Abstract
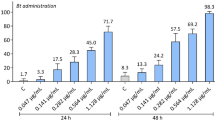
Metarhizium spp. (Hypocreales: Clavicipitaceae) are used as alternatives to hazardous pesticides. During host infection, they secrete secondary metabolites such as destruxin A. Information on the fate of those secondary metabolites in the food chain and their risk to human and animal health is scarce. In the present work, predator–prey bioassays were performed to evaluate the behavior and survival of green lacewing Chrysoperla carnea (Neuroptera: Chrysopidae) larvae when presented with armyworm Spodoptera littoralis (Lepidoptera: Noctuidae) larvae treated with Metarhizium brunneum BIPESCO5 and EAMa 01/58-Su strains. Moreover, ecotoxicological studies were done using HPLC–MS to monitor the fate of destruxin A in the prey–predator system. HPLC–MS confirmed the presence of destruxin A at low concentrations in S. littoralis larvae infected with BIPESCO5 (approximately 0.014 μg/g) and EAMa 01/58-Su (approx 0.031 μg/g) strains, whereas the metabolite was not detected in C. carnea larvae consuming M. brunneum-treated S. littoralis larvae. Furthermore, C. carnea larvae preferentially fed on healthy prey versus M. brunneum-treated prey as revealed by both the higher predator ratio feeding on S. littoralis control larvae and the higher per capita number of control larvae consumed by the predator compared to M. brunneum-treated larvae. Moreover, this preference was inversely related to the post-inoculation period of S. littoralis larvae treated with M. brunneum. Furthermore, C. carnea larvae fed on healthy prey gained more weight than those fed on treated individuals. Both M. brunneum treatments used against S. littoralis larvae may be considered low risk to C. carnea due to the lack of fungus-related mortality in the predator and the lack of movement of destruxin A from the prey to the predator. However, further studies on other nontargets and with more strains of M. brunneum are needed to evaluate their possible simultaneous use in integrated pest management.



Similar content being viewed by others
References
Anderson RD, Bell SA, Blanford S, Paaijmans PK, Thomas MB (2011) Comparative growths kinetics and virulence of four different isolates of entomopathogenic fungi in the house fly (Musca domestica L.). J Invertebr Pathol 107:179–184
Babendreier D, Jeanneret P, Pilz C, Toepfer S (2015) Non-target effects of insecticides, entomopathogenic fungi and nematodes applied against western corn rootworm larvae in maize. J Appl Entomol 139:457–467
Baverstock J, Roy HE, Pell JK (2010) Entomopathogenic fungi and insect behaviour: from unsuspecting hosts to targeted vectors. Biocontrol 55:89–102
Bischoff JF, Rehner SA, Humber RA (2009) A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 101:512–530
Campbell CL, Madden LV (1990) Introduction to plant disease epidemiology. Wiley, New York, p 532
Carpio A, Arroyo-Manzanares N, Ríos-Moreno A, Garrido-Jurado I, Gamiz-Gracia L, García-Campaña AM, Quesada-Moraga E, Arce L (2016) Development of a QuEChERS-based extraction method for the determination of destruxins in potato plants by UHPLC-MS/MS. Talanta 146:815–822
de Castro TR, Saldarriaga-Ausique JJ, Nunes DH, Ibanhes FH, Delalibera J (2013) Risk assessment of Cry toxins of Bacillus thuringiensis on the predatory mites Euseius concordis and Neoseiulus californicus (Acari: Phytoseiidae). Exp Appl Acarol 59:421–433
Dutton A, Klein H, Romeis J, Bigler F (2002) Uptake of Bt-toxin by herbivores feeding on transgenic maize and consequences for the predator Chrysoperla carnea. Ecol Entomol 27:441–447
