Abstract
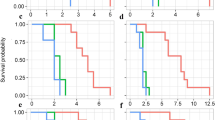
The European grapevine moth, Lobesia botrana is a major grapevine pest, but despite the abundance of vineyards it is a generalist and uses either grapes or alternative species. Given the abundance and predictability of grape, L. botrana could be expected to have evolved towards monophagy. In order to understand why this species remains polyphagous, we hypothesized that larvae reared on rare wild host plants should have higher fitness than those reared on the more abundant grape host. For this, we compared larval performance and several life history traits on three alternative host plants (Daphne gnidium, Olea europaea, Tanacetum vulgare) and three Vitaceae (Vitis vinifera), two cultivars and one wild species (Ampelopsis brevipedunculata), and two control groups raised on either a low or a high nutritive value medium. Alternative hosts are more suitable than Vitaceae for the reproductive performance of L. botrana: larval mortality and development time was reduced, while pupal weight, growth rate, female longevity, female fecundity, duration of laying and mating success were increased. High quality food ingested by larvae promotes higher adult body weight and enhances female reproductive output. This suggests that alternative hosts provide greater nutritional value for L. botrana than Vitaceae. The use of alternative host plants could thus be maintained in the host range because they offer L. botrana a better fitness than on the Vitaceae. This could typically represent an advantage for moths behaving in plant diversity grape landscapes.


Similar content being viewed by others
References
Balachowsky A, Mesnil L (1935) Les Insectes Nuisibles à la vigne, Polychrosis botrana Schiff. (Lepid. Tortricidae) In: Les insectes nuisibles aux plantes Cultivées, leurs mœurs, leur destruction, vol 1, Paris, pp 677–686
Barbosa P (1988) Some thoughts on the evolution of host range. Ecology 69:912–915
Benrey B, Denno RF (1997) The slow growth-high mortality hypothesis: a test using the cabbage butterfly. Ecology 78:987–999
Bernays EA (1989) Host range in phytophagous insects: the potential role of generalist predators. Evol Ecol 3:299–311
Bernays EA, Graham M (1988) On the evolution of host specificity in phytophagous arthropods. Ecology 69:886–892
Bernays EA, Wcislo WT (1994) Sensory capabilities, information processing, and resource specialization. Q Rev Biol 69:187–204
Bovey P (1966) Super-famille des Tortricoidea. In: Balachowsky AS (ed) Entomologie Appliquée à l’Agriculture. Vol 2 Lépidoptères, Masson et Cie, Paris, pp 456–893
Bulmer MG (1983) Models for the evolution of protandry in insects. J Theor Biol 35:195–206
Camara MD (1997) A recent host range expansion in Junonia coenia Hubner (Nymphalidae): oviposition preference, survival, growth, and chemical defense. Evolution 51:873–884
Cates RG (1980) Feeding patterns of monophagous, oligophagous, and polyphagous insect herbivores: the effect of resource abundance and plant chemistry. Oecologia 46:22–31
Courtney SP, Chen GK, Gardner A (1989) A general model for individual host selection. Oikos 55:55–65
Dall SRX, Cuthill IC (1997) The information costs of generalism. Oikos 80:197–202
Damman H (1987) Leaf quality and enemy avoidance by the larvae of a pyralid moth. Ecology 68:88–97
Dethier VG (1970) Chemical interactions between plants and insects. In: Sondheimer E, Simeone JB (eds) Chemical ecology. Academic, New York, pp 83–102
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Eichhorn KW, Lorenz DH (1977) Phonologische Entwicklumggsstadien der Rebe Nachrichtenbl. Pflanzenschutzd (Braunschweig) 29:119–120
Fagerström T, Wiklund C (1982) Why do males emerge before females? Protandry as a mating strategy in male and female butterflies. Oecologia 52:164–166
