Abstract
Dispersal is an important behavioral process that plays a significant role in, among others, speciation, population viability, and individual fitness. Despite progress in avian dispersal research, there are still many knowledge gaps. For example, it is of interest to study how dispersal propensity relates to age- and/or sex-specific patterns. Here, we investigated the role of sex and life stage on natal (i.e., movement from birth site to first breeding site) and breeding dispersal (i.e., movement between sequential breeding sites) in the Kentish Plover (Charadrius alexandrinus) for dispersal events of more than 10 km. This small and inconspicuous wader is characterized by flexible mating behavior that includes monogamy, and serial polygynandry. Using a continent-wide dataset of ringing and re-encounter data throughout the species’ range in Europe, we found that adult females generally dispersed further than adult males between seasons, but we detected no sex difference in natal dispersal distances and no general difference between natal and breeding dispersal distances. Furthermore, females were the main group exhibiting ‘long-distance’ breeding dispersal, which we defined as dispersal greater than ≥ 108 km, i.e., the upper 10% percentile of our dataset. The data set included dispersal of two females that first bred in the Mediterranean before being detected breeding at the North Sea in the subsequent year, having dispersed 1290 and 1704 km, respectively. These observations represent the longest breeding dispersal observed within the genus Charadrius. Our long-distance dispersal records are consistent with low genetic differentiation between mainland populations shown in previous work. The dispersal of the Kentish Plover is likely linked to its breeding behavior: polyandrous females exhibit extensive mate searching and habitat prospecting. We recommend that the dispersal traits of Kentish Plover be incorporated into the species’ conservation and management planning to more accurately inform models of population connectivity and metapopulation dynamics.
Zusammenfassung
Dispersion beim Seeregenpfeifer (Charadrius alexandrinus): Adulte Weibchen unternehmen die weitesten Wanderungen
Dispersion ist ein wichtiger Verhaltensprozess, der unter anderem für die Artbildung, die Lebensfähigkeit von Populationen und die individuelle Fitness eine wichtige Rolle spielt. Trotz der Fortschritte bei der Erforschung der Dispersion von Vögeln gibt es noch viele Wissenslücken. So ist es beispielsweise von Interesse zu untersuchen, wie die Dispersionsneigung mit alters- und/oder geschlechtsspezifischen Mustern zusammenhängt. Hier haben wir die Rolle des Geschlechts und des Alters für die Dispersion nach der Geburt (natal dispersal; d. h. die Bewegung zwischen Geburtsort und erstem Brutort) und die Umsiedlungen der Altvögel (breeding dispersal; d. h. die Bewegung zwischen aufeinanderfolgenden Brutplätzen) beim Seeregenpfeifer (Charadrius alexandrinus) jeweils für Distanzen von mehr als 10 km untersucht. Dieser kleine und unauffällige Regenpfeifer zeichnet sich durch ein flexibles Paarungsverhalten aus, das sowohl Monogamie als auch serielle Polygynadrie umfasst. Anhand eines kontinentweiten Datensatzes von Beringungs- und Wiederfunddaten aus dem gesamten Verbreitungsgebiet der Art in Europa haben wir festgestellt, dass sich adulte Weibchen generell weiter zwischen Brutsaisons umsiedelten als adulte Männchen. Wir haben aber keinen Geschlechtsunterschied bei den Ausbreitungsdistanzen nach der Geburt und keinen allgemeinen Unterschied zwischen den Dispersionsdistanzen nach der Geburt und als Altvögel festgestellt. Darüber hinaus waren adulte Weibchen die Hauptgruppe mit Fernumsiedlungen, die wir als Umsiedlungen von ≥ 108 km definierten (oberes 10%-Perzentil unseres Datensatzes). Im Rahmen unserer Arbeit wurden zwei Weibchen entdeckt, die im Mittelmeer brüteten, bevor sie sich im darauffolgenden Jahr an der Nordsee ansiedelten, und zwar in Entfernungen von 1.290 bzw. 1.704 km – dies sind die weitesten bekannte Brutumsiedlungen innerhalb der Gattung Charadrius. Die von uns ermittelten Fernumsiedlungen stehen im Einklang mit der geringen genetischen Differenzierung zwischen den Festlandspopulationen, die in früheren Arbeiten nachgewiesen wurde. Die hohe Dispersionsneigung des Seeregenpfeifers ist wahrscheinlich auf sein Brutverhalten zurückzuführen: Polyandrische Weibchen suchen ausgiebig nach Partnern und Lebensräumen. Wir empfehlen, die Dispersionseigenschaften des Seeregenpfeifers in die Schutz- und Managementplanungen einzubeziehen, um besser Informationen über die Populationskonnektivität und Metapopulationsdynamik zu erhalten.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dispersal plays an important role in population biology as it has many evolutionary consequences for individual fitness and population viability but also adaptation and speciation (e.g., Greenwood 1980; Greenwood and Harvey 1982; D’Urban Jackson et al. 2017). For example, dispersal can increase access to potential mates, serves to prevent inbreeding, and reduces competition between relatives or conspecifics (Greenwood 1980). At the population level, dispersal leads to range expansion, the colonization of novel habitat, and increases gene flow throughout the metapopulation (Clobert et al. 2001). Moreover, it is important to understand dispersal dynamics of threatened species so that conservation measures acknowledge a species’ ability to adapt to changing environmental conditions. Here, we study the dispersal dynamics of the Kentish Plover (Charadrius alexandrinus) and put our findings in the context of other small-bodied avian species with extensive breeding distributions and flexible mating systems—traits that characterize several demographically vulnerable Charadrius spp. worldwide.
Dispersal is often categorized by life stage. Natal dispersal refers to the movement of individuals from their birthplace to their first breeding site, whereas breeding dispersal refers to individual movements between successive reproductive attempts (Greenwood and Harvey 1982). Distinguishing between natal and breeding dispersal explains some of the observed variation in dispersal distances within species although a substantial amount of variation in dispersal is also explained by individual traits and state (e.g., genotype, phenotype, sex, and previous reproductive success) and habitat quality and availability (e.g., Clark et al. 1997; Skrade and Dinsmore 2010; Rioux et al. 2011; Pearson and Colwell 2014; Swift et al. 2021).