Dutton A, Romeis J, Bigler F (2003) Assessing the risk of insect resistant transgenic plants on entomophagous arthropods: Bt-maize expressing Cry1AB as a case study. Biocontrol 48:611–636
Eilenberg J, Zimmermann G, Butt TM, Jung K, Nielsen C, Strasser H, Typas M (2008) The fascinating true story about the famous Metarhizium anisopliae isolate Ma43, alias ATCC 90448, alias BIPESCO 5, alias F52 alias. In: 41th annual meeting of the society for invertebrate pathology and 9th international conference on Bacillus thuringiensis. University of Warwick, Coventry, UK
Favilla M, Macchia L, Gallo A, Altomare C (2006) Toxicity assessment of metabolites of fungal biocontrol agents using two different (Artemia salina and Daphnia magna) invertebrate bioassays. Food Chem Toxicol 44:1922–1931
Garrido-Jurado I, Ruano F, Campos M, Quesada-Moraga E (2011) Effects of soil treatments with entomopathogenic fungi on soil dwelling non-target arthropods at a commercial olive orchard. Biol Control 59:239–244
Garrido-Jurado I, Resquín-Romero G, Amarilla SP, Ríos-Moreno A, Carrasco L, Quesada-Moraga E (2016) Transient endophytic colonization of melon plants by entomopathogenic fungi foliar applications for the control of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). J Pest Sci 90:319–330
Gontijo PC, Moscardini VF, Michaud JP, Carvalho GA (2014) Non-target effects of chlorantraniliprole and thiamethoxam on Chrysoperla carnea when employed as sunflower seed treatments. J Pest Sci 87:711–719
Hu QB, Ren SX, An XC, Qian MH (2007) Insecticidal activity influence of destruxins on the pathogenicity of Paecilomyces javanicus against Spodoptera litura. J Appl Entomol 131:262–268
Hu QB, An XC, Jin FL, Freed S, Ren SX (2009) Toxicities of destruxins against Bemisia tabaci and its natural enemy, Serangium japonicum. Toxicon 53:115–121
Hung SY, Boucias DG (1992) Influence of Beauveria bassiana on the cellular defense response of the beet armyworm, Spodoptera exigua. J Invertebr Pathol 60:152–158
Kaur HP, Singh B, Thakur A, Kaur A, Kaur S (2015) Studies on immunomodulatory effect of endophytic fungus Alternaria alternata on Spodoptera litura. J Asia Pac Entomol 18:67–75
Kershaw MJ, Moorhouse ER, Bateman R, Reynolds SE, Charnley AK (1999) The role of destruxins in the pathogenicity of Metarhizium anisopliae for three species of insect. J Invertebr Pathol 74:213–223
Kodaira Y (1961) Studies on the New Toxic Substances to Insects, Destruxin A and B produced by Oospora destructor. Agr Biol Chem 26:36–42
Mesquita ALM, Lawrence AL (2001) Interactions among the entomopathogenic fungus, Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes), the parasitoid, Aphelinus asychis (Hymenoptera: Aphelinidae), and their aphid host. Biol Control 22:51–59
Meyling NV, Hajek AE (2010) Principles from community and metapopulation ecology: application to fungal entomopathogens. Biocontrol 55:39–54
Mudgal S, De Toni A, Tostivint C, Hokkanen H, Chandler D (2013) Scientific support, literature review and data collection and analysis for risk assessment on microbial organisms used as active substance in plant protection products—Lot 1 environmental risk characterization. EFSA supporting publication. 2013:EN-518
Obrist LB, Dutton A, Romeis J, Bigler F (2006) Biological activity of Cry1AB toxin expressed by Bt maize following ingestion by herbivorous arthropods and exposure of the predator Chrysoperla carnea. Biocontrol 51:31–84
Pedras MSC, Zaharia LI, Ward DE (2002) The destruxins: synthesis, biosynthesis, biotransformation, and biological activity. Phytochemistry 59:579–596
