Abstract
Impacts of global climate and land‐use changes on distribution patterns and breeding sites remain today poorly studied for several vulnerable emblematic bird species, including the Golden Eagle (Aquila chrysaetos). Herein, we analyzed the potential effect of global climate changes and agricultural activities on the distribution patterns of this top predator across Mexico. We assessed the long-term role of protected areas (PAs) for safeguarding the species’ overall distribution and its breeding sites. We evaluated current and future (2040s, 2060s, and 2080s) threats from global change using ecological niche modeling and geographic information system approaches to determine the percentage of the species’ distribution area that overlaps with highly human-modified areas and PAs under each climate scenario. We also used niche overlap tests to assess whether the species’ breeding sites show equivalence or similarity of climatic conditions over time. Our findings revealed shifts in the Golden Eagle’s distributional area, with an overall size reduction (by ~ 57% in the 2040s and ~ 78% in the 2080s) due to future environmental changes, mainly attributable to increasingly dry and warm conditions. Mexican PAs cover ~ 12% of the Golden Eagle’s range across country, but this decreased by > 33% on average under the species’ future distributions. Although the hypothesis of equivalent climatic conditions at breeding sites over time was rejected, those sites did have long-term climate similarity (niche overlap: 0.75–0.83; P < 0.05). Considering the species’ nest site fidelity and that colonization of new areas within Mexico seems unlikely, protection of these breeding sites is a critical step for the long-term conservation of this emblematic species in Mexico.
Zusammenfassung
Brutplätze schützen: ein wichtiges Ziel für die Erhaltung des Steinadlers in Mexiko unter den Bedingungen des globalen Wandels
Die Auswirkungen globaler Klima- und Landnutzungsänderungen auf die Verbreitungsmuster und Brutplätze mehrerer gefährdeter, symbolträchtiger Vogelarten, darunter der Steinadler (Aquila chrysaetos), sind bis heute kaum untersucht. In dieser Studie haben wir die potenziellen Auswirkungen globaler Klimaveränderungen und landwirtschaftlicher Aktivitäten auf die Verbreitungsmuster dieses Spitzenprädators in Mexiko untersucht. Wir bewerteten die langfristige Rolle von Schutzgebieten für die Sicherung der Gesamtverbreitung der Art und ihrer Brutplätze. Wir bewerteten aktuelle und zukünftige (2040, 2060 und 2080) Bedrohungen durch den globalen Wandel, indem wir ökologische Nischenmodelle und geografische Informationssysteme einsetzten, um den prozentualen Anteil des Verbreitungsgebiets der Art zu bestimmen, der sich mit stark vom Menschen veränderten Gebieten und Schutzgebieten unter jedem Klimaszenario überschneidet. Außerdem haben wir mit Hilfe von Nischenüberlappungstests untersucht, ob die Brutgebiete der Art im Laufe der Zeit gleichwertige oder ähnliche klimatische Bedingungen aufweisen. Unsere Ergebnisse zeigen, dass sich das Verbreitungsgebiet des Steinadlers aufgrund zukünftiger Umweltveränderungen insgesamt verkleinert (um ca. 57% in den 2040er Jahren und ca. 78% in den 2080er Jahren), was hauptsächlich auf zunehmend trockenere und wärmere Bedingungen zurückzuführen ist. Die mexikanischen Schutzgebiete decken landesweit etwa 12% des Verbreitungsgebiets des Steinadlers ab, doch wird dieser Anteil unter den zukünftigen Verbreitungsgebieten der Art im Durchschnitt um mehr als 33% abnehmen. Auch wenn wir die Hypothese über die Zeit gleichwertiger klimatischer Bedingungen an den Brutplätzen Zeit verwarfen, wiesen diese Standorte eine langfristige Klimaähnlichkeit auf (Nischenüberschneidung: 0,75-0,83; P < 0,05). In Anbetracht der Nistplatztreue der Art und der Tatsache, dass die Besiedlung neuer Gebiete in Mexiko unwahrscheinlich erscheint, ist der Schutz dieser Brutplätze ein entscheidender Schritt für die langfristige Erhaltung dieser emblematischen Art in Mexiko.
Similar content being viewed by others
Introduction
The Golden Eagle (Aquila chrysaetos) is one of the most widely distributed birds of prey and top predators in the world (Kovács et al. 2008; Watson 2010; Katzner et al. 2012). In North America, this species is distributed from Alaska to Central Mexico (Fig. 1) and occurs in disjunct areas in a wide variety of ecosystems, from cliffs at sea level to temperate forests nearly 3000 m s.a.l. (Bravo-Vinaja and Guzmán-Aranda 2014). Globally, it is listed as a species of “Least Concern” by the International Union for Conservation of Nature (https://www.iucnredlist.org/). However, in Mexico the Golden Eagle is restricted to severely fragmented habitats that are continuing to decline in size, connectivity, and quality due to anthropogenic activity (Campos-Rodríguez et al. 2019). It is estimated that the abundance of A. chrysaetos in the country has declined over the past 20 years, a trend that is expected to continue into the future (Bautista et al. 2022). The Golden Eagle is therefore categorized as a threatened and high-priority species at the national level in Mexico (SEMARNAT and CONANP 2008; SEMARNAT 2019). This has led to growing interest in identifying priority sites for their conservation. Despite the increase in knowledge of the distribution of Mexican Golden Eagle population over recent years, details about the combined effects of anthropogenic disturbances and global climate warming on geographic and ecological patterns for this species are scarce (Rodríguez-Estrella 2002; Bravo-Vinaja and Guzmán-Aranda 2014; D’Addario et al. 2019; Campos-Rodríguez et al. 2019).
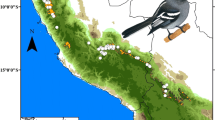
Current potential distribution model of Golden Eagles (Aquila chrysaetos) in North America and Mexico. Note an important reduction (~ 21.22%, lost areas in red) in the predicted potential distribution model when filtered to only include areas of natural forests. Numbers on the maps correspond to the main four protected areas identified within species distribution: Forest Protection Zone and Wildlife Refuge “Valle de los Cirios” (1), the Natural Resources Protection Area–Forest Protection Zone “Don Martín” (2), the Biosphere Reserve “El Vizcaíno” (3), and the Natural Resources Protection Area–Forest Protection Zone “Estado de Nayarit” (4). Dark brown shading shows areas with elevations above 1000 m asl (bird picture provided by Luis F. Lozano) (color figure online)
It is well known that strong synergy between climate change and agriculture-driven land-use change modifies the geographical distribution of suitable conditions for species’ survival, decreases connectivity among populations, and can lead to local or even global extinctions (Jetz et al. 2007; Ceballos and Ehrlich 2018; Lovejoy and Hannah 2019). This is a critical scenario for the Golden Eagle’s long-term conservation at a national scale because Mexico continues to have high annual deforestation rates (see Mendoza-Ponce et al. 2020), at the same time as temperature and precipitation patterns have reportedly changed over the past 100 years (see Cuervo-Robayo et al. 2020). Worryingly, most future estimations of the impact of these factors on Mexican biodiversity in the coming decades are not optimistic; this is the case in birds (e.g., Prieto-Torres et al. 2020, 2021a, b; Sierra-Morales et al. 2021) as well as a wide variety of mammals, amphibians, reptiles, and plants (e.g., Peterson et al. 2002; Ochoa-Ochoa et al. 2012; Ureta et al. 2018; Arenas-Navarro et al. 2020; Mayani-Parás et al. 2021). Moreover, the network of protected areas (PAs) may be less effective for conserving species under future global change scenarios, mainly because they may no longer cover species’ modified future distributions (Hannah et al. 2007; Jones et al. 2018; Maxwell et al. 2020; Prieto-Torres et al. 2020; 2021a). Thus, there is clear evidence that rapid changes in Mexico are quickly restricting our last opportunities for the implementation of effective conservation planning and mitigation policies (Hannah et al. 2007; Bitencourt et al. 2016; Pearson et al. 2019; Law et al. 2021).
Because the Golden Eagle is a territorial species exhibiting strong long-term fidelity to nesting sites (Palmer 1988; Bravo-Vinaja and Guzmán-Aranda 2014), future conservation strategies must give special priority to the long-term protection of breeding areas of this species. It is recognized that the minimum size and climate conditions of the nesting territory are limiting factors for the Golden Eagle abundance; low availability of suitable areas for breeding could cause a decrease in the reproductive rate, lead to nest abandonment, and reduce its population density (Beecham and Kochert 1975; Tavizon 2014; Bautista et al. 2022). Furthermore, the destruction of nests by human activities is currently among the most serious threats to this species’ long-term survival in Mexico (Bravo-Vinaja and Guzmán-Aranda 2014; Tavizon 2014; Campos-Rodríguez et al. 2019; Bautista et al. 2022). Therefore, determining which breeding regions are vulnerable versus stable is a critical first step in the conservation agenda for the Golden Eagle in Mexico. Forecasting the interactive effects of global changes threats, the policy makers may take advantage from selection of climatically reserves to lead optimal conservation actions for species into the future (Hannah et al. 2007; Triviño et al. 2018; Lovejoy and Hannah 2019).
In this study, we evaluate the potential impacts of climate and land-use change on Golden Eagles using ecological niche modeling and multiscale geographic information system (GIS) analyses. Specifically, we aimed to: (a) assess how direct, indirect and additive threats from global change could impact this species’ potential distribution in Mexico, and in particular its breeding sites, in future time periods; and (b) determine the role of the present network of PAs to safeguard both the species’ overall distribution and, specifically, its breeding sites.
