Abstract
The asymmetric flow field-flow fractionation (AF4) method was developed for cationic cellulose derivatives. AF4 is the method of choice especially for high-molar mass samples, which are challenging to characterize with conventional chromatographic techniques such as size-exclusion chromatography (SEC). The cationic charge of macromolecules also complicates the size-based separations where no interaction between the analytes and the column stationary phase (SEC) or membrane (AF4) should occur. However, many column matrices and membranes carry negative charge and thus preventing interactions between cationic analytes and negatively charged separation support should be taken into consideration when doing method development. In this study, two eluent compositions, neutral and acidic, were tested for AF4 separation of cationic hydroxyethyl celluloses with varying charge densities. The eluent composition with a pH below the isoelectric point of regenerated cellulose membrane, which was used in this AF4 study, enabled the size-based separation with close to 100% analysis recovery. Macromolecular parameters (molar mass and radius of gyration) and conformation were investigated by coupling a multi-angle light scattering detector and differential refractometer to the AF4 system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cationic polymers are used in various applications such as carriers of genetic material (gene delivery), constituents in cosmetics and hair care solutions, and as flocculants in waste-water purification, to name a few [1]. Currently, there is a growing demand for replacement of synthetic petroleum-based polymers with sustainable, biobased polymers. The properties and functionality of biopolymers can be altered by chemical, physical, or enzymatic modification. Adding charged groups to the polysaccharide backbone is one good example from structural tailoring. Reports on the cationization can be found for several polysaccharides, but most of the work on the cationization of polysaccharides has been conducted on starch and cellulose [1,2,3]. Both starch and cellulose have a high molar mass, which is required for certain applications. For example, only high-molar mass cationic polymers act as efficient flocculants in waste-water treatments [4]. Thus, determination of macromolecular characteristics, including molar mass, is a prerequisite for evaluation of the functionality of the modified biopolymers.
Macromolecular separation and characterization of cationic (bio)polymers is a challenging task. The most commonly used technique for separation and molar mass determination of polymers is size-exclusion chromatography (SEC). Even though SEC has been widely used for characterization of cationic polymers, the technique has some limitations. In SEC, no enthalpic interactions between the analytes and column packing material should exist. Many commonly used SEC stationary phases, however, carry a negative charge, which might contribute to the unwanted interactions between positively charged analytes and negatively charged column material. Another limitation of SEC is the incapability of the technique to characterize high-molar mass polymers and polysaccharides accurately [5, 6]. SEC has been, however, successfully used for characterization of commercial cationic hydroxyethyl cellulose derivatives [7].
Another technique for the separation of polymers is field-flow fractionation (FFF). FFF has different variants depending on the external field (flow, sedimentation, thermal, electrical), which is used to enhance the separation. Flow FFF and especially asymmetric flow FFF (AF4) is most commonly used for the separation of (bio)polymers [8]. In AF4, the analytes are injected into a thin, open channel where they separate in a parabolic flow based on their diffusion coefficients. The external flow, namely cross-flow, which is perpendicular to the main parabolic flow, enables the separation. Since the smaller molecules diffuse more quickly towards the center of the channel where the flow streams are faster, they elute from the channel earlier than the larger molecules with a lower diffusion coefficient. The AF4 channel consists of a solid top plate and porous bottom plate, which is covered by an ultrafiltration membrane with defined porosity. More information about the AF4 instrumentation and the theory can be found in the following references [9, 10]. Even though both SEC and AF4 can be used for characterization of many (bio)macromolecules, AF4 has some advantages over SEC especially for high-molar mass, charged analytes. First, AF4 is a gentler technique than SEC because separation takes place in an open channel. Second, the surface area in AF4 is around a few tens of square centimeters whereas in SEC the surface area of the porous stationary phase is in the order of the 107 cm2 [11]. Thus, the risk of interactions between charged analytes and the SEC stationary phase is greater in SEC than the risk of having interactions between the analytes and membrane in AF4.
In this study, the AF4 method was developed for cationic hydroxyethyl celluloses (HEC) with varying degrees of cationization. Two different aqueous eluents (NaNO3 solution and acidic NaCl solution) were tested and analysis recovery was monitored to see if the interactions between the charged analytes and membrane occurred. A multi-angle light scattering (MALS) detector allowed the determination of molar mass and radius of gyration (RG) for cationic HEC samples. Conformation information was obtained from the relationship between molar mass and RG across the separated molecular species.