Pourian HR, Talaei-Hassanloui A, Kosari AA, Ashouri A (2011) Effects of Metarhizium anisopliae on searching, feeding and predation by Orius albidipennis (Hem., Anthocoridae) on Thrips tabaci (Thy., Thripidae) larvae. Biocontrol Sci Technol 21:15–21
Quesada-Moraga E, Ruiz-García A, Santiago-Álvarez C (2006) Laboratory evaluation of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against puparia and adult of Ceratitis capitata (Diptera: Tephritidae). J Econ Entomol 99:1955–1966
Rännbäck LM, Cotes B, Anderson P, Rämert B, Meyling N (2015) Mortality risk from entomopathogenic fungi affects oviposition behavior in the parasitoid wasp Trybliographa rapae. J Invertebr Pathol 124:78–86
Resquín-Romero G, Garrido-Jurado I, Delso C, Ríos-Moreno A, Quesada-Moraga E (2016) Transient endophytic colonizations of plants improve the outcome of foliar applications of micoinsecticides against chewing insect. J Invertebr Pathol 136:23–31
Ríos-Moreno A, Carpio A, Garrido-Jurado I, Arroyo-Manzanares N, Lozano-Tovar MD, Arce L, Gámiz-Gracia L, García-Campaña AM, Quesada-Moraga E (2016a) Production of destruxins by Metarhizium strains under different stress conditions and their detection by using UHPLC-MS/MS. Biocontrol Sci Technol 26:1298–1311
Ríos-Moreno A, Garrido-Jurado I, Resquín-Romero G, Arroyo-Manzanares N, Arce L, Quesada-Moraga E (2016b) Destruxin A production by Metarhizium brunneum strains during transient endophytic colonization of Solanum tuberosum L. Biocontrol Sci Technol 26:574–1585
Ríos-Moreno A, Quesada-Moraga E, Garrido-Jurado I (2017) Quantification of fungal growth and destruxin A during infection of Galleria mellonella larvae by Metarhizium brunneum. J Invertebr Pathol. doi:10.1016/j.jip.2017.06.007
Rohlfs M, Churchill ACL (2011) Fungal secondary metabolites as modulators of interactions with insects and other arthropods. Fungal Genet Biol 48:23–34
Roy HE, Pell JK (2000) Interactions between entomopathogenic fungi and other natural enemies: implications for biological control. Biocontrol Sci Techn 10:737–752
Santiago-Álvarez C (1977) Virus de insectos: multiplicación, aislamiento y bioensayo de baculovirus, 43rd edn. Fundación Juan March. Serie Universitaria, Madrid
Schrank A, Vainstein MH (2010) Metarhizium anisopliae enzymes and toxins. Toxicon 56:1267–1274
Seiedy M, Saboori A, Allahyari H, Talaei-Hassanloui R, Tork M (2012) Functional response of Phytoseiulus persimilis (Acari: Phytoseiidae) on untreated and Beauveria bassiana treated adults of Tetranychus urticae (Acari: Tetranychidae). J Insect Behav 25:543–553
Shrestha G, Enkegaard A (2013) The green lacewing, Chrysoperla carnea: preference between lettuce aphids, Nasonovia ribisnigri, and western flower thrips, Frankliniella occidentalis. J Insect Sci 13:94
Shrestha G, Enkegaard A, Reddy VP, Skovgárd Steenberg T (2017) Susceptibility of larvae and pupae of the aphid parasitoid Aphelinus abdominalis (Hymenoptera: Aphelinidae) to the entomopathogenic fungus Beauveria bassiana. Ann Entomol Soc Am 110:121–127
Skrobek A, Butt TM (2005) Toxicity testing of destruxins and crude extracts from the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol Lett 251:23–28
Skrobek A, Shah FA, Butt TM (2008) Destruxin production by the entomogenous fungus Metarhizium anisopliae in insects and factors influencing their degradation. Biocontrol 53:361–373
Sowjanya KS, Padmajal V, Murthy LN (2008) Insecticidal activity of destruxin, a mycotoxin from Metarhizium anisopliae (Hypocreales), against Spodoptera litura (Lepidoptera: Noctuidae) larval stages. Pest Manag Sci 64:119–125