Methods
Species records and breeding site information
Observational records of Golden Eagles were obtained from: (1) different scientific collections and online collaborative public databases (i.e., Global Biodiversity Information Facility [GBIF; https://www.gbif.org/], Sistema Nacional de Información sobre Biodiversidad de México [SNIB; https://www.snib.mx/] and eBirds [https://ebird.org/home]); (2) > 10 years of fieldwork (2005–2020) monitoring adults and breeding sites of this species in Mexico performed by Luis F. Lozano working in the National Commission of Protected Areas (CONANP, as abbreviated from its Spanish name); and 3) specialized literature on the biology and distribution of the species (e.g., Janss et al. 1999; Rodríguez-Estrella 2002; Bravo-Vinaja and Guzmán-Aranda 2014; León-Girón et al. 2016; Campos-Rodríguez et al. 2019; Flesch et al. 2020). The GBIF information (2021) was downloaded directly using the “rgbif” library for R software (Chamberlain et al. 2019).
The monitoring program was carried out annually based on (1) linear transects of 100 km along secondary and rough terrain roads, and (2) point sampling technique via observation with binoculars/telescopes for 3–4 h (Rodríguez-Estrella et al. 2020). The monitoring effort ranged from three to ten years by locality. This allowed us to distinguish non-territorial birds (i.e., “floaters”; see Caro et al. 2011) from breeding individuals, pairs, and nests (Bibby et al. 1992; Ferrer-Sánchez and Rodríguez-Estrella 2014). The observation points were located at a minimum distance of 2 km apart to reduce the probability of double counting and achieve independent sampling. In addition, the content of each nest (eggs, chicks, etc.) and status of the nest (active or not active) was determined (Rodríguez-Estrella et al. 2020).
Then, in view of the shortcomings of GBIF data (Yesson et al. 2007) and the need for good quality data for optimal model performance (Beck et al. 2014; Perez-Navarro et al. 2021), we performed a data cleaning process. This data cleaning consisted of four steps: (a) removing records that lacked lat–long coordinates or contained data transcription errors; (b) excluding records that lacked data for the bioclimatic variables used; (c) restricting our data to those collected between 1970 and 2021; and (d) eliminating localities whose coordinates (longitude and latitude) had less than three decimal places. Moreover, for records from 2001 to 2021 (i.e., without the same temporality as climatic layers) we performed an outlier exclusion procedure in the environmental space by removing points whose annual mean temperature (Bio 01), annual precipitation (Bio 12), or precipitation seasonality (Bio 15) values fell beyond the upper and lower quartiles of the set of species’ records within the time range (1970–2000) of bioclimatic variables (Robertson et al. 2016; Prieto-Torres et al. 2020). Then, we removed records that were present in more than one source or were spatial duplicates, retaining only information corresponding to unique localities within a vicinity of ~ 10 km2 (based on the known home range for the species; see McGrady et al. 2002; Tapia et al. 2007). This was done to avoid biases derived from spatial autocorrelation in areas that are heavily represented in the data (Peterson et al. 2011). Besides, we also discarded records outside the documented geographic and elevational ranges of the species’ distribution (BirdLife International 2022). After all of these steps, we retained a total of 4877 unique locality records (including 152 Mexican breeding sites [i.e., territories occupied by pairs with nests]).
Environmental variables and future climate scenarios
To characterize the species’ potential distribution, we downloaded the 19 ‘bioclimatic variables’ (at ~ 5 km2 cell size resolution) from the WorldClim 2.1 database (Fick and Hijmans 2017). We excluded the four variables that combine temperature and precipitation (bio 8, bio 9, bio 18 and bio 19), owing to known artifacts (Escobar et al. 2014; Booth 2022). To avoid overfitting and reduce the dimensionality of the climatic variables, we derived the set of four variables that explained 95% of the total variance, using a Principal Components Analysis (Hanspach et al. 2011) implemented in the “ENMGadgets” R package (Barve and Barve 2016). These Worldclim bioclimatic variables were complemented with two topographic (elevation and slope) datasets downloaded from the Hydro1k project (see USGS 2001) and a vegetation dissimilarity index layer that provide information on the spatial variation of habitats (see Tuanmu and Jetz 2015). Although these variables are not commonly used in correlative modeling studies, we decided to include them because they are considered important drivers of the habitat preferences for distribution and breeding ecology of Golden Eagles (see Tapia et al. [2007] and Fielding et al. [2019] for more details).
For models based on future climate projections (2040s, 2060s, and 2080s), we used climate data from the Coupled Model Intercomparison Project 6 (CMIP6; Stoerk et al. 2018). Five general circulation models (CanESM5, MIROC6, BCC-CSM2-MR, CNRM-CM6-1, and IPSL-CM6A-LR) were selected based on the results from GCM compare R’s web application (Fajardo et al. 2020), adopting the “storyline” approach (Shepherd et al. 2018). Besides, these models showed improvements in the estimation of zonal-mean atmospheric fields, equatorial ocean subsurface fields, precipitation values. and the simulation of El Niño-Southern Oscillation in the Americas (Zelinka et al. 2020). All projections were performed using an intermediate Shared Socio-economic Pathway scenario (SSP 3.70), which assumes high greenhouse gas emission and low climate change mitigation policies (Riahi et al. 2017), which seems to be the most likely scenario in the future (Stocker et al. 2013; Pandit et al. 2021). All global climate models were downloaded from the WorldClim website (https://www.worldclim.org/data/cmip6/cmip6_clim2.5m.html) as digital layers (at ~ 5 km2 cell size resolution).
Ecological niche and species distribution models
Because several authors have been reported that there are uncertainties linked to the implemented algorithm (e.g., Qiao et al. 2015; Hao et al. 2019), we decided to use the bio-assembly of models’ approach forecasting the species distribution. To do this, we used the “modleR” library in R (Sánchez-Tapia et al. 2020), which involves four steps: data setup, fit and projection of the model, partition union, and consensus between algorithms. Models for A. chrysaetos were obtained using four algorithms: Bioclim (Beaumont et al. 2005; Booth et al. 2014), Mahalanobis distance (Hijmans et al. 2017), Maxent (Elith et al. 2006), and Maxnet (Phillips et al. 2017). These algorithms were selected over others because they have been shown to perform well using presence-only data, as is the case here (Elith et al. 2011; Qiao et al. 2015), and they also had the best predictive performance in terms of kappa, TSS, and ROC test evaluations (see below).
Given that dispersal plays a crucial role in the distribution of organisms and must be considered in the development of such models (Barve et al. 2011), we created an area for model calibration, known as “M” according to the BAM diagram (Soberón and Peterson 2005). This area (a GIS mask or polygon) was defined by intersecting the species’ records with maps of terrestrial ecoregions (Dinerstein et al. 2017) and neotropical biogeographic provinces (Morrone et al. 2022), including a 50-km buffer (based on the natal dispersal distance [NDD] reported in previous works [see Millsap et al. 2014; Murphy et al. 2019; Whitfield et al. 2023]). The area defined as “M” thus represents a biogeographical hypothesis about the sites that have been historically accessible to the species, and to which we therefore restricted our modeling distribution (Peterson et al. 2011). This consideration assumes that these regions define the area that has historically been accessible to the species in geographical space (because there are no ecological or geographical barriers that prevent access; Soberón and Peterson 2005).
The models were generated by partitioning the localities into calibration and evaluation datasets, using the n-fold cross-validation option, as implemented in the “partition_type” function of the “modleR” library (Sánchez-Tapia et al. 2020). Each run was performed with a different selection of the calibration and evaluation datasets, and the proportion of data for calibration was set to 70%. In addition, a set of 10,000 pseudo-absence datasets was randomly generated inside the calibration area (M). We repeated these steps ten times for each algorithm to ensure that the evaluation procedure was independent of the random splitting procedure. The prevalence was set to 0.5 to give the presences and absences the same importance in the calibration process. All other parameters in “modleR” were kept at default settings (see Sánchez-Tapia et al. 2020).
Then, we used a true skill statistic (TSS) protocol to convert the probabilities of occurrence into presences and absences (Allouche et al. 2006). To generate a consensus map for the species, we added all models’ outputs and calculated the relative number of times that species records were predicted by each model in each cell. Then, a final consensus presence/absence map was generated using a minimum congruence threshold of 0.5 (i.e., at least 50% of maps agreed on their predictions; Araújo et al. 2005; Sánchez-Tapia et al. 2020). The performance of the final map under current climate conditions was evaluated by calculating the values and significance of omission error (i.e., percentage of records erroneously omitted by the model; Anderson et al. 2003) and the partial-ROC test (Lobo et al. 2008; Peterson et al. 2008). The models were calibrated using the available data for the entire range of the species, then cropped to the geographic extent of Mexico for subsequent analyses (Fig. 1).
For the future climate scenarios, we generated a total of 40 maps of the species’ potential distribution (i.e., four algorithms × two time points × five global climate models). Following the same procedures as described above for the current climate scenario, these future maps were used to produce a consensus map (i.e., threshold > 0.5) for each global climate model. Then, the future geographic distributions (for years 2040, 2060, and 2080) were obtained by overlaying the binary projections from the five global climate models, determining the “presence” to any pixel where ≥ 80% of predictive models coincided (i.e., suitable in 4 or more models = presence). This resulted in a single consensus map for each of the scenarios forecasted, for a total of four maps: current, 2040s, 2060s, and 2080s.
Finally, to measure the risk of strict extrapolation into future species’ models resulting from projections to non-analogous conditions, we performed mobility-oriented parity (MOP; Owens et al. 2013)—as implemented in the “ntbox” R package (Osorio‐Olvera et al. 2020). MOP consists of measuring the similarity between the closest 30% of the environmental conditions of the calibration area to each environmental condition in the area of transference (see Owens et al. 2013; Alkishe et al. 2017). Areas of projection with values of similarity of zero indicate higher uncertainty given the presence of non-analogous environmental conditions, as suitability in those regions derives from model extrapolation only, and caution is required when interpreting the likelihood of the species’ presence in such areas (Alkishe et al. 2017). Those areas were deleted from our binary results (suitable areas) for the subsequent analyses. This step is important for proposing conservation areas, since it is most beneficial to protect areas where there is a high degree of certainty that the species of interest will be found (see Velazco et al. 2020).