Materials and Methods
Materials and Reagents
The cationic HEC derivatives (quaternary ammonium salt of hydroxyethylcelluloses) with different degrees of cationization were obtained from The Dow Chemical Company (Amerchol Corp., Philadelphia, USA). The trade names for the samples used here are Polymer JR-400 (N% 1.5–2.2), Polymer LR-400 (N% 0.8–1.1), and Polymer LK (N% 0.4–0.6). The nitrogen content indicates the charge density (i.e., cationic group per repeating unit) of the polymers. According to the manufacturer, all samples have similar viscosities, which indicates similarity in molar mass (however, as can be seen from the results of this study; molar mass differences could be detected due to differences in the lengths of the branches). Sodium nitrate and sodium chloride were purchased from Merck (Darmstadt, Germany).
Determination of Refractive Index Increment (∂n/∂c)
Specific refractive index increment (∂n/∂c) values for the cationic HEC samples were determined offline using a T-rEX differential refractometer (vacuum wavelength of light λ0 = 658 nm, Wyatt Technology Co., Santa Barbara, USA). Samples were dissolved in eluent (0.8 M NaNO3 or 0.135 M NaCl in 0.012 M HNO3) at five concentrations of approximately 0.5, 0.75, 1, 1.5, and 2 mg mL−1, and each solution was injected directly into the refractometer cell using disposable 1 mL syringes. The temperature of the refractometer was set to 30 °C. ASTRA software (Wyatt Technology Co.) was used for data collection and processing. The ∂n/∂c values obtained from the slope of a plot of concentration versus differential refractive index are presented in Fig. 1 and Table 1.
Refractive index increment (∂n/∂c) values for cationic hydroxyethylcelluloses (HEC) with varying degree of cationization (black solid line for the sample with N% 1.5–2.2, green dotted line for sample with N% 0.8–1.1, and red dashed line for the sample with N% 0.4–0.6). Measurements were performed in acidic aqueous solution (0.135 M NaCl in 0.012 M HNO3)
AF4 Experiments
FFF experiments were carried out using an AF2000 MT instrument (including software, Postnova Analytics, Landsberg/Lech, Germany) equipped with MALS (Brookhaven Instruments Corporation, Holtsville, NY, USA), and differential refractive index, DRI (PN 3150, Postnova Analytics) detectors. The MALS detector contains a 30 mW laser as the light source operating at λ0 = 660 nm with seven scattering angles (35°, 50°, 75°, 90°, 105°, 130°, 145°). Two eluents were tested in the FFF analyses: 0.8 M NaNO3 and 0.135 M NaCl in 0.012 M HNO3. These two eluents were chosen based on earlier studies on the macromolecular characterization of cationic polymers: 0.8 M NaNO3 was used successfully for size-exclusion chromatographic analyses of cationic hydroxyethylcellulose derivatives and nitric acid-based eluent for flow-FFF analysis of polyvinylpyridine [7, 11]. A regenerated cellulose (RC) membrane with the cut-off value of 10 kg mol−1 and spacer (trapezoidal shape with tip-to-tip length of 28 cm and cross-sectional width at focusing zone of 2 cm) with a thickness of 350 µm were used in a separation channel. The detector flow rate was 1 mL min−1. The DRI and MALS detectors were calibrated and normalized according to instructions from Postnova Analytics (2009) using the bovine serum albumin and polystyrene sodium sulfonate standards. The injection volume was 100 µL. An exponentially decaying cross-flow gradient was used (Fig. 2) and the focusing time was 5 min. The samples were dissolved in the eluent at a concentration of ~ 1 mg mL−1. Debye formalism was used for fitting of AF4 data.
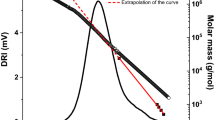
Fractograms (LS signal at 90°) of cationic HEC with N% 1.5–2.2 analyzed using 0.8 M NaNO3 (red line) and 0.135 M NaCl in 0.012 M HNO3 (black, dotted line) as eluent. Exponentially decaying cross-flow gradient (exponent 0.2) was used in the AF4 analyses (dashed grey line). Open symbols represent molar mass (red squares for data obtained using eluent of 0.8 M NaNO3 and black triangles for data obtained using eluent of 0.135 M NaCl in 0.012 M HNO3)
Results and Discussion
Comparison of Two Eluents for AF4 Separation of Cationic HEC Samples
Eluent composition plays an important role in the separation of cationic macromolecules. The SEC column stationary phases and AF4 membrane materials commonly carry a weak negative charge. To mask the anionic sites at the column packing material or at the surface of the AF4 membrane, mobile phases with relatively high salt content are used to reduce the possible ionic interactions between the analytes and the stationary phase/membrane. Thus, we decided to test 0.8 M NaNO3, which has been successfully used for SEC separation of cationic celluloses [7]. The other eluent tested was acidic salt solution (0.135 M NaCl in 0.012 M HNO3, pH ~ 2). In acidic conditions (below pH of 3.4 which is the isoelectric point for the RC membrane), the RC membrane is weakly positively charged [12, 13]. The repulsion between the positively charged membrane and cationic analytes likely prevents unwanted interactions between the membrane and the cationic HEC molecules.