Feeny P (1976) Plant apparency and chemical defense. In: Wallace JW, Mansell RL (eds) Biochemical interactions between plants and insects. Recent Advances in Phytochemistry. Plenum, New York, pp 1–40
Gabel B (1995) Tansy flowers attract European grapevine moth females, Lobesia botrana den. & Schiff. (Lep. Tortricidae). J Appl Entomol 113:153–158
Gabel B, Roehrich R (1995) Sensitivity of grapevine phenological stages to larvae of European grapevine moth, Lobesia botrana Den. et Schiff. (Lep., Tortricidae). J Appl Entomol 119:127–130
Gabel B, Thiéry D (1994) Non-host plant odor (Tanacetum vulgare; Asteracea) affects the reproductive behaviour of Lobesia botrana Den. et Schiff. (Lepidoptera Tortricidae). J Insect Behav 7:149–157
Häggström H, Larsson S (1995) Slow larval growth on a suboptimal willow results in high predation mortality in the leaf beetle Galerucella lineola. Oecologia 104:308–315
Haukioja E, Neuvonen S (1985) Induced long-term resistance of birch foliage against defoliators: defensive or incidental? Ecology 66:1303–1308
Jaenike J (1978) On optimal oviposition behaviour in phytophagous insects. Theor Popul Biol 14:350–356
Jaenike J (1990) Host specialization in phytophagous insects. Annu Rev Ecol Syst 21:243–273
Janzen DH (1985) A host is more than its chemistry. Illinois Nat Hist Surv Bull 33:141–175
Kaspi R, Mossinson S, Drezner T, Kamensky B, Yuval B (2002) Effects of larval diet on development rates and reproductive maturation of male and female Mediterranean fruit flies. Physiol Entomol 27:29–38
Keese MC (1997) Does escape to enemy-free space explain host specialization in two closely related leaf-feeding beetles (Coleoptera: Chrysomelidae)? Oecologia. DOI 10.1007/s004420050286
Landolt PJ, Phillips TW (1997) Host plant influences on sex pheromone behaviour of phytophagous insects. Annu Rev Entomol 42:371–391
Lustner G (1914) Das Verhalten der Raupen des ein-und zweibindingen Traubenwicklers zu den Weinbergskraüten und anderen Pflanzen. Z Weinbau Weinbehandl 1:3–35
Maher N, Toulouse ME, Jolivet J, Thiéry D (2000) Oviposition preference of the European grape vine moth, Lobesia botrana (Lepidoptera: Tortricidae) for host and non host plants present in Bordeaux area. IOBC/wprs Bull 23(4):131–134
Marchal P (1912) Mission d’étude de la Cochylis et de l‘Eudemis pendant l’année 1911. Librairie Polytechnique. Paris
Nylin S, Wiklund C, Wickman PO, Garcia-Barros E (1993) Absence of trade-offs between sexual size dimorphism and early male emergence in a butterfly. Ecology 74:1414–1427
Ohsaki N, Sato Y (1994) Food plant choice of Pieris butterflies as a trade-off between parasitoids avoidance and quality of plants. Ecology 75:59–68
Parry D, Spence JR, Volney WJA (1998) Budbreak phenology and natural enemies mediate survival of first-instar forest tent caterpillar (Lepidoptera: Lasiocampidae). Environ Entomol 27:1368–1374
Rausher MD (1985) Variability for host preference in insect populations: mechanistic and evolutionary models. J Insect Physiol 31:873–889
Ringo J (1996) Sexual receptivity in insects. Annu Rev Entomol 41:473–494
Roditakis NE (1988) Factors affecting population size of grapes berry moth Lobesia botrana Den. & Schiff. in Crete. In: Cavalloro R (ed) Influence of environmental factors on the control of grape pests diseases and weeds. ECC publications, Balkema, Amsterdam, pp 69–76
Roehrich R, Boller E (1991) Tortricids in vineyards. In: Van der Gesst LPS, Evenhuis HH (eds) Tortricid pests, their biology natural enemies and control. Amsterdam, Elsevier, pp 507–514
Savopoulou-Soultani M, Tzanakakis ME (1987) Comparison of olive flowers with vine flowers and leaves as food for larva of Lobesia botrana. In: Cavalloro R (ed) Influence of environmental factors on the control of grape pest diseases and weeds. Commision of the European Communities, Rotterdam, pp 63–67