Spatio-temporal analyses and summary metrics
We determined the loss and gain of suitable habitats by comparing the geographic projections of niche models in current versus future scenarios (following Thuiller et al. [2005]). This comparison allowed us to identify areas of climatic stability (i.e., places where the conditions were suitable under the current and all future models). When the loss of suitable areas was predicted in future-projected models, we calculated the differences (current vs. future) in values of the bioclimatic and elevation variables (Cobos and Bosch 2018; Atauchi et al. 2020).
We assessed the effects on our models of current habitat loss (i.e., human modified areas that may be unsuitable for some species) using the 2017 land cover and vegetation map generated by the Mexican Instituto Nacional de Estadística y Geografía (available on: https://www.inegi.org.mx/temas/usosuelo/). We reclassified this map using the “majority” resampling technique at ~ 5 km2 in ArcMap 10.2.2 (ESRI 2010) to discriminate pixels representing extremely disturbed landscapes (i.e., areas occupied by crops, deforested areas, farming areas, pastures, and urban settlements) as “highly human-modified areas”. We then calculated the average extent of species distribution under the current climate scenario that overlapped with these highly human-modified areas. For future scenarios, we repeated this process for the land-use and land-cover change scenarios (from 2015 to 2100) modeled by Chen et al. (2022), which are based on the projected demand of the latest IPCC coupling socio-economic and climate change scenarios, SSP–RCP, using the maps corresponding to each of our time points (2040, 2060 and 2080).
Finally, to estimate the importance of the existing PA network for Golden Eagle distribution, we calculated the proportion of the species’ distribution area that fell within these conservation areas by overlapping the raster of current Mexican PAs with the binary species distribution maps. The shapefile of the boundaries of terrestrial PAs was obtained from the CONANP website (available on: http://sig.conanp.gob.mx/website/pagsig/info_shape.htm), selecting both official PAs and voluntary conservation areas. All of these post-modeling analyses and statistical calculations were performed using the “maptools” (Bivand et al. 2016), “raster” (Hijmans et al. 2016) and “LetsR” (Vilela and Villalobos 2015) R packages.
Characterization of the impact of climate change on breeding sites
The potential impacts of global climate change on Golden Eagle’ breeding sites across Mexico were assessed (following Cobos and Bosch 2018) by comparing the climatic data between the current and future (2040s, 2060s, and 2080s) scenarios using a principal component analysis (PCA; Pearson 1901; Karamizadeh et al. 2013) of the six uncorrelated (r < 0.8; Dormann et al. 2013) bioclimatic variables: bio 02, bio 07, bio 10, bio 13, bio 14, and bio 15. To do this analysis, values of current and future bioclimatic variables were extracted for 10,000 random points for the available climatic space (within the area defined as M) and the known breeding sites of the species in Mexico. The results were then represented in a biplot of the two first principal components (PCs), using ellipsoids to represent the conditions (ecological niche) of breeding sites. We assessed whether the species’ breeding sites show equivalent climatic conditions over time using niche overlap tests (see Warren et al. 2008; Broennimann et al. 2012). The hypotheses of niche equivalence and similarity (i.e., conservatism of ecological conditions) for breeding sites among climate scenarios was evaluated by performing statistical tests to compare the empirically observed distributions of Schoener’s D to 1000 randomly generated simulated values (see Warren et al. 2008; Broennimann et al. 2012).
Results
Model statistics and current spatial patterns of the Golden Eagle
Models obtained for the individual algorithms showed highly significant values of the partial ROC test (ranging from 1.07 to 1.43; P < 0.05) and low omission errors (mean of 11.0 ± 7.9%). These performance values (Table 1) indicated that our species’ distribution models were statistically better than random expectations. Therefore, we considered our models to have good discrimination capacity for recovering the ecological niche and geographical range of the species.
The consensus map showed that the current potential distribution area of the Golden Eagle has a size of ca. 1,027,400 km2 in Mexico (Fig. 1; Table 2). On average, this estimated range represent the 7.2% of species’ whole distribution into North American region. The suitability areas for the Golden Eagle in Mexico are concentrated mainly in the north central region, including large areas in the states of Chihuahua (26.35%), Coahuila (17.83%), Durango (9.88%), Baja California (8.44%), and Zacatecas (7.92%). A total of six natural ecosystems were identified as important in the Golden Eagle’s distribution across Mexico (Table 3). The three more important ecosystems were xerophytic scrub (644,600 km2; 62.74%), grassland (179,750 km2; 17.50%), and coniferous-oak forest (175,350 km2; 17.07%). The model showed an important degree of overlap (21.22%) between the species distribution and current highly human modified areas (red color in Fig. 1; Table 2), including 11.84% of recorded breeding sites. The sites of strongest overlap between the Golden Eagle distribution and human-modified areas were across the northwestern Chihuahua, northeastern Nuevo León, and a largely continuous stretch from Durango–Sinaloa to Tlaxcala–Puebla (Fig. 1).
The model also showed a relatively high proportion (ca. 12%) of the species’ potential distributional areas within existing PAs. The PAs with the largest areas of conditions suitable for species were the Forest Protection Zone and Wildlife Refuge “Valle de los Cirios” (29,880 km2), the Natural Resources Protection Area–Forest Protection Zone “Don Martín” (17,800 km2), the Biosphere Reserve “El Vizcaíno” (11,780 km2), and the Natural Resources Protection Area–Forest Protection Zone “Estado de Nayarit” (7285 km2). These four PAs together account for more than 55.26% of the portion of the species’ range that falls within PAs, and a total of 26 records (17.11%) of breeding sites were reported within the limits of these PAs.
Impacts of global changes on species distribution patterns
Our model projections showed that the distribution area of the Golden Eagle across Mexico could decrease significantly in the future (Table 2; Fig. 2). Furthermore, our results suggest that the Golden Eagle will not be able to offset this lost distribution area by colonizing areas that become newly suitable in the future, since < 1% of the distribution areas under the future scenarios corresponded to newly colonized areas (Table 2). The potential distribution areas decreased under future scenarios by ~ 49% [2040s] and ~ 76% [2080s] relative to the present. This loss of area occurred mainly across the Mexican Plateau in the states of Chihuahua, Durango, and Zacatecas (Fig. 2). In general, all projections showed decreases for the Golden Eagle’s distribution across the six natural ecosystems identified in Mexico (Table 3). The most significant losses (> 77% of the current available suitability areas) were detected in seasonally tropical dry forests, thorn forests, and grasslands, leaving up to 89% of the species’ remaining distribution in the future in Xerophytic scrub and coniferous-oak forests. In addition, the models showed that on average only 23.61% (242,600 km2) of the potential distribution could be considered environmentally stable across time (i.e., was predicted to occur under all scenarios analyzed). These areas of stability (with respect to both climate and topographic conditions) overlapped with 27.63% of the breeding sites recorded for the species in Mexico, while 49.34% of recorded breeding sites corresponded to areas where suitable conditions were predicted to loss in the future.
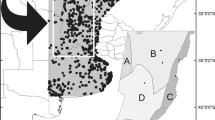
Golden Eagle (Aquila chrysaetos) distribution projected to 2040, 2060, and 2080 under an intermediate Shared Socio-economic Pathway climate scenario: SSP 3.70. Colors in maps correspond to the predictions of range expansion (green), loss (blue), and stable areas (red) under future projections relative to the current scenario. The black-hatched polygons in the maps represent the protected areas. Dark brown shading shows area with elevations above 1000 m asl. The bar graph in the bottom right panel shows the area of the species’ distribution that falls within the four protected areas identified as the most important under the current scenario: Forest Protection Zone and Wildlife Refuge “Valle de los Cirios” (1), the Natural Resources Protection Area–Forest Protection Zone “Don Martín” (2), the Biosphere Reserve “El Vizcaíno” (3), and the Natural Resources Protection Area–Forest Protection Zone “Estado de Nayarit” (4) (color figure online)
The MOP analyses (Electronic Supplementary Appendix S1) indicated that areas where strict extrapolation occurred outside of the potential distribution/suitable areas predicted by models in the future across Mexico. Thus, general shifts in species ranges corresponded to changes in the extent and quality of suitable climate conditions (i.e., extension and quality) within the range of conditions currently used by the species. Areas where the species was expected to be present in the current scenario but not in the future (Fig. 2), showed significant increases in average temperature annual (> 2.6 °C), temperature seasonality (> 1.4 °C) and maximum temperature of the warmest quarter (> 2.9 °C), as well as reductions in precipitation seasonality (> 2.2 [2040]–3.4 [2080] mm).
On the other hand, the combined effects of future climate change and habitat loss could represent a still-greater risk, leading to an average reduction of 57% (2040s)–78% (2080s) in the area of the Golden Eagle’s potential distribution (Table 2). Furthermore, current PAs cover between 15.30% (2040s) and 20.24% (2080s) of the species’ projected future distribution. However, this results in fact correspond to a reduction of conservation sites because the surface (km2) within PAs decreased by > 33% on average under the species’ future distributions (see Table 2). Indeed, the four most important PAs in the species’ current distribution showed important reductions in their areas of overlap with Golden Eagle distribution in the future (decreasing by 38.52% [2040s]–56.34% [2080s]) in the future.
Climate global change impacts on breeding sites
In the PCA, 67.44% of the total variance was explained by the two first PCs (Fig. 3a). Precipitation of wettest month (bio 13) and precipitation seasonality (bio 15) were more closely correlated with each other than with the other variables, while the temperature annual range (bio 07) and the mean temperature of warmest quarter (bio 10) variables followed opposite directions in the biplot (Fig. 3b). The test of equality of climate conditions for the Golden Eagle’s breeding sites in Mexico among years rejected that hypothesis (P > 0.05). However, the similarity background test showed that the hypothesis of niche similarity cannot be rejected, since the observed D values were significantly (P < 0.05) greater than random expectation (Fig. 3). Therefore, a higher conservatism of ecological conditions for these sites is expected. In fact, and despite those climatic conditions characterizing the niche of breeding sites trend—as expected—to shift its position into ecological space (Fig. 3), we observed (according to Rödder and Engler [2011] proposal) a high–very high overlap values (0.75–0.83) among the available climates by years for these reproductive sites of the species.
