiser A, Rid M, Biancu S, Gross J, Hoffmann C (2018) Physical factors influencing the oviposition behaviour of European grapevine moths Lobesia botrana and Eupoecilia ambiguella. J Appl Entomol 142:201–210. https://doi.org/10.1111/jen.12423
Martín-Vertedor D, Ferrero-García JJ, Torres-Vila LM (2010) Global warming affects phenology and voltinism of Lobesia botrana in Spain. Agric For Entomol 12:169–176. https://doi.org/10.1111/j.1461-9563.2009.00465.x
Meier U et al (2009) The BBCH system to coding the phenological growth stages of plants–history and publications. Journal für Kulturpflanzen 61:41–52
Mondy N, Corio-Costet MF (2000) The response of the grape berry moth (Lobesia botrana) to a dietary phytopathogenic fungus (Botrytis cinerea): the significance of fungus sterols. J Insect Physiol 46:1557–1564. https://doi.org/10.1016/s0022-1910(00)00085-8
Mondy N, Charrier B, Fermaud M, Pracros P, Corio-Costet MF (1998) Mutualism between a phytopathogenic fungus (Botrytis cinerea) and a vineyard pest (Lobesia botrana). Positive effects on insect development and oviposition behaviour. C R Acad Sci Paris Sci e la vie Life Sci 321:665–671. https://doi.org/10.1016/s0764-4469(98)80006-1
Moreau J, Benrey B, Thiéry D (2006) Grape variety affects larval performance and also female reproductive performance of the European grapevine moth Lobesia botrana (Lepidoptera: Tortricidae). Bull Entomol Res 96:205–212. https://doi.org/10.1079/ber2005417
Moreau J, Monceau K, Thiéry D (2016) Larval food influences temporal oviposition and egg quality traits in females of Lobesia botrana. J Pest Sci 89:439–448. https://doi.org/10.1007/s10340-015-0695-6
Müller C (2006) Plant–insect interactions on cuticular surfaces. In: Riederer M, Müller C (eds) Annual plant reviews biology of the plant cuticle, vol 23. Blackwell, Oxford, pp 398–417
Müller C, Hilker M (2001) Host finding and oviposition behavior in a chrysomelid specialist—the importance of host plant surface waxes. J Chem Ecol 27:985–994. https://doi.org/10.1023/a:1010343205114
Müller C, Riederer M (2005) Plant surface properties in chemical ecology. J Chem Ecol 31:2621–2651. https://doi.org/10.1007/s10886-005-7617-7
Oksanen J et al (2017) ‘vegan’. Community Ecology Package. R package version 2.4-3. https://CRAN.R-project.org/package=vegan
Palliotti A, Cartechini A (2001) Developmental changes in gas exchange activity in flowers, berries, and tendrils of field-grown Cabernet Sauvignon. Am J Enol Vitic 52:317–323
Pensec F et al (2014) Changes in the triterpenoid content of cuticular waxes during fruit ripening of eight grape (Vitis vinifera) cultivars grown in the upper rhine valley. J Agric Food Chem 62:7998–8007. https://doi.org/10.1021/jf502033s
Possingham JV, Chambers TC, Radler F, Grncarevic M (1967) Cuticular transpiration and wax structure and composition of leaves and fruit of Vitis vinifera. Aust J Biol Sci 20:1149–1154
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org
Radler F (1965) The surface waxes of the sultana vine (Vitis vinifera cv. Thompson Seedless). Aust J Biol Sci 18:1045–1056
Radler F, Horn D (1965) The composition of grape cuticle wax. Aust J Chem 18:1059–1069
Ramaswamy SB (1988) Host finding by moths: sensory modalities and behaviours. J Insect Physiol 34:235–249
Reineke A, Thiéry D (2016) Grapevine insect pests and their natural enemies in the age of global warming. J Pest Sci 89:313–328. https://doi.org/10.1007/s10340-016-0761-8
Savopoulou-Soultani M, Tzanakakis ME (1988) Development of Lobesia botrana (Lepidoptera: Tortricidae) on Grapes and Apples Infected with the Fungus Botrytis cinerea. Environ Entomol 17:1–6. https://doi.org/10.1093/ee/17.1.1
Savopoulou-Soultani M, Stavridis DG, Tzanakakis ME (1990) Development and reproduction of Lobesia botrana on vine and olive inflorescences. Entomol Hell 8:29–36
Schoonhoven LM, van Loon JJA, Dicke M (2005) Insect–plant biology, 2nd edn. Oxford University Press, New York
Sharon R, Zahavi T, Soroker V, Harari AR (2009) The effect of grape vine cultivars on Lobesia botrana (Lepidoptera: Tortricidae) population levels. J Pest Sci 82:187–193. https://doi.org/10.1007/s10340-008-0238-5