Strasser H, Hutwimmer S, Burgstaller W (2011) Metabolite toxicology of fungal biocontrol agents. In: Ehlers R-U (ed) Regulation of biological control agents. Springer, Dordrecht, pp 191–213
Strasser H, Vey A, Butt TM (2000) Are there any risks in using entomopathogenic fungi for pest control, with particular reference to the bioactive metabolites of Metarhizium, Tolypocladium and Beauveria species? Biocontrol Sci Technol 10:717–735
Strauch O, Strasser H, Hauschild R, Ehlers R-U (2011) Proposals for bacterial and fungal biocontrol agents. In: Ehlers R-U (ed) Regulation of biological control agents. Springer, Dordrecht, pp 267–288
Stroup WW (2012) Generalized linear mixed models: modern concepts, methods and applications. CRC Press, Boca Raton, p 555
Taibon J, Sturm S, Seger C, Strasser H, Stuppner H (2015) Quantitative assessment of destruxins from strawberry and maize in the lower parts per billion range: combination of a QuEChERS-based extraction protocol with a fast and selective UHPLC-QTOF-MS assay. J Agric Food Chem 63:5707–5713
Tauber MJ, Tauber CA, Danne KM, Hagen KS (2000) Commercialization of predators: recent lessons from green lacewings (Neuroptera: Chrysopidae: Chrysoperla). Am Entomol 46:26–38
Thungrabeab M, Tongma S (2007) Effect of entomopathogenic fungi, Beauveria bassiana (Balsam) and Metarhizium anisopliae (Metsch) on non target insects. KMITL Sci Technol 7:8–12
Traugott M, Weissteiner S, Strasser H (2005) Effects of the entomopathogenic fungus Beauveria brongniartii on the non-target predator Poecilus versicolor (Coleoptera: Carabidae). Biol Control 33:107–112
Villani MG, Allee LL, Preston-Wilsey L, Consolie N, Xia Y, Brandenburg RL (2002) Use of radiography and tunnel castings for observing mole cricket (Orthoptera: Gryllotalpidae) behavior in soil. Am Entomol 48:42–50
Wang Ch, Skrobek A, Butt TM (2004) Investigations on the destruxin production of the entomopathogenic fungus Metarhizium anisopliae. J Invertebr Pathol 85:168–174
Wang S, Fang W, Wang Ch, St Leger RJ (2011) insertion of an esterase gene into a specific locust pathogen (Metarhizium acridum) enables it to infect caterpillars. PLoS Pathog 7:e1002097
Yousef M, Lozano-Tovar MD, Garrido-Jurado I, Quesada-Moraga E (2013) Biocontrol of Batrocera oleae (Dipetera: Tephritidae) with Metarhizium anisopliae and its extracts. J Econ Entomol 106:1118–1125
Zimmermann G (2007) Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci Technol 17:879–920
Acknowledgements
Ríos-Moreno gratefully acknowledges “SENACYT and IFARHU from Panama” for a doctoral grant. We thank the Central Service of Research Support from the University of Cordoba (SCAI) for analyses of destruxin A.
Funding information
A grant from the European Community’s Seventh Framework Program (FP7-ENV.2011.3.1.9-1 ECO-INNOVATION, INBIOSOIL, Grant Agreement No. 282767) and a grant from the Ministerio de Economía y Competitividad, Spain, Project AGL 2016-80483-R, supported this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Communicated by Michael Traugott.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ríos-Moreno, A., Quesada-Moraga, E. & Garrido-Jurado, I. Treatments with Metarhizium brunneum BIPESCO5 and EAMa 01/58-Su strains (Ascomycota: Hypocreales) are low risk for the generalist predator Chrysoperla carnea . J Pest Sci 91, 385–394 (2018). https://doi.org/10.1007/s10340-017-0905-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-017-0905-5