Savopoulou-Soultani M, Tzanakakis ME (1988) Development of Lobesia botrana (Lepidoptera: Tortricidae) on grapes and apples infected with the fungus Botrytis cinerea. Environ Entomol 17:1–6
Savopoulou-Soultani M, Stavridis DG, Tzanakakis ME (1990) Development and reproduction of Lobesia botrana on vine and olive inflorescences. Entomologia Hellenica 8:29–35
Savopoulou-Soultani M, Stavridis DG, Vassiliou A, Stafilidis JE, Iraklidis I (1994) Response of Lobesia botrana (Lepidoptera: Tortricidae) to levels of sugar and protein in artificial diets. J Econ Entomol 87:84–90
Schultz JC (1988) Many factors influence the evolution of herbivore diets, but plant chemistry is central. Ecology 69:896–897
Singer MC (1982) Sexual selection for small size in male butterflies. Am Nat 119:440–443
Slansky F (1976) Phagism relationship among butterflies. J N Y Entomol S 84:91–105
Southwood TRE (1972) The insect/plant relationship—an evolutionary perspective. Symp R Entomol Soc Lond 6:3–30
Stoeva R (1982) Les hôtes de la teigne bariolée des vignes Lobesia botrana Schiff en Bulgarie. Horticult Viticult Sci 19:83–89
Strong DL, Lawton JH, Southwood R (1984) Insects on plants. Harvard University Press, Cambridge
Swain T (1977) Secondary plant compounds as protective agents. Annu Rev Plant Physiol 42:55–302
Thiéry D (2005) Les vers de la grappe. Guide Pratique, Vigne & Vins Publ. Int. (in press)
Thiéry D, Xuéreb A (2003) Relative abundance of several larval parasitoids of Lobesia botrana on different varieties of grapes. IOBC/wprs Bull 26:135–139
Thiéry D, Xuéreb A, Villemant C, Sentenac G, Delbac L, Kuntzman P (2001) Larval parasites of vineyards tortricids: a brief overview from 3 french vine growing areas. IOBC/wprs bull 24:13–142
Thomas AW (1989) Food consumption and utilization by 6th-instar larvae of spruce budworm, Choristoneura fumiferana: a comparison on three Picea (spruce) species. Entomol Exp Appl 52:205–214
Torres-Vila LM, Stockel J, Roehrich R (1995) Le potentiel reproducteur et ses variable biotiques associées chez le mâle de l’Eudémis de la vigne Lobesia botrana. Entomol Exp Appl 77:105–119
Torres-Vila LM, Rodriguez-Molina MC, Roehrich R, Stockel J (1999) Vine phenological stage during larval feeding affects male and female reproductive output of Lobesia botrana (Lepidoptera: Tortricidae). Bull Entomol Res 89:549–556
Weseloh RM, Andreadis TG, Moore REB, Andersson JF, Dubois NR, Lewis FB (1983) Field confirmation of a mechanism causing synergism between Bacillus thuringiensis and the gypsy moth parasitoid, Apanteles melanoscelus. J Invertebr Pathol 41:99–103
West SA, Cunningham JP (2002) A general model for host plant selection in phytophagous insects. J Theor Biol 214:499–513
Wiklund C, Fagerström T (1977) Why do males emerge before females? Oecologia 31:153–158
Wiklund C, Kaitala A, Lindfors V, Abenius J (1993) Polyandry and its effect on female reproduction in the green-veined white butterfly (Pieris napi L.). Behav Ecol Sociobiol 33:25–33
Acknowledgements
We thank X. Arruego, C. Couranjou and E. Richard for their experimental contribution. Authors are indebted to Drs R. Naisbit, B. Benrey and D. Bailey for valuable discussions and comments on the earlier version of manuscript and to E. Haine for language correction. The second author was supported by a Swiss national Foundation grant NCCR. All experiments described in this paper were done in France according to the rules of the ethical board for animal experiments complying with the current laws of this country.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Christian Koerner.
Rights and permissions
About this article
Cite this article
Thiéry, D., Moreau, J. Relative performance of European grapevine moth (Lobesia botrana) on grapes and other hosts. Oecologia 143, 548–557 (2005). https://doi.org/10.1007/s00442-005-0022-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0022-7