Biplot of the two first principal component analysis axes (PC1 and PC2), showing the climatic niche of Golden Eagle (Aquila chrysaetos) breeding sites under current and future conditions. We showed the complete panorama for the available climates and the niches of breeding sites for the species. (a) Inset: variables, correlations, and directions in the biplot; and (b) PCA-env showing changes in bioclimatic variables in the ecological conditions for breeding sites from current to future scenarios. Ellipsoids enclose 100% of the values that represent the climates of the breeding sites of the species under each climate scenario. Color shading shows the density of the occurrence records by cell, with the solid and dashed contour lines indicating 100% and 50% isopleths, respectively. The individual overlap values observed among comparisons were: current–2040s = 0.79; current–2060s = 0.83; and current–2080s = 0.75. All comparisons were statistically significant in both niche equivalence and niche similarity tests (color figure online)
Discussion
In recent years, there has been an increase in the number of studies focused on the impact of rapidly changing environmental conditions on biodiversity. Several studies have evaluated the potential additive effects of these threats on the conservation of a number of species, biological assemblages, and ecosystems across Mexico (e.g., Peterson et al. 2002; Ochoa-Ochoa et al. 2012; Rojas-Soto et al. 2012; Prieto-Torres et al. 2016, 2020, 2021b; Mayani-Parás et al. 2021; Sierra-Morales et al. 2021; Ureta et al. 2022). The current study is the first to explore the impact of both climate and land-use changes on a top predator species such as the Golden Eagle, even providing novel information about breeding parameters for its population over time. Worryingly, our results suggest a concerning scenario for this emblematic species due to the reduction of range size and the increase in fragmentation across Mexico (Rodríguez-Estrella 2002; Campos-Rodríguez et al. 2019; D’Addario et al. 2019; Bautista et al. 2022). This is not substantially improved by the existing PA network; on one hand, Mexican PAs are not fully exempt from human disturbance and climate change, and on the other, most of the sites (including breeding areas) that are highly resilient to climate and land-use changes are located outside of current PAs. Therefore, it is imperative that policy-makers promote new and more effective efforts for long-term conservation planning for this species as soon as possible. Our results provide critical insights on which areas should be prioritized to achieve those goals. Information derived from these studies will be crucial for proposing feasible and effective actions to conserve this emblematic species at both national and international scales.
Impacts of global changes on species distribution patterns
Landscape modifications have accelerated in Mexico in the last 20 years (see Mendoza-Ponce et al. 2020), which is an important concern from a conservation point of view. New infrastructure (e.g., roads, wind farms, mining projects, etc.) and intensive farming (especially in open land and grasslands) are increasingly fragmenting and reducing suitable habitats for the Golden Eagle and, therefore, constitute a direct threat to this species (Marzluff et al. 1997; SEMARNAT and CONANP 2008; Lozano and Ávila-Villegas 2009; De León Girón 2017; Campos-Rodríguez et al. 2019; Bautista et al. 2022). This degradation, fragmentation, and loss of tropical forests has led to strong reductions of bird abundance due to edge effects and possibly local-scale climate change. All of these factors contribute to a reduction of the quality of the Golden Eagle’s food supply (e.g., Stouffer 2020; Sherry 2021), including its most important prey such as rodents, lagomorphs, and smaller birds (Di Vittorio and López-López 2014; D’Addario et al. 2019). Furthermore, these local changes can favor the emergence of new diseases that negatively impact the demography and population dynamics (Selwood et al. 2014; Fecchio et al. 2019). Habitat fragmentation is a process that is too rapid to allow for an adaptive response (Lande 1988), and it is perhaps the mechanisms that most strongly increases a population’s risk of reaching critical states of loss of genetic variability, demographic and environmental stochasticity, and vulnerability to catastrophic events. Therefore, there is an urgent need for conservation measures to combat habitat fragmentation, as has been suggested in other countries for this and other raptor species (e.g., Gil-Sánchez et al. 2004; Cadahía et al. 2010; D’Addario et al. 2019).
We found that global warming is expected to generate future conditions that will sharply decrease the availability of suitable habitats for Golden Eagle in Mexico. Such changes could be attributable mainly to increasingly dry and warm conditions, which may promote alterations in the location and size of areas suitable occupied by the species (including breeding sites). Certainly, this result is not unique. It is now well established that climate change impacts species’ ranges—especially latitudinal and altitudinal movements (Lovejoy and Hannah 2019). Anthropogenic influence on the climate system has already altered the frequency and intensity of extreme weather events (Estrada et al. 2023), and lowland areas may experience persistent extreme heat or drought as the climate continues to warm (e.g., Prieto-Torres et al. 2016). This is consistent with previous studies in Mexican seasonally dry forests that suggest higher vulnerability to GCC for these biota (Prieto-Torres et al. 2016, 2020, 2021a) and, therefore, represent a major problem for the Golden Eagle’s survival in this ecosystem. Although this species is often considered a generalist species that is adaptable to a wide variety of conditions (Kochert et al. 2002; Watson 2010), a preference for grasslands and xerophytic scrub climates has been recorded in Mexico (Campos-Rodríguez et al. 2019; BirdLife International 2022; Bautista et al. 2022). Thus, when temperature is already near the species’ maximum tolerance levels, it cannot persist unless it moves to higher elevations or higher latitudes with more optimal temperatures (Sergio et al. 2022).
Although our results are generally consistent, they should be interpreted with caution because it is unknown to what extent the species will be able to adapt to climate changes. A species’ adaptive potential is determined by its own evolutionary rate and ability to respond to rapid environmental change, which depend directly on factors such as geographic range size, dispersal ability, reproductive rates, and degree of specialization of habitat requirements (Ortega et al. 2019; Silva et al. 2019). None of these parameters were evaluated here, so the impacts of environmental changes on Golden Eagle population dynamics are still unknown. Although our model did take into account climatic, soil, and topographical factors, species distributions can be constrained by a variety of other factors such as interspecific interactions, human activities, and the quality of foraging opportunities. Therefore, to develop more suitable adaptation strategies, further research on demographic parameters and the effects of extreme weather events are urgently needed. Medium and long-term monitoring programs are essential for answering these questions, especially in zones that—based on our models (Fig. 2)—are more likely to suffer drastic decreases.
Finally, it is important to note that when both climate and land-use changes are considered together, the area of suitable habitats for Golden Eagle will further significantly reduce. In this sense, the identification of “refugia areas” or “safe places” (i.e., locations that are highly resilient to both GCC and land-use changes) is a crucial strategy for planning by managers and conservation practitioners faced with these threats that already taking place (see Jones et al. 2016; Velazco et al. 2019). From this perspective, the identified climatic stable areas should be prioritized to protect and even using to establish corridors that would support climate-induced dispersal from high risk areas to suitable ones (Struebig et al. 2015; Triviño et al. 2018). Because these areas may remain well-preserved in the future even under agricultural expansion, resources and efforts should be dedicated to their long-term maintenance. Future actions should also focus on maintaining suitable habitats in unprotected areas, mitigation of global climate change impacts, and assessing the effectiveness of conservation efforts under different anthropogenic practices (e.g., Sánchez-Romero et al. 2021; Bautista et al. 2022).
Climate global change impacts on breeding sites
Our results suggest a drastic effect of future climatic conditions on breeding areas for Golden Eagle population across country. The Golden Eagle is a territorial species that exhibits a long-term fidelity to nesting sites (Bravo-Vinaja and Guzmán-Aranda 2014), so these negative impacts could compromise future population viability. Previous works found evidence that environmental conditions—specifically, extended periods of low temperatures during the incubation and early nestling period—affect the probability of nesting as well as fledgling production and survival (Steenhof et al. 1997). For top predators like the Golden Eagle, drastic changes in temperatures can reduce breeding performance by increasing energy demands and/or influencing the behavior of prey in ways that decrease food availability (Steenhof et al. 1997; Watson 2010; Sergio et al. 2022). So, long-term shifts to more extreme weather conditions in the region could result in decreased reproductive output by Golden Eagles.
For long-lived predators such as Golden Eagle and the Red Kite (Milvus milvus), a decrease in most components of the breeding performance has been suggested due to drought (see Millsap et al. 2015; Sergio et al. 2022). Such impact could include a 126% increase in the probability of skipping reproduction, 37% reduction in the number of breeding pairs, and 46% reduction in the number of fledglings raised which decreased the total number of fledglings produced, but also a reduction in nestling body condition and in survival (Sergio et al. 2022). These negative impacts, consequently, dictated the survival of all future breeding adults, implying a delayed negative effect on abundance that is still visible decades later (e.g., Martín et al. 2021; Sergio et al. 2022). In this sense, climate change may erode populations more quickly and severely than currently appreciated, especially if strong philopatry limits the ability of reproductively active individuals to relocate their breeding territories to locations with more suitable conditions despite experiencing low breeding success. Therefore, protecting current breeding sites is a critical goal toward the long-term conservation of this species in Mexico.
Although our predictions within Mexico are quite pessimistic, it is important to highlight a potentially hopeful scenario for the long-term survival of this species. Several authors suggest that long-lived predators, such as Golden Eagles, exhibit wide thermal tolerance associated with latitudinal gradients and may therefore be able to breed in climates that are currently cooler than their physiological optima (Deutsch et al. 2008; Martín et al. 2021; Sergio et al. 2022). For these species, climate warming is expected to particularly benefit animals occurring at higher latitudes by increasing population growth and carrying capacity (e.g., Deutsch et al. 2008; Martín et al. 2014). In fact, several works in Scotland shows successful results of colonization, reintroduction and even translocation of Golden Eagle population (O’Toole et al. 2002; Whitfield et al. 2007, 2008). Therefore, under future climates, it is possible that the species’ distribution would be displaced northward in the USA and Canada, facilitating the increased recruitment at these sites (see Martín et al. 2021; Sergio et al. 2022). However, we did not assess this possibility in this study due to a lack of access to nesting data outside of Mexico. More research and fieldwork testing this hypothesis are needed to obtain reliable knowledge about the species’ dynamic responses to future environmental scenarios.