The overlay of fractograms (light scattering signal at 90 °C) obtained with two eluent conditions for cationic HEC with nitrogen content of 1.5–2.2% is presented in Fig. 2. As can be seen from both fractograms, elution within 20 min could be achieved using the exponentially decaying cross-flow gradient. Molar masses across the peaks are also presented in Fig. 2. In the case of acidic eluent, the molecules elute according to the AF4 principles (e.g., smaller molecules with higher diffusion coefficient elutes before the larger molecules with lower diffusion coefficients). The elution of sample in 0.8 M NaNO3, however, seemed to be biased. The elution order of molecules for most of the peak was opposite to that observed in acidic eluent (and opposite to what is suggested by the AF4 theory). In addition, molar mass across the peak was higher in 0.8 M NaNO3 than in acidic eluent, indicating that the cationic HEC sample was not dissolved as the level of individual molecules but existed in the form of aggregates. The intensity difference of light scattering signals shown in Fig. 2 also indicates the difference in the molar masses of the sample analyzed in two eluent conditions. The AF4 analysis recovery was determined based on the refractive index signals and measured ∂n/∂c values. The recovery in the 0.8 M NaNO3 eluent was low at 17%, whereas recoveries for all the samples in acidic eluent were close to 100% (Table 1). Based on these AF4 trials on the two eluents, the acidic eluent was proven to be superior for the characterization of cationic HEC samples.
Molar Mass and Size of Cationic HEC with Different Charge Densities
To obtain reliable molar mass information, ∂n/∂c values for all samples dissolved in acidic eluent (0.135 M NaCl in 0.012 M HNO3) were measured by off-line refractometry. As can be seen in Fig. 1 and Table 1, the ∂n/∂c values for samples varied from 0.132 to 0.141 mL g−1. This range is typical for polysaccharides in aqueous solution [14, 15]. No clear correlation between the degree of cationization and ∂n/∂c was observed. For comparison, ∂n/∂c of the HEC sample with a nitrogen content of 1.5–2.2% dissolved in 0.8 M NaNO3 was measured but eluent composition had no effect on the measured ∂n/∂c values (similar ∂n/∂c values of 0.141 for both eluents).
Molar masses across the DRI signals and radii of gyration across the light scattering signal (at 90°) for all cationic HEC samples are presented in Fig. 3. As can be seen in the figure, separation for all samples follows the elution order predicted by AF4 theory. The molar mass averages in Table 1 reveal that molar mass increases with increasing degree of cationization. This increase in molar mass is most likely due to the presence of long-chain hydroxyethyl chains in the samples with a higher degree of cationization (please see next section for further discussion). In addition, the radius of gyration (RG) increases with increasing molar mass (Table 1).
Differential refractive index (DRI) signals and molar masses (a) and light scattering signals at 90° (LS 90°) and radius of gyration (b) for hydroxyethyl cellulose samples with varying degrees of cationization. Red lines and symbols represent the sample with N% of 0.4–0.6, green represents the sample with N% of 0.8–1.1 and black for the sample with the highest N% of 1.5–2.2
Conformation of Cationic HEC
Information regarding conformation of the macromolecules can be obtained by plotting the logarithm of the radius of gyration against the logarithm of molar mass. Depending on the shape of the curves, one or more straight lines can be fitted to the data points, and slopes of these straight lines reveal conformational and even structural information of the samples. As can be seen in Fig. 4, the slopes of the straight lines fitted to different samples differ from each other. For samples with the lowest charge densities (N% 0.4–0.6 and N% 0.8–1.1), the slopes for the molecular population below ~ 500 kg mol−1 are 0.57 and 0.49, respectively. Values close to 0.5 are typical for random coils in solution [16]. The slope for the sample with the highest charge density (N% 1.5–2.2) is, however, lower (0.38) compared to the other two samples. A slope of 0.38 indicates a compact solution conformation and cannot solely be explained by the higher number of cationic groups. In the etherification reaction of celluloses, the hydroxyl groups of glucose units are replaced by the hydroxyethyl groups, which can undergo further elongation resulting in side chains with varying lengths [17]. Longer side chains might explain the low slope of the conformation plot, which is a common indication of the presence of long-chain branching in the macromolecular sample [16]. In the high molar mass region (> 500 kg mol−1), the conformation plots start to bend, which again indicates the more compact conformation of these high-molar mass and probably long-chain branched species.