Shin KH, Choi IM, Chung KH, Lee JM, Park HS (2010) Morphological development and chemical composition of epicuticular wax crystals in ‘Campbell Early’ grape. Hortic Environ Biotechnol 51(4):253–256
Silva-Moreno E, Brito-Echeverría J, López M, Ríos J, Balic I, Campos-Vargas R, Polanco R (2016) Effect of cuticular waxes compounds from table grapes on growth, germination and gene expression in Botrytis cinerea. World J Microbiol Biotechnol 32:74
Sprute D, Greif A, Gross J, Hoffmann C, Rid M, König M (2016) Schädlingsmonitoring des Traubenwicklers durch Auswertung einer Motten-Eiablage-Karte mittels Smartphone-Anwendung. GIL Jahrestagung 2016:201–204
Städler E (2002) Plant chemical cues important for egg deposition by herbivorous insects. In: Hilker M, Meiners T (eds) Chemoecology of insect eggs and egg deposition. Blackwell Publishing, Berlin, pp 171–197
Svobodová E, Trnka M, Dubrovský M, Semerádová D, Eitzinger J, Štěpánek P, Žalud Z (2014) Determination of areas with the most significant shift in persistence of pests in Europe under climate change. Pest Manag Sci 70:708–715. https://doi.org/10.1002/ps.3622
Tasin M et al (2005) Antennal and behavioral responses of grapevine moth Lobesia botrana females to volatiles from grapevine. J Chem Ecol 31:77–87. https://doi.org/10.1007/s10886-005-0975-3
Tasin M, Anfora G, Leonardelli E, Ioriatti C, Lucchi A, De Cristofaro A, Pertot I (2009) A bioassay-based approach for the evaluation of host-plant cues as oviposition stimuli in grapevine moth. IOBC/wprs Bull 41:83–86
Tasin M, Lucchi A, Ioriatti C, Mraihi M, De Cristofaro A, Boger Z, Anfora G (2011) Oviposition response of the moth Lobesia botrana to sensory cues from a host plant. Chem Senses 36:633–639. https://doi.org/10.1093/chemse/bjr027
Tasin M, Knudsen GK, Pertot I (2012) Smelling a diseased host: grapevine moth responses to healthy and fungus-infected grapes. Anim Behav 83:555–562. https://doi.org/10.1016/j.anbehav.2011.12.003
Thiéry D, Moreau J (2005) Relative performance of European grapevine moth (Lobesia botrana) on grapes and other hosts. Oecologia 143:548–557. https://doi.org/10.1007/s00442-005-0022-7
Thiéry D, Monceau K, Moreau J (2014) Different emergence phenology of European grapevine moth (Lobesia botrana, Lepidoptera: Tortricidae) on six varieties of grapes. Bull Entomol Res 104(3):277–287. https://doi.org/10.1017/S000748531300031X
Udayagiri S, Mason CE (1997) Epicuticular wax chemicals in Zea mays influence oviposition in Ostrinia nubilalis. J Chem Ecol 23:1675–1687. https://doi.org/10.1023/B:JOEC.0000006443.72203.f7
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Witzgall P, Kirsch P, Cork A (2010) Sex pheromones and their impact on pest management. J Chem Ecol 36:80–100. https://doi.org/10.1007/s10886-009-9737-y
Acknowledgements
We thank Svenja Stein and Sandra Schubach (JKI, Dossenheim, Germany) for excellent laboratory assistance. We are grateful to Adrian Brückner (Technical University of Darmstadt, Germany) and Doreen Gabriel (JKI, Braunschweig, Germany) for statistical advices. We thank Sandra Biancu and Claudia Vogel (JKI, Siebeldingen, Germany) for cultivation of insects. The authors thank Patricia Mohr (Keyence GmbH, Neu-Isenburg, Germany) for providing the digital microscope free of charge. MR and AM were supported by funds of the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) under the innovation support Program Number 2814701611.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. G. Becher.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Chromatograms of berry wax extracts from V. vinifera ‘Regent’ at BBCH 77 (above) and BBCH 89 (bottom, mirrored) obtained after derivatization and GC-FID analysis. IS = Internal standard = Tetracosane, OA = Oleanolic acid, MA = Montanic acid. (TIFF 37 kb)
Rights and permissions
About this article
Cite this article
Rid, M., Markheiser, A., Hoffmann, C. et al. Waxy bloom on grape berry surface is one important factor for oviposition of European grapevine moths. J Pest Sci 91, 1225–1239 (2018). https://doi.org/10.1007/s10340-018-0988-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-018-0988-7