In birds, natal dispersal distances are often larger than breeding dispersal distances (Greenwood and Harvey 1982). For example, Paradis et al. (1998) found larger natal than breeding dispersal distances in 61 of 69 studied British bird species. After having settled as a breeder, site fidelity can be beneficial in terms of familiarity with former mates, predator avoidance, food resources, and other habitat characteristics of a site (e.g., Greenwood and Harvey 1982), which may explain why breeding dispersal is often less pronounced than natal dispersal. For example, many young colonial seabirds disperse to another colony, but movements of established adult breeders between colonies are rare (e.g., Greenwood and Harvey 1982). However, inbreeding avoidance of young birds might play a key role, too. Alternatively, in nomadic species, natal and breeding dispersal are often equally pronounced in all age and sex classes: probably because all birds change sites when habitat conditions deteriorate (e.g., Greenwood and Harvey 1982; Robinson and Oring 1997).
A substantial part of dispersal variation within species is explained by differences between males and females. In mate-competition systems, site familiarity is of greater benefit for the sex that invests more in parental care (e.g., the female), whereas individuals of the other sex may explore different breeding sites in search of potential mates (e.g., Kempenaers and Valcu 2017). In birds, most species show female-biased dispersal as males typically defend territories or resources to attract females. However, in some avian clades, such as Anatidae, males are the more dispersive sex (Greenwood 1980; Clark et al. 1997; Mabry et al. 2013). Male-biased natal dispersal was also found in spotted sandpipers Actitis macularia, in which polyandrous females compete over resources (Oring and Lank 1982; Reed and Oring 1993). The mating system itself may be associated with sex-biased dispersal and breeding site fidelity (Kwon et al. 2022), but this relationship is generally weak across birds (Mabry et al. 2013; Trochet et al. 2016).
Despite the aforementioned progress in avian dispersal research, there are still many knowledge gaps—some of which are touched upon in our study. First, it is unclear whether breeding dispersal propensity is high in small-bodied species inhabiting unpredictable habitats, as this relationship has mainly been documented in larger and more conspicuous species (e.g., Robinson and Oring 1997; Donald et al. 2021). Particularly, it is of interest whether dispersal propensity relates to age- and/or sex-specific patterns (e.g., Greenwood and Harvey 1982). Second, dispersal patterns may be even more complex in species with widely spaced breeding distributions and flexible mating systems including serial polygamy with brood desertion and subsequent re-mating. This behavior is seen in several Charadrius plover species breeding in temperate and tropical latitudes with extended breeding seasons (Eberhart-Phillips 2019; Stenzel and Page 2019). Plover species exhibiting this breeding behavior are often characterized by male–male resource competition in combination with male-biased brood care, which may lead to strong sex differences in dispersal. However, patterns may also be population specific due to local constraints such as density dependence and operational sex ratio (Eberhart-Phillips et al. 2018). Third, estimating dispersal rates from spatially restricted study sites may underestimate true dispersal patterns and mislead inference (Barrowclough 1978; Greenwood and Harvey 1982; Paradis et al. 1998). For example, many avian study sites are spatially smaller than the distance that a bird can move within a day (Paradis et al. 1998). Thus, more analyses on broad geographical scale are necessary to cover the full range of dispersal in a species (see Paradis et al. 1998).
To address the aforementioned research gaps in avian dispersal, the Kentish Plover (Charadrius alexandrinus) serves as an ideal study system. Kentish Plovers, nesting solitarily or semi-colonially, exhibit a particularly diverse mating system that includes monogamy and serial polygamy with many females deserting early broods soon after hatching to re-mate for another breeding attempt (Rittinghaus 1961; Lessells 1984; Székely and Lessells 1993; Amat et al. 1999; Kosztolányi et al. 2009; Eberhart-Phillips 2019). Additionally, the large breeding range of Kentish Plovers includes temperate and subtropical zones of Eurasia and northern Africa where it inhabits coastal and (often saline) inland habitats (BirdLife International 2022). Within Europe, the breeding distribution is scattered along the Atlantic and Mediterranean coasts, the Black Sea and Caspian Sea, and inland areas in central and eastern Europe (Keller et al. 2020) with a breeding population of approximately 21,500–34,800 pairs (BirdLife International 2015). Especially in its European range, the distribution is rather patchy nowadays (e. g., Thorup and Bregnballe 2021). The species is listed as threatened or declining in many European countries because of degradation and loss of wetland habitat and human disturbance (e.g., destruction of nests) on beaches (e.g., Gomez-Serrano 2021; Thorup and Bregnballe 2021; BirdLife International 2022). While breeders of northern populations perform long-distance migration, breeders of southern populations are either short-distance migrants or residents (BirdLife International 2022; Spina et al. 2022). Population genetic analyses show that Kentish Plovers across continental Eurasia are near panmictic (Küpper et al. 2012; Sadanandan et al. 2019): high gene flow is female biased and likely driven by serially polyandrous females that are capable of dispersing long distances between breeding attempts (Küpper et al. 2012). However, the genetic data evaluated to-date do not allow for the distinction between natal and breeding dispersal, hindering genetic-based investigations of sex-specific dispersal at different life-history stages. Re-encounter data of previously marked birds enable more detailed investigations of dispersal, but previous studies in this species were restricted to local or regional scales (e.g., Székely and Lessels 1993; Foppen et al. 2006).
To characterize the dispersal movements of Kentish Plovers in a more comprehensive manner, we utilized a European-wide bird ringing database provided by EURING. We predicted that natal and breeding dispersal distances would not differ in Kentish Plovers: dispersal should remain high across both life-history stages due to frequent re-mating in the species and the widely spaced breeding distribution in Europe leading to regular movements of individuals between breeding sites in search of new partners or higher quality habitat. Although both males and females can be serially polygamous, we predicted that breeding dispersal would be higher in females than in males because males acquire nesting territories and invest more in brood care than females, and serial polyandry is more common than serial polygyny in this species (Lessells 1984; Amat et al. 1999; Kosztolányi et al. 2009).
We aimed to use our results gained from the Kentish Plover for a comparison with other Charadrius plovers, as this genus offers a wide range of different breeding systems that might influence dispersal behavior.
Methods
Source of data and fundamental data categorization
The primary source for our analyses was the ‘EURING Data Bank’ (obtained 9/10/2019; du Feu et al. 2009), which contained 12,057 records of Kentish Plovers: 9651 re-encounters (i.e., re-captures, re-sightings, and dead recoveries) of 2406 individually ringed birds. Of those, we excluded entries of individual plovers that were hand reared or moved before release (13 individuals) and imprecise entries. Imprecise entries were those without a precision of less than 1 week for the date (35 individuals), or had imprecise geographical locations with an uncertainty of more than 5 km (544 data points of 264 individuals). Finally, we excluded re-encounters of dead plovers except those that were found freshly dead (86 individuals).