Our results have important conservation implications. Frequently, the protection of long-lived species focuses on adult survival as the main target of management action, but as we discussed herein breeding site management have more complex and protracted impacts on population dynamics, potentially carrying over for decades and becoming apparent only after it may be too late. In this sense, different conservation strategies should be developed for areas with different conditions (following Hole et al. 2011): (1) in those breeding areas with less native vegetation but low climatic anomaly, restoration is an important strategy to increase connectivity and population size; (2) in areas with less native vegetation and high climatic variation, populations should be monitored to identify those that are most vulnerable and require assisted conservation interventions (e.g., future translocations to refugia areas); and (3) in areas where native vegetation is abundant but that are projected to face high climatic anomaly, it is important to reduce the existing climate change factors and protect the current vegetation so that the species has opportunities to adapt to the changing local climate or disperse to areas with more suitable climate. In order to encourage the protection of these critical areas, it is important to financially support landowners—especially smallholders—who preserve remnant vegetation. Future conservation proposals protecting breeding sites and/or adult survival cannot be developed without these financial actions, especially considering the current anthropogenic/land-use planning systems (e.g., Baranovskis et al. 2022). Without this support, it could be very difficult, if not impossible, to halt the downward spiral of Golden Eagle abundance. All of these measures (e.g., payments or tax relief in exchange for environmental services, ecological long-term studies, etc.) should also be accompanied by continuous educational activities that could allow Golden Eagle to function as an umbrella species, thereby protecting other poorly known species that share its habitat (SEMARNAT and CONANP 2008; Bautista et al. 2022).
Of course, recognition of the relevance of climate impacts on breeding sites does not imply that protecting breeding sites alone is sufficient to conserve the long-term presence of the Golden Eagle in Mexico. Conservation frameworks based on demographic targets beyond breeding productivity are also needed. Considerable benefits would also result from a program that monitors subadult ecology and adult survival, so that measures of all three of the main demographic parameters became accessible (Whitfield et al. 2006). This is relevant because mechanisms underlying Allee effects (e.g., reduced availability of mates, sexual selection and foraging efficiency or increased risk of predation) can occur in areas far away from breeding territories, which increases their likelihood of extinction because of a decrease in reproduction and/or survival (Penteriani et al. 2008). In fact, the absence of information on the location of settlement areas and on the dynamics of individuals within them (as for the majority of species) means we are unprepared to halt population declines (Penteriani et al. 2005, 2008).
It is important to note that the conservation strategies outlined in our study are broad and generic suggestions, rather than detailed prescriptions for specific locations. Given the low resolution of the climatic models at our scale of analysis, it is not possible to evaluate if our refugia areas have local characteristics (e.g., streams, lakes, cold air drains and topographic exposure to radiation and wind) which could create micro-refugia on an even finer scale that could favor the species survival (Ashcroft 2010; Gavin et al. 2014). For example, maximum wind speed and sunshine duration (both of which increase the costs of thermoregulation) had a negative effect on the probability that chicks successfully fledged. Thus, specific sites within the defined refugia region may present important changes that some species will not be able to tolerate. Further threats causing loss of eggs and nestlings in this eagle are agricultural measures such as human disturbance (Wiggins et al. 2014; Fernández-Bellon et al. 2019). Considering the potential for high levels of variation in fine-scale landscape scenarios, future studies should focus on gaining a more spatially and temporally detailed models. Although it could be a major research challenge, we also recommend studies on prey availability and diet in Golden Eagle nesting areas.
Conclusion
In summary, both future climate change and habitat loss were shown to be major threats to the future distribution and survival of the Golden Eagle. Information derived from this study will be crucial for proposing feasible and effective actions to conserve this species, especially at its breeding sites. This integrative analysis approach provides novel evidence and key guidelines for conservation planning of resilient areas, as well as the importance of expanding the network of protected/conservation areas. However, only protecting breeding sites is insufficient to ensure the long-term presence of this species. More research about floaters and settlement areas is also needed. We hope that our findings will motivate conservationists and policymakers to implement changes in the near future to favor the long-term conservation of this vulnerable species.
Data availability
All R scripts and input data for ecological niche analysis and species distribution modelling are available in https://github.com/davidprietorres/GoldenEagleMx_globalChange. Additional data are available from the Corresponding author upon request.
References
Alkishe AA, Peterson AT, Samy AM (2017) Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus. PLoS ONE 12:e0189092. https://doi.org/10.1371/journal.pone.0189092
Allouche O, Tsoar A, Kadmon R (2006) Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43:1223–1232. https://doi.org/10.1111/j.1365-2664.2006.01214.x
Anderson R, Lew D, Peterson A (2003) Evaluating predictive models of species’ distributions: criteria for selecting optimal models. Ecol Model 162:211–232. https://doi.org/10.1016/S0304-3800(02)00349-6
Araújo MB, Pearson RG, Thuiller W, Erhard M (2005) Validation of species–climate impact models under climate change. Global Chang Biol 11:1504–1513. https://doi.org/10.1111/j.1365-2486.2005.01000.x
Arenas-Navarro M, García-Oliva F, Torres-Miranda A, Téllez-Valdés O, Oyama K (2020) Environmental filters determine the distribution of tree species in a threatened biodiversity hotspot in western Mexico. Bot Sci 98:219–237. https://doi.org/10.17129/botsci.2398
Ashcroft MB (2010) Identifying refugia from climate change. J Biogeogr 37:1407–1413. https://doi.org/10.1111/j.1365-2699.2010.02300.x
Atauchi JP, Constantino CC, Ferro G, Prieto-Torres DA (2020) Present and future potential distribution of the endangered Anairetes alpinus (Passeriformes: Tyrannidae) under global climate change scenarios. J Ornithol 161:723–738. https://doi.org/10.1007/s10336-020-01762-z
Baranovskis Ģ, Nikodemus O, Brūmelis G, Elferts D (2022) Biodiversity conservation in private forests: factors driving landowner’s attitude. Biol Conserv 266:109441. https://doi.org/10.1016/j.biocon.2021.109441
Barve N, Barve V, Jiménez-Valverde A, Lira-Noriega A, Maher SP, Peterson AT et al (2011) The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol Model 222:1810–1819. https://doi.org/10.1016/j.ecolmodel.2011.02.011
Bautista J, Otero M, Gómez GJ, Garrido JR (2022) Águilas reales y humanos. Expansión a paisajes dominados por humanos [Golden eagles and humans. Expansion to human dominated landscapes]. Wilder South, Granada, Spain
Beaumont L, Hughes L, Poulsen M (2005) Predicting species distributions: use of climatic parameters in BIOCLIM and its impact on predictions of species’ current and future distributions. Ecol Model 186:251–270. https://doi.org/10.1016/j.ecolmodel.2005.01.030
Beck J, Böller M, Erhardt A, Schwanghart W (2014) Spatial bias in the GBIF database and its effect on modeling species’ geographic distributions. Ecol Inf 19:10–15. https://doi.org/10.1016/j.ecoinf.2013.11.002
Beecham JJ, Kochert MN (1975) Breeding biology of the Golden Eagle in southwestern. Wilson Bull 87:506–513. http://www.jstor.org/stable/4160686
Bibby CJ, Burgess ND, Hill DA (1992) Bird census techniques, Primera Edición. Londres: Academic Press
BirdLife International (2022) Species factsheet: Aquila chrysaetos. http://www.birdlife.org. Accessed 15 Feb 2021
Bitencourt C, Rapini A, Damascena LS, Junior PDM (2016) The worrying future of the endemic flora of a tropical mountain range under climate change. Flora-Morphol Distrib Funct Ecol Plants 218:1–10. https://doi.org/10.1016/j.flora.2015.11.001
Bivand R, Lewin-Koh N, Pebesma E, Archer E, Baddeley A, Bearman N, Golicher D (2016) Package ‘maptools’: tools for handling spatial objects. https://cran.r-project.org/web/packages/maptools/index.html. Accessed 30 Mar 2022
Booth TH (2022) Checking bioclimatic variables that combine temperature and precipitation data before their use in species distribution models. Austral Ecol 47:1506–1514. https://doi.org/10.1111/aec.13234
Booth T, Nix H, Busby J, Hutchinson M (2014) Bioclim: the first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Divers Distrib 20:1–9. https://doi.org/10.1111/ddi.12144
Barve N, Barve, V (2016) ENMGadgets: tools for pre and post processing in ENM workflow. https://github.com/narayanibarve/ENMGadgets. R package version 0.0.14
Bravo-Vinaja MG, Guzmán-Aranda JC (2014) Distribución potencial del águila real (Aquila crhysaetos) en el Altiplano Mexicano a través de monitoreo y modelos HSI basados en Sistemas de Información Geográfica. Informe Final GT028 SNIB-CONABIO, proyecto No. GT028. Ciudad de México, México
Broennimann O, Fitzpatrick MC, Pearman PB, Petitpierre B, Pellissier L, Yoccoz NG et al (2012) Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecol Biogeogr 21:481–497. https://doi.org/10.1111/j.1466-8238.2011.00698.x
Cadahía L, López-López P, Urios V, Negro JJ (2010) Satellite telemetry reveals individual variation in juvenile Bonelli’s eagle dispersal áreas. Eur J Wildl Res 56:923–930. https://doi.org/10.1007/s10344-010-0391-z
Campos-Rodríguez J, Flores-Leyva X, Pérez-Valera D, García-Martínez D (2019) Anidación del águila real en el sureste de Zacatecas, México. Huitzil Rev Mex De Ornitol 20:1–13. https://doi.org/10.28947/hrmo.2019.20.1.394
Caro J, Ontiveros D, Pizarro M, Pleguezuelos JM (2011) Habitat features of settlement areas used by floaters of Bonelli’s and Golden Eagles. Bird Conserv Int 21:59–71. https://doi.org/10.1017/S0959270910000213
Ceballos G, Ehrlich PR (2018) The misunderstood sixth mass extinction. Science 360:1080–1081. https://doi.org/10.1126/science.aau0191
Chamberlain S, Barve V, Mcglinn D, Oldoni D, Desmet P, Geffert L et al (2019) Rgbif: interface to the global biodiversity information facility API. R package version 1.2.0. https://cran.r-project.org/package=rgbif. Accessed 10 Feb 2021
Chen G, Li X, Liu X (2022) Global land projection based on plant functional types with a 1-km resolution under socio-climatic scenarios. Sci Data 9:1–18. https://doi.org/10.1038/s41597-022-01208-6
Cobos ME, Bosch RA (2018) Recent and future threats to the Endangered Cuban toad Peltophryne longinasus: potential additive impacts of climate change and habitat loss. Oryx 52:116–125. https://doi.org/10.1017/S0030605316000612
Cuervo-Robayo AP, Ureta C, Gómez-Albores MA, Meneses-Mosquera AK, Téllez-Valdés O, Martínez-Meyer E (2020) One hundred years of climate change in Mexico. PLoS ONE 15:e0209808. https://doi.org/10.1371/journal.pone.0209808
D’Addario M, Monroy-Vilchis O, Zarco-González MM, Santos-Fita D (2019) Potential distribution of Aquila chrysaetos in Mexico: implications for conservation. Avian Biol Res 12:33–41. https://doi.org/10.1177/1758155918823424
De León Girón G (2017) Abundancia, ecología reproductiva, dieta, uso de hábitat y amenazas del águila real (Aquila chrysaetos canadensis) en Baja california, México. Propuestas para su conservación. Dissertation, Centro de Investigaciones Biológicas del Noroeste, S.C.