Conformation plots for cationic HEC samples with varying slopes. Color coding is identical to Fig. 3
Conclusions
AF4 is a separation technique especially for high-molar mass macromolecules which are challenging to separate using conventional chromatographic techniques. In this study, the AF4 method was developed for cationic hydroxyethyl cellulose (quaternary ammonium salt of hydroxyethyl celluloses) samples with varying degrees of cationization. The eluent compositions tested were: 0.8 M NaNO3 and 0.135 M NaCl in 0.012 M HNO3. The separation in the neutral eluent resulted in poor sample recovery, which was likely due to the unwanted interactions between the cationic analytes and membrane carrying a negative charge. In addition, the separation behavior clearly deviated from what was expected based on the AF4 retention theory. In acidic eluent (pH below 3.4), the regenerated cellulose membrane carries a weak positive charge, which seemed to prevent the interactions between the membrane and cationic molecules. The analysis recovery in acidic eluent was near 100% for all the samples and molecules eluted in the order suggested by AF4 theory (molecules with higher translational diffusion coefficients elutes before the molecules with lower diffusion coefficients). MALS/DRI detection allowed determination of molar mass, size, and conformation of the cationic hydroxyethyl celluloses. Interestingly, the conformation plots suggested that the sample with the highest nitrogen content contains elongated side chains in contrast to the samples with lower nitrogen content. The methodology presented here can be used for separation and characterization of other cationic polysaccharides, which might have functions in several applications such as acting as flocculating agents in waste-water purification.
References
Prado HJ, Matulewicz MC (2014) Cationization of polysaccharides: a path to greener derivatives with many industrial applications. Eur Polym J 52:53–75
Krentz D, Lohmann C, Schwarz S, Bratskaya S, Liebert T, Laube J, Heinze T, Kulicke W (2006) Properties and flocculation efficiency of highly cationized starch derivatives. Starch/Stärke 58:161–169
Parviainen H, Hiltunen M, Maunu SL (2014) Preparation and flocculation behavior of cellulose-g-PMOTAC copolymer. J Appl Polym Sci 131:40448
Levine S, Friesen WI (1987) In: Attia YA (ed) Flocculation in biotechnology and separation science. Elsevier, Amsterdam
Striegel AM, Yau WW, Kirkland JJ, Bly DD (2009) Modern size-exclusion liquid chromatography. Practice of gel permeation and gel filtration chromatography. Wiley, New York
Striegel AM, Isenberg SL, Cote GL (2009) An SEC/MALS study of alternan degradation during size-exclusion chromatographic analysis. Anal Bioanal Chem 394:1887–1893
Liu XM, Gao W, Maziarz EP, Salamone JC, Duex J, Xia E (2006) Detailed characterization of cationic hydroxyethylcellulose derivatives using aqueous size-exclusion chromatography with on-line triple detection. J Chromatogr A 1104:145–153
Messaud FA, Sanderson RD, Runyon JR, Otte T, Pasch H, Williams SKR (2009) An overview on field-flow fractionation techniques and their applications in the separation and characterization of polymers. Prog Polym Sci 34:351–368
Giddings JC, Yang FJ, Myers MN (1976) Theoretical and experimental characterization of flow field-flow fractionation. Anal Chem 48:1126–1132
Wahlund K (2013) Flow field-flow fractionation: critical overview. J Chromatogr A 1287:97–112
Benincasa MA, Giddings JC (1997) Separation and characterization of cationic, anionic, and nonionic water-soluble polymers by flow FFF: sample recovery, overloading, and ionic strength effects. J Microcolumn Sep 9:479–495
Pontié M (1999) Effect of aging on UF membranes by a streaming potential (SP) method. J Membr Sci 154:213–220
Wagner M, Pietsch C, Tauhardt L, Schallon A, Schubert US (2014) Characterization of cationic polymers by asymmetric flow field-flow fractionation and multi-angle light scattering—a comparison with traditional techniques. J Chromatogr A 1325:195–203
Theisen A, Johann C, Deacon MP, Harding SE (2000) Refractive increment data-book. Nottingham University Press, Nottingham
Terada E, Samoshina Y, Nylander T, Lindman B (2004) Adsorption of cationic cellulose derivatives/anionic surfactant complexes onto solid surfaces. I. Silica surfaces. Langmuir 20:1753–1762
Burchard W (1999) Solution properties of branched macromolecules. Adv Polym Sci 143:113–194
Abdel-Halim ES (2014) Chemical modification of cellulose extracted from sugarcane bagasse: preparation of hydroxyethyl cellulose. Arab J Chem 7:362–371
Acknowledgements
Open access funding provided by Aalto University. The authors would like to express their gratitude to Vladimir Aseyev for guidance with refractive index increment analyses. Magnus Ehrnroot Foundation is also acknowledged for funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Human and animal rights statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pitkänen, L., Tenkanen, M. Field-Flow Fractionation of Cationic Cellulose Derivatives. Chromatographia 82, 1827–1832 (2019). https://doi.org/10.1007/s10337-019-03800-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03800-2