To separate dispersal from other movements, we classified the status of each ringing or re-encounter data point. We defined ‘certain breeding’ as either being classified as ‘nesting or breeding’ (status ‘N’) or being caught at a nest (catching method ‘N’). We assigned ‘possible breeding’ for all other data with status ‘K’ (‘in colony’) or birds being ringed or re-encountered during the core breeding period between May and June for European populations (see Rittinghaus 1975). As the base data for natal dispersal, we took plovers being ringed as a chick (EURING code ‘1’ for age), because those chicks certainly hatched close to their ringing sites. In total, 5428 ringing or re-encounter data of 1906 individuals applied to one of those definitions (i.e., certainly breeding, possibly breeding or ringed as a chick).
We aligned ringing and re-encounter data to identify data that fulfilled one of our breeding status criteria for both the ringing and re-encounter data of an individual. This resulted in 3223 data points of 1070 individuals after removing re-encounters of first calendar year birds. Consequently, ‘certain dispersal’ refers to cases of ‘certain breeding’ (including data of birds being ringed as chicks) of an individual at the ringing and re-encounter date, whereas the status ‘possible dispersal’ was given for cases with only ‘possible breeding’ either at the ringing or re-encounter stage. We pooled certain and possible breeding dispersal for our analyses to have a larger sample size, but we provide results based on certain dispersal data only if sample sizes were sufficient (e.g., only for between-season breeding dispersal, see below and Fig. S1 in the online supplement).
Definitions of dispersal types and further data selection
For our analyses, we excluded all dispersal movements within a 10 km radius around the ringing place, and generally dispersal distances up to 10 km, because re-encounters within 10 km were not consistently reported from ringers to national ringing schemes or from national ringing schemes to EURING, and the spatial precision (e.g., data reported on site level) of most data was too low for fine-scale analyses using the EURING data. Hence, our analyses ignore short-distance dispersal movements below 10 km although we acknowledge that such movements may represent a large proportion of ‘dispersal events’ (see Table 1). Here, we focus on medium- to long-distance dispersal, which we defined in this study as comprising distances of > 10–107 km and ≥ 108 km, respectively. As there is no general threshold for long-distance dispersal in animals and plants (Nathan et al. 2003), and it has, therefore, to be defined on a case-by-case basis, we classified movements equal or greater than 108 km as long-distance dispersal for our focal group of birds (between-season breeding dispersal) because this threshold represents the upper 10% percentile of our dataset (see Fig. 1b). We did not use the threshold of 50 km used by Stenzel et al. (1994) for long-distance dispersal in the closely related Snowy Plover because their threshold was case specific to the US Pacific Coast and was not informed by a statistical distribution. We obtained distances between ringing and re-encounter locations directly from the EURING database or measured in QGIS version 3.16.10-Hannover (QGIS Development Team 2020) using its ‘Shape Tools’ extension.
‘Natal dispersal’ is referred to as movements by individuals that were ringed as chicks and re-encountered for the first time in their first year after ringing (i.e., the birds’ second calendar year of life, which is the typical age of first breeding in Kentish Plovers, cf. Rittinghaus 1961). Furthermore, the status of their first re-encounter following their hatch year had to be either possible or certain breeding (n = 13 males and 21 females, of which 3 males and 9 females performed certain dispersal). We referred to ‘breeding dispersal’ here for birds that were ringed as adults and re-encountered at least possibly breeding in their first calendar year after ringing (n = 6 males and 33 females, of which only 10 females were categorized as certain dispersal). This made it possible to compare general patterns of ‘natal dispersal’ with ‘breeding dispersal’.
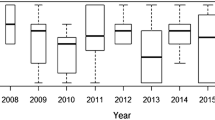
Distribution of sex-specific dispersal distances in European Kentish Plovers (Charadrius alexandrinus) based on the EURING database: a comparison of all sex-type combinations of natal and breeding dispersal (one data point for each individual within the first year after ringing, see Methods); b sex differences in between-season breeding dispersal without reference to elapsed time since ringing (1–13 years). The two recently observed cases of dispersal from the Mediterranean Sea to the North Sea (see text) are indicated by asterisks. Horizontal lines in boxplots show median values, box shows the 25 and 75 percentiles, whiskers the 1.5 times interquartile range
For a more comprehensive analysis of sex differences in between-season breeding dispersal, we included all re-encounter data of an individual without reference to elapsed time since ringing (see Stenzel et al. 1994; Pakanen et al. 2015). Those birds were re-encountered 1–13 years after being ringed as an adult (n = 33 males, 87 females, of which 4 males and 22 females referred to certain dispersal). We did so because many individuals were not detected within 1 year after ringing. However, many of those birds were re-sighted in later years (e.g., over-seen in their old or new breeding site in the meantime). There was no effect of elapsed time since ringing on the observed distances (see Fig. S2 in the online supplement). Only two individuals were detected at more than two places (including the ringing place) > 10 km away from each other in different breeding seasons (i.e., those birds performed more than one dispersal movement). For consistency, we used the first observed dispersal movement after ringing for all individuals. Our data included between-season movements of seven birds back to the area around their ringing place (< 10 km) following within-season dispersal to another place. We refer to between-season dispersal distances ≥ 108 km as ‘long-distance’ breeding dispersal (i.e., the upper 10% percentile of our dataset).
We refer to within-season breeding dispersal for adult birds that were found at a second place within the same breeding season either in the year of ringing or in subsequent years. However, our sample size was low for this subset of our data (n = 20 males, 24 females, of which 6 females referred to certain dispersal).
Additional data from color-ringing projects
We used own color-ringing data that are not yet included in the EURING dataset, to enlarge the sample size for between-season long-distance breeding dispersal (≥ 108 km). We color-ringed Kentish Plovers at the Tagus estuary in Portugal (n = 135 males, 161 females; ringing period 2007–2021), Brittany, NW France (n = 95 males, 191 females; ringing period 2008–2018), the Marker Wadden in The Netherlands (n = 7 males, 17 females; ringing period 2020–2021), and Schleswig–Holstein, Germany (n = 92 males, 127 females; ringing period 2009–2021). Of those, 12 plovers (2 males and 10 females) were found ≥ 108 km to have dispersed in later breeding seasons until 2022.
Data analyses
All analyses were performed in R (version 4.1.1, R Core Team 2021). We checked the fit of the data with and without transformation in competing models using the fitdistrplus package (Dutang 2015) and diagnostic plots in R. We then chose appropriate linear or generalized linear models, or non-parametric tests for hypothesis testing. We performed a linear model with log-transformed dispersal distances to evaluate sex- and stage-specific differences. As the observed dispersal distances were strongly skewed and the fit of tested generalized linear models was poor, we used the non-parametric Kruskal–Wallis test to examine differences in breeding dispersal distances between the sexes because of its robustness (i.e., making no assumptions about the distribution of the data). To compare sex-specific proportions of long-distance breeding dispersal, we used Fisher’s exact test. We used QGIS (v. 3.16; QGIS Development Team 2020) for visualization of long-distance breeding dispersal movements.