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA 105:6668–6672. https://doi.org/10.1073/pnas.0709472105
Di Vittorio M, López-López P (2014) Spatial distribution and breeding performance of Golden Eagles Aquila chrysaetos in Sicily: implications for conservation. Acta Ornithol 49:33–45. https://doi.org/10.3161/000164514X682878
Dinerstein E, Olson D, Joshi A, Vynne C, Burgess ND, Wikramanayake E et al (2017) An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 67:534–545. https://doi.org/10.1093/biosci/bix014
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G et al (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x
Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A et al (2006) Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29:129–151. https://doi.org/10.1111/j.2006.0906-7590.04596.x
Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ (2011) A statistical explanation of MaxEnt for ecologists. Divers Distrib 17:43–57. https://doi.org/10.1111/j.1472-4642.2010.00725.x
Escobar LE, Lira-Noriega A, Medina-Vogel G, Peterson TA (2014) Potential for spread of the white-nose fungus (Pseudogymnoascus destructans) in the Americas: use of Maxent and Niche A to assure strict model transference. Geospat Health 9:221. https://doi.org/10.4081/gh.2014.19
ESRI (2010) ArcMap 10.0. Environmental System Research Institute Inc, New York
Estrada F, Perron P, Yamamoto Y (2023) Anthropogenic influence on extremes and risk hotspots. Sci Rep 13:35. https://doi.org/10.1038/s41598-022-27220-9
Fajardo J, Corcoran D, Roehrdanz P, Hannah L, Marquet P (2020) GCM compare R: a web application to assess differences and assist in the selection of general circulation models for climate change research. Methods Ecol Evol 11:656–663. https://doi.org/10.1111/2041-210X.13360
Fecchio A, Wells K, Bell JA, Tkach VV, Lutz HL, Weckstein JD et al (2019) Climate variation influences host specificity in avian malaria parasites. Ecol Lett 22:547–557. https://doi.org/10.1111/ele.13215
Fernández-Bellon D, Wilson MW, Irwin S, O’Halloran J (2019) Effects of development of wind energy and associated changes in land use on bird densities in upland areas. Conserv Biol 33:413–422. https://doi.org/10.1111/cobi.13239
Ferrer-Sánchez Y, Rodríguez-Estrella R (2014) Man-made environments relationships with island raptors: endemics do not cope with habitat changes, the case of the island of Cuba. Biodivers Conserv 24:407–425. https://doi.org/10.1007/s10531-014-0819-y
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. https://doi.org/10.1002/joc.5086
Fielding AH, Haworth PF, Anderson D, Benn S, Dennis R, Weston E et al (2019) A simple topographical model to predict Golden Eagle Aquila chrysaetos space use during dispersal. Ibis 162:400–415. https://doi.org/10.1111/ibi.12718
Flesch EP, Graves TA, Thomson JM, Proffitt KM, White PJ, Stephenson TR et al (2020) Evaluating wildlife translocations using genomics: a bighorn sheep case study. Ecol Evol 10:13687–13704. https://doi.org/10.1002/ece3.6942
Gavin DG, Fitzpatrick MC, Gugger PF, Heath KD, Rodríguez-Sánchez F, Dobrowski SZ et al (2014) Climate refugia: joint inference from fossil records, species distribution models and phylogeography. New Phytol 204:37–54. https://doi.org/10.1111/nph.12929
GBIF.org (2021) GBIF occurrence download Aquila chrysaetos Linnaeus, 1758. https://doi.org/10.15468/dl.sazav7. Accessed 11 Feb 2021
Gil-Sanchez JM, Moleon M, Otero M, Bautista J (2004) A nine-year study of successful breeding in a Bonelli’s eagle population in southeast Spain: a basis for conservation. Biol Conserv 118:685–694. https://doi.org/10.1016/j.biocon.2003.10.017
Hannah L, Midgley G, Andelman S, Araújo M, Hughes G, Martinez-Meyer E et al (2007) Protected area needs in a changing climate. Front Ecol Environ 5:131–138. https://doi.org/10.1890/1540-9295(2007)5[131:PANIAC]2.0.CO;2
Hanspach J, Kühn I, Schweiger O, Pompe S, Klotz S (2011) Geographical patterns in prediction errors of species distribution models. Global Ecol Biogeogr 20:779–788. https://doi.org/10.1111/j.1466-8238.2011.00649.x
Hao T, Elith J, Guillera-Arroita G, Lahoz-Monfort JJ (2019) A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers Distrib 25:839–852. https://doi.org/10.1111/ddi.12892
Hijmans RJ, Phillips S, Leathwick J, Elith J (2021) Dismo: species distribution modeling. R package version 1.1-4. R package version 1.3-9. Retrieved from https://cran.r-project.org/web/packages/dismo/index.html. Accessed 15 Jul 2022
Hijmans R, van Etten J, Sumner M et al (2016) raster: geographic data analysis and modeling. R package version 2.3-40. https://cran.r-project.org/web/packages/raster/index.html. Accessed 15 July 2022
Hole DG, Huntley B, Arinaitwe J, Butchart SH, Collingham YC, Fishpool LD et al (2011) Toward a management framework for networks of protected areas in the face of climate change. Conserv Biol 25:305–315
Janss G, Lazo A, Ferrer M (1999) Use of raptor models to reduce avian collisions with powerlines. J Raptor Res 33:154–159
Jetz W, Wilcove DS, Dobson AP (2007) Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol 5:6. https://doi.org/10.1371/journal.pbio.0050157
Jones KR, Watson JE, Possingham HP, Klein CJ (2016) Incorporating climate change into spatial conservation prioritisation: a review. Biol Conserv 194:121–130. https://doi.org/10.1016/j.biocon.2015.12.008
Jones KR, Venter O, Fuller RA, Allan JR, Maxwell SL, Negret PJ et al (2018) One-third of global protected land is under intense human pressure. Science 360:788–791. https://doi.org/10.1126/science.aap9565
Karamizadeh S, Abdullah SM, Manaf AA, Zamani M, Hooman A (2013) An overview of principal component analysis. J Signal Inf Process 4:173–175. https://doi.org/10.4236/jsip.2013.43B031
Katzner T, Smith BW, Miller TA, Brandes D, Cooper J, Lanzone M et al (2012) Status, biology and conservation priorities for North America´s Eastern Golden Eagle (Aquila chrysaetos) population. Auk 129:168–176. https://doi.org/10.1525/auk.2011.11078
Kochert MN, Steenhof K, McIntyre CL, Craig EH (2002) Golden Eagle (Aquila chrysaetos), version 2.0. In: Poole AF, Gill FB (eds) The birds of North America. Ithaca, NY, USA, Cornell Lab of Ornithology
Kovács A, Mammen UCC, Wernham CV (2008) European monitoring for raptors and owls: state of the art and future needs. Ambio 37:408–412. https://doi.org/10.1579/0044-7447(2008)37[408:EMFRAO]2.0.CO;2
Lande R (1988) Demographic models of the northern spotted owl (Strixoccidentalis caurina). Oecologia 75:601–607. https://www.jstor.org/stable/4218620
Law EA, Macchi L, Baumann M, Decarre J, Gavier-Pizarro G, Levers C et al (2021) Fading opportunities for mitigating agriculture-environment trade-offs in a south American deforestation hotspot. Biol Conserv 262:109310. https://doi.org/10.1016/j.biocon.2021.109310
León-Girón G, Rodríguez-Estrella R, Ruiz-Campos G (2016) Current distribution status of Golden Eagle (Aquila chrysaetos) in Northwestern Baja California, Mexico. Rev Mex Biol 87:1328–1335. https://doi.org/10.1016/j.rmb.2016.10.003
Lobo JM, Jiménez-Valverde A, Real R (2008) AUC: a misleading measure of the performance of predictive distribution models. Global Ecol Biogeogr 17:145–151. https://doi.org/10.1111/j.1466-8238.2007.00358.x
Lovejoy TE, Hannah L (2019) Biodiversity and climate change: transforming the biosphere. Yale University Press, USA
Lozano LF, Ávila-Villegas L (2009) Águila Real, el símbolo nacional de México en riesgo. Instituto del Medio Ambiente (IMAE) de Aguascalientes. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad
Martín B, Onrubia A, Ferrer M (2014) Effects of climate change on the migration behavior of the Common Buzzard (Buteo buteo). Clim Res 60:187–197. https://doi.org/10.3354/cr01233
Martín B, Onrubia A, Ferrer M (2021) Climate change and the spatiotemporal variation in survival of a long-distance migrant (White Stork, Ciconia ciconia) across Western Europe. Birds 2:362–380. https://doi.org/10.3390/birds2040027
Marzluff JM, Knick ST, Vekasy MS, Schueck LS, Zarriello TJ (1997) Spatial use and habitat selection of golden eagles in southwestern Idaho. Auk 114:673–687. https://doi.org/10.2307/4089287
Maxwell SL, Cazalis V, Dudley N et al (2020) Area-based conservation in the twenty-first century. Nature 586:217–227. https://doi.org/10.1038/s41586-020-2773-z
Mayani-Parás F, Botello F, Castañeda S, Munguía-Carrara S, Sánchez-Cordero V (2021) Cumulative habitat loss increases conservation threats on endemic species of terrestrial vertebrates in Mexico. Biol Conserv 253:108864. https://doi.org/10.1016/j.biocon.2020.108864