Results
Medium- to long-distance natal vs. breeding dispersal distances in the first year after ringing
Median natal dispersal distance > 10 km of Kentish Plovers in the EURING dataset was 18 km (interquartile range (IQR) = 16–84 km, maximum = 201 km; n = 13) for males and 21 km (IQR = 16–53 km, maximum = 449 km; n = 21) for females (Fig. 1a). Median breeding dispersal distance > 10 km was 14 km (IQR = 12–17.5 km, maximum = 66 km; n = 6) for males and 22 km (IQR = 16–76 km, maximum = 1,704 km; n = 33) for females (Fig. 1a). We found no statistically clear effect of sex (F1,70 = 0.841, p = 0.36, 95% CI = − 0.436–0.161 for males), stage (F1,70 = 0.073, p = 0.79, 95% CI = − 0.299–0.227 for natal dispersal) or the interaction of sex and stage (F1,69 = 2.068, p = 0.15, 95% CI = − 0.170–1.048 for natal dispersal of males) on dispersal distance. There were not enough data for ‘certain dispersal’ for adult males to run the same model with this data set alone.
Sex differences in between-season breeding dispersal distances (> 10 km) and in the proportion of long-distance breeding dispersal (≥ 108 km) 1+ years after ringing
Males had shorter dispersal distances (median = 18 km, IQR = 12–38 km, maximum = 1058 km; n = 33) than females (median = 28 km, IQR = 16.5–70 km, maximum = 1704 km, n = 87; Kruskal–Wallis Chi-squared = 4.779, df = 1, p = 0.03; Fig. 1b) considering all distances > 10 km. This sex difference is consistent if only certain dispersal data are used (see Fig. S1 in the online supplement), but this difference is not statistically significant due to the more limited sample size (n = four males, 22 females).
The proportion of long-distance breeding dispersal (≥ 108 km) in relation to all observed movements > 10 km between seasons tended to be lower in adult males than in adult females: only 1 of 33 adult males (3.0%) compared to 11 of 87 adult females (12.6%) moved ≥ 108 km, even though the relationship was not significant (Fisher test, p = 0.18). Similarly, the proportion of males moving ≥ 108 km in relation to total ringing numbers with given sex (0.2%, 1 of 403) was about an order of magnitude lower than in females (1.7%, 11 of 656; Fisher’s exact test: p = 0.04). In our additional data not included in the EURING dataset, the proportion of color-ringed males with long-distance breeding dispersal (0.6%, 2 of 329) was also lower than the proportion of females (2.0%, 10 of 496) although the difference was not statistically significant (Fisher’s exact test: p = 0.14).
Extent of long-distance breeding dispersal (≥ 108 km)
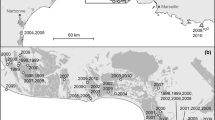
Adult Kentish Plovers occasionally moved large distances among breeding sites (Fig. 2). Of the 1,059 adult Kentish Plovers that were sexed when ringed, 12 (1.1%) dispersed ≥ 108 km. Dispersal between inland and coastal regions or between different seas (e.g., Atlantic and Mediterranean Sea) was observed in four cases: one male and female certainly dispersed from inland breeding sites in eastern Austria to coastal sites at the Mediterranean Sea in France (1058 km) or Italy (669 km), respectively, within 1–3 years after ringing (Fig. 2). From the Mediterranean Sea, two females certainly dispersed to the North Sea over 1290 and 1,704 km, respectively, between two successive breeding seasons (Fig. 2).
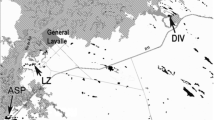
Long-distance breeding dispersal (≥ 108 km) in European Kentish Plovers (Charadrius alexandrinus): a map with data from EURING database (see Methods for details). Places of re-encounters are shown by blue circles (males) and red circles (females). Additional re-encounters from color-ringing studies in Portugal, France, The Netherlands, and Germany (see Methods) are supplemented here for visualization with blue triangles (males) and red triangles (females). Independently of data source, the lines connect those places with original ringing places (males: dashed lines, females: solid lines). The ringing places of two females which dispersed from the Mediterranean Sea to the North Sea between the breeding seasons, 2017 and 2018 (see panels b and c), are indicated by asterisks. b Female “Red 024” was ringed during incubation at the salines of Pesquieres near Hyères, France, in 2017 and was re-caught 1290 km away at its nest in Beltringharder Koog, Germany in 2018 (picture taken by Diana Nett). c This female dispersed over 1704 km from the Spanish island of Mallorca to the sandbank of Sankt Peter-Ording, Germany, between the breeding seasons, 2017 and 2018 (picture taken by Rainer Schulz)
Within-season breeding dispersal (> 10 km)
Dispersal distances of > 10 km within a breeding season were not significantly different between males (median = 18.5 km, IQR = 17.75–19.5 km, maximum = 49 km; n = 20) and females (median = 18.5 km, IQR = 12–35.75 km, maximum = 146 km, n = 24; Kruskal–Wallis Chi-squared = 0.009, df = 1, p = 0.92). The variance of the distances was higher in females than in males, with distances of ≥ 108 km only found in two females but no males. There were not enough data for a separate analysis for the category ‘certain dispersal’ (see Methods), as only data for six females and no data for males were available.
Discussion
Natal vs. breeding dispersal in the first year after ringing
Our data show similar distances for natal and breeding dispersal events of more than 10 km (Fig. 1a), indicating that there are no age-dependent differences for Kentish Plovers in these movements. Moreover, breeding dispersal at the continental scale (i.e., > 1000 km) within the first year after ringing was exclusively detected in adult females (Fig. 1a).
Generally, natal dispersal is typically more pronounced in birds than breeding dispersal (Greenwood and Harvey 1982; Paradis et al. 1998, but see Dale et al. 2005). However, we acknowledge that we were unable to examine potential differences in small-scale (< 10 km) dispersal events, which typically comprise the majority of all dispersal movements (cf. Foppen et al. 2006; Skrade and Dinsmore 2010; Pakanen et al. 2015), because of limitations of the EURING dataset (see Methods). Consequently, our results are meaningful with regard to medium- to long-distance dispersal. This could explain why local-scale dispersal distances of Kentish Plovers studied by Foppen et al. (2006) in the Dutch Wadden Sea were further for juveniles than for adults, which is in contrast to our results.