McGrady MJ, Grant JR, Bainbridge IP, McLeod DRA (2002) A model of Golden eagle (Aquila chrysaetos) ranging behaviour. J Raptor Res 36:62–69
Secretaría del Medio Ambiente y Recursos Naturales [SEMARNAT] (2019) Modificación del Anexo Normativo III, Lista de especies en riesgo de la Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Diario Oficial de la Federación, 14 de noviembre de 2019, México
Secretaría del Medio Ambiente y Recursos Naturales [SEMARNAT] and Comisión Nacional de Áreas Naturales Protegidas [CONANP] (2008) Programa de Acción para la Conservación de la Especie (PACE): Águila Real (Aquila chrysaetos): 50. México
Mendoza-Ponce AV, Corona-Núñez RO, Kraxner F, Estrada F (2020) Spatial conservation prioritization for biodiversity in a megadiverse. Anthropocene 32:100267. https://doi.org/10.1016/j.ancene.2020.100267
Millsap BA, Harmata AR, Stahlecker DW, Mikesic DG (2014) Natal dispersal distance of bald and golden eagles originating in the coterminous United States as inferred from band encounters. J Rapt Res 48:13–23. https://doi.org/10.3356/JRR-13-00005.1
Millsap BA, Grubb TG, Murphy RK, Swem T, Watson JW (2015) Conservation significance of alternative nests of golden eagles. Global Ecol Conserv 3:234–241. https://doi.org/10.1016/j.gecco.2014.11.017
Morrone JJ, Escalante T, Rodríguez-Tapia G, Carmona A, Arana M, Mercado-Gómez, JD (2022) Biogeographic regionalization of the Neotropical region: New map and shapefile. An Acad Bras Ciênc 94:e20211167. https://doi.org/10.1590/0001-3765202220211167
Murphy RK, Stahlecker DW, Millsap BA, Jacobson KV, Johnson A, Smith CS et al (2019) Natal dispersal distance of Golden eagles in the southwestern United States. J Fish Wildl Manag 10:213–218. https://doi.org/10.3996/052018-JFWM-039
O’Toole L, Fielding AH, Haworth PF (2002) Re-introduction of the golden eagle into the Republic of Ireland. Biol Conserv 103:303–312. https://doi.org/10.1016/S0006-3207(01)00141-0
Ochoa-Ochoa LM, Rodríguez P, Mora F, Flores-Villela O, Whittaker RJ (2012) Climate change and amphibian diversity patterns in Mexico. Biol Conserv 150:94–102. https://doi.org/10.1016/j.biocon.2012.03.010
Ortega JC, Machado N, Diniz-Filho JAF, Rangel TF, Araújo MB, Loyola R et al (2019) Meta-analyzing the likely cross-species responses to climate change. Ecol Evol. https://doi.org/10.1002/ece3.5617
Osorio-Olvera L, Lira-Noriega A, Soberón J, Peterson AT, Falconi M, Contreras-Díaz RJ et al (2020) ntbox: an r package with graphical user interface for modelling and evaluating multidimensional ecological niches. Method Ecol Evol 11:1199–1206. https://doi.org/10.1111/2041-210X.13452
Owens HL, Campbell LP, Dornak LL, Saupe EE, Barve N, Soberón J et al (2013) Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol Model 263:10–18. https://doi.org/10.1016/j.ecolmodel.2013.04.011
Palmer RS (1988) Handbook of North American birds: volume 5, diurnal Raptors (Part 2) family ‘“Accipitridae”’ (concluded): buteos, Golden Eagle. Family ‘‘Falconidae”. Yale University Press, New Haven
Pandit R, Pörtner HO, Scholes RJ, Agard J, Archer E, Arneth A et al (2021) Scientific outcome of the IPBES-IPCC co-sponsored workshop on biodiversity and climate change. Report. Bonn, Germany: Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. https://www.publicgardens.org/resources/scientific-outcome-ipbes-ipcc-co-sponsored-workshop-biodiversity-and-climate-change. Accessed 6 Mar 2022
Pearson K (1901) On lines and planes of closest fit to systems of points in space. Philos Mag 2:559–572
Pearson R, Martínez-Meyer E, Andrade Velázquez M, Caron M, Corona-Núñez R, Davis K et al (2019) Research priorities for maintaining biodiversity’s contributions to people in Latin America. UCL Open: Environment 1, 02. https://doi.org/10.14324/111.444/ucloe.000002
Penteriani V, Otalora F, Sergio F, Ferrer M (2005) Environmental stochasticity in dispersal areas can explain the ‘mysterious’ disappearance of breeding populations. Proc R Soc Lond B Biol Sci 272:1265–1269. https://doi.org/10.1098/rspb.2005.3075
Penteriani V, Otalora F, Ferrer M (2008) Floater mortality within settlement areas can explain the Allee effect in breeding populations. Ecol Model 213:98–104. https://doi.org/10.1016/j.ecolmodel.2007.11.009
Perez-Navarro MA, Broennimann O, Esteve MA, Moya-Perez JM, Carreño MF, Guisan A, Lloret F (2021) Temporal variability is key to modelling the climatic niche. Res Biodivers 27:473–484. https://doi.org/10.1111/ddi.13207
Peterson AT, Ortega-Huerta MA, Bartley J, Sánchez-Cordero V, Soberón J, Buddemeier RH, Stockwell DR (2002) Future projections for Mexican faunas under global climate change scenarios. Nature 416:626. https://doi.org/10.1038/416626a
Peterson AT, Papeş M, Soberón J (2008) Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol Model 213:63–72. https://doi.org/10.1016/j.ecolmodel.2007.11.008
Peterson AT, Soberon J, Pearson RG, Anderson RP, Martínez-Meyer E, Nakamura M et al (2011) Ecological niches and geographic distributions. Princeton University Press, Princeton, New Jersey, USA
Phillips SJ, Anderson RP, Dudík M, Schapire RE, Blair ME (2017) Opening the black box: an open-source release of Maxent. Ecography 40:887–893. https://doi.org/10.1111/ecog.03049
Prieto-Torres DA, Navarro-Sigüenza AG, Santiago-Alarcon D, Rojas-Soto O (2016) Response of the endangered tropical dry forests to climate change and the role of Mexican Protected Areas for their conservation. Global Change Biol 22:364–379. https://doi.org/10.1111/gcb.13090
Prieto-Torres DA, Lira-Noriega A, Navarro-Sigüenza AG (2020) Climate change promotes species loss and uneven modification of richness patterns in the avifauna associated to Neotropical seasonally dry forests. Perspect Ecol Conserv 18:19–30. https://doi.org/10.1016/j.pecon.2020.01.002
Prieto-Torres DA, Nori J, Rojas-Soto OR, Navarro-Sigüenza AG (2021a) Challenges and opportunities in planning for the conservation of Neotropical seasonally dry forests into the future. Biol Conserv 257:109083. https://doi.org/10.1016/j.biocon.2021.109083
Prieto-Torres DA, Nuñez LE, Remolina-Figueroa D, Arizmendi MC (2021b) Most Mexican hummingbirds lose under climate and land-use change: long-term conservation implications. Perspect Ecol Conserv 19:487–499. https://doi.org/10.1016/j.pecon.2021.07.001
Qiao H, Soberón J, Peterson AT (2015) No silver bullets in correlative ecological niche modelling: insights from testing among many potential algorithms for niche estimation. Methods Ecol Evol 6:1126–1136. https://doi.org/10.1111/2041-210X.12397
Riahi K, van Vuuren DP, Kriegler E, Edmonds J, O’Neill BC, Fujimori S et al (2017) The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: an overview. Global Environ Change 42:153–168. https://doi.org/10.1016/j.gloenvcha.2016.05.009
Robertson MP, Visser V, Hui C (2016) Biogeo: an R package for assessing and improving data quality of occurrence record datasets. Ecography 39:394–401. https://doi.org/10.1111/ecog.02118
Rödder D, Engler JO (2011) Quantitative metrics of overlaps in Grinnellian niches: advances and possible drawbacks. Global Ecol Biogeogr 20:915–927. https://doi.org/10.1111/j.1466-8238.2011.00659.x
Rodríguez-Estrella R (2002) A Survey golden eagles in northern Mexico in 1984 and recent records in central and southern Baja California Peninsula. J Raptor Res 36:3–9
Rodríguez-Estrella R., Lafón A, de León G, Nocedal J, Chapa L, Eccardi F et al (2020) Informe del Programa de Monitoreo del Águila Real en México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad & Centro de Investigaciones Biológicas del Noroeste S.C. México
Rojas-Soto OR, Sosa V, Ornelas JF (2012) Forecasting cloud forest in eastern and southern Mexico: conservation insights under future climate change scenarios. Biodivers Conserv 21:2671–2690. https://doi.org/10.1007/s10531-012-0327-x
Sánchez-Romero R, Balvanera P, Castillo A, Mora F, García-Barrios LE, González-Esquivel CE (2021) Management strategies, silvopastoral practices and socioecological drivers in traditional livestock systems in tropical dry forests: an integrated analysis. For Ecol Manage 479:118506. https://doi.org/10.1016/j.foreco.2020.118506
Sánchez-Tapia A, Mortara SR, Rocha DSB, Barros FSM, Gall GM, De Siqueira MF (2020) modleR: a modular workflow to perform ecological niche modeling in R. BioRxiv. https://doi.org/10.1101/2020.04.01.021105
Selwood KE, McGeoch MA, Nally RM (2014) The effects of climate change and land-use change on demographic rates and population viability. Biol Rev 90:837–853. https://doi.org/10.1111/brv.12136
Sergio F, Tavecchia G, Blas J, Tanferna A, Hiraldo F, Korpimaki E, Beissinger SR (2022) Hardship at birth alters the impact of climate change on a long-lived predator. Nat Commun 13:5517. https://doi.org/10.1038/s41467-022-33011-7