A general limitation of large-scale ringing data is that we might have not detected all dispersal movements of an individual, potentially leading to false categorizations (e.g., mixing of early breeding and natal dispersal or within-season and between-season breeding dispersal). Moreover, an observed dispersal movement might be the product of several successive movements. However, as we found no relationship between the time gap and between-season breeding dispersal distance (see Fig. S2), we believe our ringing data reflect true dispersal behavior of the study species.
Sex-biased breeding dispersal
Observed medium- to long-distance dispersal distances were smaller in adult males than in adult females (Fig. 1a, b), and the proportion of dispersing males with long-distance breeding dispersal was nearly ten times lower than the proportion of females compared to ringing numbers. The additional color-ringing data showed a similar pattern, but the difference was not statistically significant and sample size was limited. Females seem to be the main drivers of breeding dispersal over larger distances in Kentish Plovers, which is consistent with earlier genetic studies (see section “Genetic consequences” below).
Female-biased breeding dispersal is likely linked to the mating system of the Kentish Plover, which is dominated by sequential polyandry and reduced parental care in females (e.g., Amat et al. 1999): long-distance dispersal by females could be related to their extensive search for new partners. Because females are rarer than males among breeding Kentish Plovers (e.g., 42% in Turkey, Eberhart-Phillips et al. 2018; Eberhart-Phillips 2019), in principle they have the opportunity to re-mate quicker than males (Székely et al. 1999). Long-distance dispersal can enable such females to exploit heterogenous environments and find optimal breeding habitat for the sequential breeding attempts. By contrast, male Kentish Plovers need to acquire and defend a breeding territory, with site fidelity providing advantages in local resource competition. Both explanations, female brood desertion for re-mating and male–male resource competition, are not mutually exclusive but likely drive the sex-biased breeding dispersal that we documented.
Despite observing that breeding dispersal was female-biased, we also documented a number of adult males with medium-distance dispersal movements (i.e., > 10–107 km; Fig. 1a), and one adult male certainly moved > 1000 km within 3 years after ringing (Figs. 1b; 2). Consequently, adult males also make a meaningful contribution to dispersal in this species. Unpaired males, who cannot attract a partner locally, potentially due to male-biased operational sex ratio (i.e., lower mate availability for males; Eberhart-Phillips et al. 2018), may disperse to other sites where the breeding phenology is more favorable to find a suitable mate. Additionally, sometimes Kentish Plover pairs have been observed relocating together to an alternative breeding site (Székely and Lessels 1993). Similarly, we observed a breeding pair that moved together over 94 km after nest predation by a crow from a German to a Danish breeding site within one breeding season according to sequential observations of both partners together in three sites (beach of Sank-Peter Ording, Germany; Beltringharder Koog, Germany; island of Rømø, Denmark; Cimiotti et al. 2015). Breeding failure has generally been shown to trigger breeding dispersal for both sexes in the Kentish Plover (Foppen et al. 2006) and other closely related plover species (e.g., Skrade and Dinsmore 2010; Rioux et al. 2011; Pearson and Colwell 2014).
High dispersal propensity in the Kentish Plover
Despite the above-mentioned sex differences, we detected medium- to long-distance dispersal in all sex–age combinations (Fig. 1a), and some individuals even switched between inland and coastal breeding sites and between the seas (Fig. 2). Habitat preferences may explain the high dispersal propensity in Kentish Plovers. Like the congeneric Snowy Plover, Kentish Plovers breed in highly variable habitats such as saline lakes in steppe environments, on sand banks, in primary dunes, in recently embanked areas, or even in heavily altered and intensely used areas (e.g., salt pans, Rocha et al. 2016) in coastal environments (BirdLife International 2022). Breeding conditions in those habitats can change quickly, for example when temporary salt lakes or lagoons dry out in early summer or storms alter the habitat (Convertino et al. 2011; Cruz-López et al. 2017). Because of the stochastic nature of these breeding habitats, adult Kentish Plovers must be flexible throughout their lifetime to breed successfully. This may explain the high dispersal potential we documented in our study, which is typical for ecologically similar steppe and coastal birds (e.g., avocets, stilts, and the Sociable Lapwing Vanellus gregarius; Donald et al. 2021; Robinson and Oring 1997; Pigniczki et al. 2019; Joest et al. 2021). Similarly, plover populations breeding in riverine habitats or man-made secondary habitats, such as the Little Ringed Plover, face unstable breeding conditions, and therefore are dispersive in all age classes (Pakanen et al. 2015). Our findings support those of Paradis et al. (1998) that dispersal distances are higher in wetland species and in species with small, scattered, or declining populations. Interestingly, Dale et al. (2005) found even higher breeding dispersal than natal dispersal distances in a fragmented passerine bird population. Here, the authors defined breeding dispersal as the movement between two successively occupied territories, irrespectively whether a male attracted a partner or not. In their study, breeding dispersal mainly took place in the early period of adult life. The authors concluded that their observed strategy (i.e., first returning to the place of birth, followed by opportunistic dispersal) might be advantageous for similarly patchy distributed populations in other bird species.
Additionally, dispersal propensity can be associated with migratory behavior, as Paradis et al. (1998) found larger dispersal distances in migratory compared to resident bird species. In European Kentish Plovers, northern and southern breeding sites are connected by migration (e.g., Bairlein et al. 2014; Spina et al. 2022). Plovers may explore alternative breeding sites during migration or wintering (see Stenzel et al. 1994, Hillman et al. 2012). Conversely, immigrating individuals might affect the migratory composition of populations.
Genetic consequences
Our findings that Kentish Plovers exhibit female-driven long-distance breeding dispersal supports the results of Küpper et al. (2012) showing female-biased gene flow within a near panmictic population throughout continental Eurasia. Küpper et al. (2012) suggested that this female-biased gene flow could be the result of strongly female-biased breeding dispersal and male-biased natal dispersal. This was partially supported by our data, as we observed further breeding dispersal in females than in males. However, we did not detect significant sex differences in natal dispersal. One reason for this difference could be that the data sets are not comparable with respect to sex differences. In particular, the study by Küpper et al. (2012) included many island populations (e.g., from Azores, Madeira, and Cape Verde) that may show different dispersal behavior due to the closed nature of these isolated populations.
Comparison with other plover species
Dispersal patterns in our study (i.e., similar natal and breeding dispersal distances) differed from those found in several Charadriidae species including studies in the Kentish Plover restricted to a few locations (Table 1). Mean, median or maximum natal dispersal reported here were greater than breeding dispersal distances in seven species of Charadrius plovers (see Table 1) and in the northern lapwing (Thompson et al. 1994; Lislevand et al. 2009), another member of the Charadriidae family. For the Red-breasted Plover (C. obscurus), Marchant and Higgins (1993) reported a larger maximal breeding than natal dispersal distance, but in a more comprehensive study of Dowding (2001), mean and median dispersal distances were larger for natal than breeding dispersal in accordance with other Charadrius plovers (Table 1). The observed unusual pattern in our study is possibly a result of methodological differences. Dispersal distances up to 10 km, which might comprise most dispersal movements, are missing in our analyses leading to a strong bias toward longer dispersal distances. Studies on local populations usually only anecdotally detect long-distance dispersal. In contrast, the EURING dataset covered a large geographical range allowing us to examine the differences between demographic classes and sexes with a focus toward the upper range of realized dispersal movements. Thereby, the known maximum breeding dispersal distance of the Kentish Plover increased tenfold from 170 km (Székely and Lessels 1993) to 1704 km.
The observed long-distance dispersal in Kentish Plovers includes the farthest recorded breeding dispersal movements within the genus Charadrius (see Table 1). Movements between breeding sites of more than 1000 km were only published for two other species: a female piping plover dispersed from Michigan, USA, over 1208 km into the range of the Atlantic population in North Carolina, USA (Table 1; Hillman et al. 2012) and a female Snowy Plover from Monterey Bay, California, USA, dispersed 1140 km northward to Washington state (Stenzel et al. 1994).
A role for sexual selection on shaping sex-biased dispersal has been invoked in explaining observed differences in male and female dispersal in the polygamous Snowy Plover C. nivosus of North America (Stenzel et al. 1994; Pearson and Colwell 2014). In Snowy Plovers, long-distance breeding dispersal is similarly female-biased. Female-biased breeding dispersal is also present in Little Ringed Plovers C. dubius (Pakanen et al. 2015), a species with male-biased brood care and occasional brood desertion by the female (Stenzel and Page 2019). In contrast, no sex differences were found in Piping Plover C. melodus and Mountain Plover C. montanus, two species in which female brood desertion or polyandry is extremely rare (Haig and Oring 1988; Skrade and Dinsmore 2010)—indicating that sex-biased dispersal likely may go hand-in-hand with breeding system variation in Charadrius plovers and other shorebirds (Kwon et al. 2022).
Monitoring implications
The exchange of individuals among breeding sites may occasionally lead to an over-estimation of population sizes, but also mortality rates (e.g., quantifying true mortality is complicated by permanent emigration when basing inference on local mark-recapture observations), which should be considered when interpreting local population trends and forecasting population viability (Eberhart-Phillips and Colwell 2014). To avoid double counts of dispersing individuals, population counts should be synchronized on a geographical scale that acknowledges movement tendencies (Eberhart-Phillips et al. 2015).
Conclusion
The dispersal propensity of the Kentish Plover is high—an encouraging sign that the species can find patchy and far-distant suitable breeding habitats despite human-induced habitat destruction. Hence, conservation efforts such as the maintenance or restoration of suitable habitat should include abandoned or potential breeding areas (e.g., in Great Britain and southern Scandinavia) in contrast to more site-faithful species, for which conservation efforts should be focused on presently used areas and their surroundings. Even if recently used breeding areas are managed and kept intact for Kentish Plovers, alternative breeding sites should remain available for dispersing individuals, especially when conditions worsen elsewhere within the species’ range.
However, it is essential to preserve key breeding areas (e.g., in southern Europe) to maintain source populations (Tittler et al. 2006). Breeding success should be enhanced by conservation measures (e.g., Cimiotti and Hötker 2013) even at productive breeding sites to produce a surplus of young birds for the colonization of other areas. Potentially misleading interventions such as nest protection measures in areas with low chick survival should be carefully weighted with respect to the high dispersal propensity of the species. The aim should be a network of well-managed breeding sites with a coordinated population monitoring throughout the species’ range. Illustrative cases of exceptional long-distance dispersal, as observed in our study, can be used for raising public awareness for endangered species, and thereby increase public support to respect protection zones for beach-nesting birds.
Our data support recent findings that high dispersal propensity is intertwined with sex differences in breeding biology (d’Urban-Jackson et al. 2017, Kwon et al. 2022). This is especially true for species that are not classically nomadic: other factors such as the mating system can lead to high dispersal propensity. Therefore, a simple classification into nomadic vs. philopatric species has to be treated with care.
Data availability
Data used in the study can be obtained after request at the EURING Data Bank, http://www.euring.org/ (Accessed date 9 October 2019). The additional data used for the Discussion can be obtained from the first author.