Shepherd TG, Boyd E, Calel RA, Chapman SC, Dessai S, Dima-west IM et al (2018) Storylines: an alternative approach to representing uncertainty in physical aspects of climate change. Clim Change 151:555–571. https://doi.org/10.1007/s10584-018-2317-9
Sherry TW (2021) Sensitivity of tropical insectivorous birds to the Anthropocene: a review of multiple mechanisms and conservation implications. Front Ecol Evol 9:662873. https://doi.org/10.3389/fevo.2021.662873
Sierra-Morales P, Rojas-Soto O, Ríos-Munoz CA, Ochoa-Ochoa LM, Flores-Rodríguez P, Almazan-Núñez C (2021) Climate change projections suggest severe decreases in the geographic ranges of bird species restricted to Mexican humid mountain forests. Global Ecol Conserv 30:e01794. https://doi.org/10.1016/j.gecco.2021.e01794
Silva JLSE, Cruz-Neto O, Peres CA, Tabarelli M, Lopes AV (2019) Climate change will reduce suitable Caatinga dry forest habitat for endemic plants with disproportionate impacts on specialized reproductive strategies. PLoS ONE 14:e0217028. https://doi.org/10.1371/journal.pone.0217028
Soberón J, Peterson AT (2005) Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers Inf 2:1–10. https://doi.org/10.17161/bi.v2i0.4
Steenhof K, Kochert MN, Macdonald TL (1997) Interactive effects of prey and weather on Golden Eagle reproduction. J Anim Ecol 66:350–362. https://doi.org/10.2307/5981
Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J et al (2013) Climate Change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, United Kingdom and New York
Stoerk T, Wagner G, Ward RE (2018) Policy brief—Recommendations for improving the treatment of risk and uncertainty in economic estimates of climate impacts in the sixth Intergovernmental Panel on Climate Change assessment report. Rev Environ Econ Policy 12:371–376. https://doi.org/10.1093/reep/rey005
Stouffer PC (2020) Birds in fragmented Amazonian rainforest: lessons from 40 years at the Biological Dynamics of Forest Fragments Project. The Condor 122:duaa005. https://doi.org/10.1093/condor/duaa005
Struebig MJ, Wilting A, Gaveau DLA, Meijaard E, Smith RJ (2015) Targeted conservation to safeguard a biodiversity hotspot from climate and land-cover change. Curr Biol 25:372–378. https://doi.org/10.1016/j.cub.2014.11.067
Tapia L, Domínguez J, Rodríguez L (2007) Modelling habitat use and distribution of golden eagles Aquila chrysaetos in a low-density area of the Iberian Peninsula. Biodivers Conserv 16:317–332. https://doi.org/10.1007/978-1-4020-6865-2_22
Tavizon J (2014) Dinámica Poblacional y Viabilidad Espacio Temporal del Águila Real. Dissertation, Universidad Autónoma de Nuevo León
Thuiller W, Lavorel S, Araújo MB (2005) Niche properties and geographic extent as predictors of species sensitivity to climate change. Global Ecol Biogeogr 14:347–357. https://doi.org/10.1111/j.1466-822X.2005.00162.x
Triviño M, Kujala H, Araújo MB, Cabeza M (2018) Planning for the future: identifying conservation priority areas for Iberian birds under climate change. Landsc Ecol 33:659–673. https://doi.org/10.1007/s10980-018-0626-z
Tuanmu MN, Jetz W (2015) A global, remote sensing-based characterization of terrestrial habitat heterogeneity for biodiversity and ecosystem modelling. Global Ecol Biogeogr 24:1329–1339. https://doi.org/10.1111/geb.12365
Ureta C, Cuervo-Robayo AP, Calixto-Pérez E, González-Salazar C, Fuentes-Conde E (2018) A first approach to evaluate the vulnerability of islands’ vertebrates to climate change in Mexico. Atmosfera 31:221–254. https://doi.org/10.20937/ATM.2018.31.03.0
Ureta C, Ramírez-Barrón M, Sánchez-García EA, Cuervo-Robayo AP, Munguía-Carrara M, Mendoza-Ponce A et al (2022) Species, taxonomic, and functional group diversities of terrestrial mammals at risk under climate change and land-use/cover change scenarios in Mexico. Global Change Biol 28:6992–7008. https://doi.org/10.1111/gcb.16411
USGS (2001) HYDRO1k elevation derivate database, sioux falls, SD: U.S. geologica survey earth resources observation and science (EROS) center. https://lta.cr.usgs.gov/HYDRO1K. Accessed 20 Oct 2021
Velazco SJE, Villalobos F, Galvão F, De Marco JP (2019) A dark scenario for Cerrado plant species: effects of future climate, land use and protected areas ineffectiveness. Divers Distrib 25:660–673. https://doi.org/10.1111/ddi.12886
Velazco SJE, Ribeiro BR, Laureto O, Maira L, De Marco JP (2020) Overprediction of species distribution models in conservation planning: a still neglected issue with strong effects. Biol Conserv 252:108822. https://doi.org/10.1016/j.biocon.2020.108822
Vilela B, Villalobos F (2015) letsR: a new R package for data handling and analysis in macroecology. Methods Ecol Evol 6:1229–1234. https://doi.org/10.1111/2041-210X.12401
Warren DL, Glor RE, Turelli M (2008) Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62:2868–2883. https://doi.org/10.1111/j.1558-5646.2008.00482.x
Watson J (2010) The Golden Eagle. T. & A.D. Poyser, London
Whitfield DP, Fielding AH, McLeod DR, Haworth PF, Watson J (2006) A conservation framework for the Golden Eagle in Scotland: refining condition targets and assessment of constraint influences. Biol Conserv 130:465–480. https://doi.org/10.1016/j.biocon.2006.01.008
Whitfield DP, Fielding AH, Gregory MJ, Gordon AG, McLeod DR, Haworth PF (2007) Complex effects of habitat loss on Golden Eagles Aquila chrysaetos. Ibis 149:26–36. https://doi.org/10.1111/j.1474-919X.2006.00591.x
Whitfield DP, Fielding AH, McLeod DR, Haworth PF (2008) A conservation framework for golden eagles: implications for their conservation and management in Scotland. Scottish National Heritage
Whitfield DP, Fielding AH, Anderson D, Benn S, Reid R, Tingay R, Weston E (2023) Sex difference in natal dispersal distances of Golden Eagles Aquila chrysaetos in Scotland. Ibis. https://doi.org/10.1111/ibi.13225
Wiggins DA, Schnell GD, Agustín DJ (2014) Distribution and nesting success of ferruginous hawks and Swainson’s hawks on an agricultural landscape in the Great Plains. Southwest Nat 59:356–363. https://doi.org/10.1894/MCG-01.1
Yesson C, Brewer PW, Sutton T, Caithness N, Pahwa JS, Burgess M et al (2007) How global is the global biodiversity information facility? PLoS ONE 2:e1124. https://doi.org/10.1371/journal.pone.0001124
Zelinka D, Myers TA, McCoy DT, Po-Chedley S, Caldwell PM, Ceppi P et al (2020) Causes of higher climate sensitivity in CMIP6 models. Geophys Res Lett 47:e2019GL085782. https://doi.org/10.1029/2019GL085782
Acknowledgements
A version of this study was submitted by AMG-R as a written thesis in partial fulfillment of the requirement for a bachelor’s degree at the Instituto Politécnico Nacional (IPN, Mexico). We appreciate the financial and logistical support provided by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (DGAPA-UNAM; PAPIIT projects: IA202822 [DAP-T]) and Programa de Investigación en Cambio Climático (PINCC-UNAM), which allowed us to perform this study. DAP-T also extends his gratitude to the Rufford Foundation (project 28502-B) for financial resources for the development of courses and workshops during which students (including AMG-R) acquired the tools and skills necessary to carry out this kind of research. We also appreciate the efforts of CONANP and the Programa de Monitoreo del Águila Real en México to generate more than 15 years of information about the breeding ecology of this species, including the open access data that allowed us to perform this study. We also thank Javier Fajardo for providing technical assistance using the GCM compareR’s web application based on data from the Coupled Model Intercomparison Project 6 (CMIP6). We also thank to Lynna M. Kiere for feedback on English language editing and manuscript proofreading.
Author information
Authors and Affiliations
Contributions
AMG-R and DAP-T conceived and designed the study. AMG-R and LFL compiled the database of available records and performed the fieldwork. AMG-R and DAP-T performed the ecological niche models and spatial analyses. All authors contributed to the analysis and interpretation of results and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have declared that no competing interests exist.
Additional information
Communicated by O. Krüger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gama-Rodríguez, A.M., García, J.A., Lozano, L.F. et al. Protecting breeding sites: a critical goal for the conservation of the golden eagle in Mexico under global change scenarios. J Ornithol (2024). https://doi.org/10.1007/s10336-024-02168-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10336-024-02168-x