References
Amat JA, Fraga RM, Arroyo GM (1999) Brood desertion and polygamous breeding in the Kentish Plover Charadrius alexandrinus. Ibis 141:596–607
Amirault-Langlais DL, Imlay TL, Boyne AW (2014) Dispersal patterns suggest two breeding populations of piping plovers in Eastern Canada. Wilson J Ornithol 126:352–359. https://doi.org/10.1676/13-056.1
Bairlein F, Dierschke J, Dierschke V et al (2014) Atlas des Vogelzugs. Ringfunde deutscher Brut- und Gastvögel. Aula-Verlag, Wiebelsheim
Barrowclough GF (1978) Sampling bias in dispersal studies based on finite area. Bird-Banding 49:333–341
Berthouly-Salazar C, Hui C, Blackburn TM et al (2013) Long-distance dispersal maximizes evolutionary potential during rapid geographic range expansion. Mol Ecol 22:5793–5804. https://doi.org/10.1111/mec.12538
BirdLife International (2015) European red list of birds. Office for Official Publications of the European Communities, Luxembourg
BirdLife International (2022) Species factsheet: Charadrius alexandrinus. http://www.birdlife.org. (Accessed 14 Jan 2022)
Cimiotti DV, Hötker H (2013) Conservation of Kentish Plovers in NW Europe: results of a workshop in N Germany. Wader Study Group Bull 120:218–220
Cimiotti D, Schulz R, Klinner-Hötker B, Hötker H (2015) Seltene Vogelarten in Deutschland: Seeregenpfeifer. Der Falke 62:24–29
Cimiotti DV, Audevard A, Garcias Salas PJ et al (2018) Umsiedlungen bei Seeregenpfeifern: Nordfriesland statt Mallorca und Côte d’Azur. Der Falke 65:27–29
Clarke AL, Sæther B-E, Røskaft E et al (1997) Sex Biases in Avian Dispersal: a Reappraisal. Oikos 79:429. https://doi.org/10.2307/3546885
Clobert J, Wolff JO, Nichols JD et al (2001) Indtroduction. In: Clobert J, Danchin E, Dhondt AA, Nochols JD (eds) Dispersal. Oxford University Press, New York, pp xvii–xxi
Colwell MA, Reynolds JD, Gratto CL et al (1988) Phalarope philopatry. Proc Int Ornithol Congr 19:585–593
Convertino M, Elsner JB, Muñoz-Carpena R et al (2011) Do tropical cyclones shape shorebird habitat patterns? Biogeoclimatology of Snowy Plovers in Florida. PLoS One 6:1–9. https://doi.org/10.1371/journal.pone.0015683
Cramp S, Simmons KEL, Brooks DJ et al (eds) (1983) The birds of the western Palearctic, vol 3. Oxford University Press, Oxford
Cruz-López M, Eberhart-Phillips LJ, Fernández G et al (2017) The plight of a plover: viability of an important snowy plover population with flexible brood care in Mexico. Biol Conserv 209:440–448. https://doi.org/10.1016/j.biocon.2017.03.009
D’Urban Jackson J, dos Remedios N, Maher KH et al (2017) Polygamy slows down population divergence in shorebirds. Evolution 71:1313–1326. https://doi.org/10.1111/evo.13212
Donald PF, Kamp J, Green RE et al (2021) Migration strategy, site fidelity and population size of the globally threatened Sociable Lapwing Vanellus gregarius. J Ornithol 162:349–367. https://doi.org/10.1007/s10336-020-01844-y
Dowding JE (2001) Natal and breeding dispersal of northern New Zealand dotterels. Conservation Advisory Science Notes No. 338. Wellington, New Zealand
Dutang C (2015) fitdistrplus: an R package for fitting distributions. J Stat Softw. https://doi.org/10.18637/jss.v064.i04
Eberhart-Phillips L (2019) Plover breeding systems—diversity and evolutionary origins. In: Colwell MA, Haig SM (eds) The population ecology and conservation of Charadrius Plovers. CRC Press, Boca Raton, pp 65–88
Eberhart-Phillips LJ, Colwell MA (2014) Conservation challenges of a sink: the viability of an isolated population of the Snowy Plover. Bird Conserv Int 24:327–341. https://doi.org/10.1017/S0959270913000506
Eberhart-Phillips LJ, Hudgens BR, Colwell MA (2015) Spatial synchrony of a threatened shorebird: regional roles of climate, dispersal and management. Bird Conserv Int 26:119–135. https://doi.org/10.1017/S0959270914000379
Eberhart-Phillips LJ, Küpper C, Carmona-Isunza MC et al (2018) Demographic causes of adult sex ratio variation and their consequences for parental cooperation. Nat Commun. https://doi.org/10.1038/s41467-018-03833-5
du Feu CR, Joys AC, Clark JA et al (2009) EURING Data Bank geographical index 2009. http://www.euring.org/edb
Flynn L, Nol E, Zharikov Y (1999) Philopatry, Nest-Site Tenacity, and Mate Fidelity of Semipalmated Plovers. J Avian Biol 30:47. https://doi.org/10.2307/3677242
Foppen RPB, Majoor FA, Willems FJ et al (2006) Survival and emigration rates in Kentish Charadrius alexandrinus and Ringed Plovers Ch. hiaticula in the Delta area. SW-Netherlands Ardea 94:159–173
Gómez-Serrano MÁ (2021) Four-legged foes: dogs disturb nesting plovers more than people do on tourist beaches. Ibis 163:338–352. https://doi.org/10.1111/ibi.12879
Greenwood PJ (1980) Mating systems, philopatry, and dispersal in birds and mammals. Anim Behav 28:1140–1162
Greenwood PJ, Harvey PH (1982) The natal and breeding dispersal of birds. Annu Rev Ecol Syst 13:1–21. https://doi.org/10.1146/annurev.es.13.110182.000245
Haig SM, Oring LW (1988) Distribution and dispersal in the Piping Plover. Auk 105:630–638
Hillman MD, Karpanty SM, Fraser JD et al (2012) Evidence for long-distance dispersal and successful interpopulation breeding of the endangered piping plover. Waterbirds 35:642–644. https://doi.org/10.1675/063.035.0414
Joest R, Hälterlein B, Klinner-Hötker B et al (2021) Population, breeding success as well as feeding ecology and breeding settlement of the young birds of Pied Avoced Recurvirostra avosetta in the North Frisian ‘Naturschutzköge’ Beltringharder Koog and Fahretofter Westerkoog 1991 to 2019 [in German]. Corax 24:481–497
Keller V, Herrando S, Voríšek P et al (2020) European breeding bird atlas 2: distribution, abundance and change. Lynx Edicions/European Bird Census, Barcelona
Kempenaers B, Valcu M (2017) Breeding site sampling across the Arctic by individual males of a polygynous shorebird. Nature 541:528–531. https://doi.org/10.1038/nature20813
Kosztolańyi A, Javed S, Küpper C et al (2009) Breeding ecology of Kentish Plover Charadrius alexandrinus in an extremely hot environment. Bird Study 56:244–252. https://doi.org/10.1080/00063650902792106
Küpper C, Edwards SV, Kosztolányi A et al (2012) High gene flow on a continental scale in the polyandrous Kentish plover Charadrius alexandrinus. Mol Ecol 21:5864–5879. https://doi.org/10.1111/mec.12064
Kwon E, Valcu M, Cragnolini M et al (2022) Breeding site fidelity is lower in polygamous shorebirds and male-biased in monogamous species. Behav Ecol 33:592–605. https://doi.org/10.1093/beheco/arac014
Lessells CM (1984) The mating system of Kentish Plovers Charadrius alexandrinus. Ibis 126:474–483. https://doi.org/10.1111/j.1474-919X.1984.tb02074.x
Lislevand T, Byrkjedal I, Grønstøl GB (2009) Dispersal and age at first breeding in Norwegian Northern Lapwings (Vanellus vanellus). Ornis Fenn 86:11–17
Mabry KE, Shelley EL, Davis KE et al (2013) Social mating system and sex-biased dispersal in mammals and birds: a phylogenetic analysis. PLoS One 8:1–9. https://doi.org/10.1371/journal.pone.0057980
Marchant S, Higgins PJ (1993) Handbook of Australian, New Zealand and Antarctic birds, vol 2. Oxford University Press, Melbourne
Mead CJ, Clark JA (1988) Report on bird ringing in Britain and Ireland for 1987. Ringing Migr 9:169–204
Nathan R, Cronin JT, Strand AE et al (2003) Methods for estimating long-distance dispersal. Oikos 103:261–273
Oring LW, Lank DB (1982) Sexual selection, arrival times, philopatry and site fidelity in the polyandrous spotted sandpiper. Behav Ecol Sociobiol 10:185–191. https://doi.org/10.1007/BF00299684
Pakanen VM, Lampila S, Arppe H, Valkama J (2015) Estimating sex specific apparent survival and dispersal of Little Ringed Plovers (Charadrius dubius). Ornis Fenn 92:172–186
Paradis E, Baillie SR, Sutherland WJ, Gregory RD (1998) Patterns of natal and breeding dispersal in birds. J Anim Ecol 67:518–536. https://doi.org/10.1046/j.1365-2656.1998.00215.x
Pearson WJ, Colwell MA (2014) Effects of nest success and mate fidelity on breeding dispersal in a population of Snowy Plovers Charadrius nivosus. Bird Conserv Int 24:342–353. https://doi.org/10.1017/S0959270913000403
Pigniczki C, Nagy T, Olah J et al (2019) Breeding, dispersal, migration and conservation of the Black-winged Stilt (Himantopus himantopus) in Hungary. Ornis Hungarica 27:1–19. https://doi.org/10.2478/orhu-2019-0013
QGIS Development Team (2020) QGIS Geographic Information System (http://qgis.osgeo.org). Open Source Geospatial Foundation Project.
R Core Team (2021) R: A language and environment for statistical computing (https://www.R-project.org/)
Reed JM, Oring LW (1993) Philopatry, site fidelity, dispersal, and survival of spotted sandpipers. Auk 110:541–551
Rioux S, Amirault-Langlais DL, Shaffer F (2011) Piping Plovers make decisions regarding dispersal based on personal and public information in a variable coastal ecosystem. J F Ornithol 82:32–43. https://doi.org/10.1111/j.1557-9263.2010.00305.x
Rittingshaus H (1961) Der Seeregenpfeifer. A. Ziemsen Verlag, Wittenberg
Rittinghaus H (1975) Charadrius alexandrinus Linné 1758 – Seeregenpfeifer. In: Glutz von Blotzheim UN (ed) Handbuch der Vögel Mitteleuropas, vol. 6 (Charadriiformes, part 1). Aula-Verlag, Wiebelsheim
Robinson JA, Oring LW (1997) Natal and breeding dispersal in American avocets. Auk 114:416–430
Rocha AD, Fonseca D, Masero JA, Ramos JA (2016) Coastal saltpans are a good alternative breeding habitat for Kentish plover Charadrius alexandrinus when umbrella species are present. J Avian Biol 47:824–833. https://doi.org/10.1111/jav.00883
Sadanandan KR, Küpper C, Low GW et al (2019) Population divergence and gene flow in two East Asian shorebirds on the verge of speciation. Sci Rep 9:1–9. https://doi.org/10.1038/s41598-019-44996-5
Skrade PDB, Dinsmore SJ (2010) Sex-related dispersal in the mountain plover (Charadrius montanus). Auk 127:671–677. https://doi.org/10.1525/auk.2010.09059
Spina F, Baillie SR, Bairlein F et al (eds) (2022) The Eurasian African Bird Migration Atlas (https://migrationatlas.org). EURING/CMS
Stenzel LE, Page GW (2019) Breeding biology of Charadrius Plovers. In: Colwell MA, Haig SM (eds) The population ecology and conservation of Charadrius Plovers. CRC Press, Boca Raton, pp 91–125
Stenzel LE, Warriner JC, Warriner JS et al (1994) Long-distance breeding dispersal of Snowy Plovers in Western North America. J Anim Ecol 63:887. https://doi.org/10.2307/5266
Stenzel LE, Page GW, Warriner JC et al (2007) Survival and natal dispersal of juvenile snowy plovers (Charadrius alexandrinus) in central coastal California. Auk 124:1023–1036. https://doi.org/10.1642/0004-8038(2007)124[1023:SANDOJ]2.0.CO;2
Swift RJ, Anteau MJ, Ellis KS et al (2021) Dispersal distance is driven by habitat availability and reproductive success in Northern Great Plains piping plovers. Mov Ecol 9:1–15. https://doi.org/10.1186/s40462-021-00293-3
Szekely T, Lessels CM (1993) Mate Change by Kentish Plovers Charadrius alexandrinus. Ornis Scand 24:317–322. https://doi.org/10.2307/3676794
Thompson PS, Baines D, Coulson JC, Longrigg G (1994) Age at first breeding, philopatry and breeding site-fidelity in the Lapwing Vanellus vanellus. Ibis (Lond 1859) 136:474–484. https://doi.org/10.1111/j.1474-919X.1994.tb01124.x
Thorup O, Bregnballe T (2021) Bestandsudvikling hos Hvidbrystet Præstekrave i Danmark og nabolandene. Dansk Orn Foren Tidsskr 115:285–300
Tittler R, Fahrig L, Villard MA (2006) Evidence of large-scale source-sink dynamics and long-distance dispersal among Wood Thrush populations. Ecology 87:3029–3036. https://doi.org/10.1890/0012-9658(2006)87[3029:EOLSDA]2.0.CO;2
Trochet A, Stevens VM, Baguette M (2016) Evolution of sex-biased dispersal. Q Rev Biol 91:297–320
Acknowledgements
We are grateful to the European Union for Bird Ringing (EURING) which made the recovery data available through the EURING Data Bank and to the many ringers and ringing scheme staff who have gathered and prepared the data. We are grateful to Dagmar S. Cimiotti, Phillip Gienapp, and Krisztina Kupán for the statistical advice.
Funding
Open Access funding enabled and organized by Projekt DEAL. The Ministry for Energy Transition, Climate Protection, Environment and Nature of Schleswig–Holstein (Germany) financed a previous study on the species. CK was supported by the Max-Planck Society, LEH was funded by the German Science Foundation (DFG Eigene Stelle grant: EB 590/1-1).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by N. Chernetsov.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In memorial of Brigitte Klinner-Hötker (1956–2021), who observed a female Kentish Plover dispersing nearly 1300 km over the course of her valuable long-term engagement in our population study in northern Germany.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cimiotti, D.V., Eberhart-Hertel, L., Audevard, A. et al. Dispersal in Kentish Plovers (Charadrius alexandrinus): adult females perform furthest movements. J Ornithol 165, 301–314 (2024). https://doi.org/10.1007/s10336-023-02120-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02120-5