Abstract
Background
Breeding dispersal is an important ecological process that affects species’ population dynamics and colonization of new suitable areas. Knowledge of the causes and consequences of breeding dispersal is fundamental to our understanding of avian ecology and evolution. Although breeding success for a wild and reintroduced population of the Crested Ibis (Nipponia nippon) has been reported, the relationships between individuals’ breeding dispersal and their breeding success, age and sex remain unclear.
Methods
Ibises’ breeding dispersal distance, which is the distance moved by adults between sites of reproduction, was estimated based on the observations of consecutive breeding sites of marked ibis individuals. From observational and capture-recapture data (n = 102) over 9 years, individuals’ breeding dispersal probability in relation to age, sex, and reproductive success was analyzed via a generalized linear mixed effect modeling approach.
Results
Our results show that 55% males and 51% females keep their previous territories following nesting success. Failed breeding attempts increased dispersal probabilities. Both females and males failed in breeding were more likely to disperse with greater distances than successful birds (females: 825 ± 216 m vs 196 ± 101 m, males: 372 ± 164 m vs 210 ± 127 m). Crested Ibis exhibited a female-biased dispersal pattern that the mean dispersal distance of females (435 ± 234 m) was much larger than that of males (294 ± 172 m).
Conclusion
Our results are fundamental to predict the patterns of breeding dispersal related to reproductive success under different release sites. From the conservation point of view, landscape connectivity between the reintroduced populations should be taken into account in accordance with the distance of breeding dispersal.
Similar content being viewed by others
Background
Breeding dispersal, defined as the net movement between two successive breeding sites, is an important life history trait related to population viability (Greenwood and Harvey 1982). Understanding the causes and consequences of the dispersal movements is a central issue for a broad range of biological disciplines, ranging from conservation biology to population ecology, evolution and communities of species (Clobert et al. 2001). Dispersal strategies affect the dynamics and the genetic and demographic structure of populations (Hanski and Gaggiotti 2004), especially for rare or endangered species (Pearson and Colwell 2014). On the other hand, breeding dispersal of birds may be affected by various factors, such as sex, age (Andreu and Barba 2006; Eeva et al. 2008), and breeding success (Pasinelli et al. 2007; Bötsch et al. 2012). For instance, breeding dispersal may occur frequently after breeding failure (Wiklund 1996; Forero et al. 1999), indicating that the probability of breeding dispersal declines with increasing reproductive success. Breeding dispersal allows individuals to gain higher quality territories or mates (Greenwood and Harvey 1982; Blakesley et al. 2006) and improve future breeding performance (Payne and Payne 1993). With respect to sex bias, it is generally thought that a male bird usually competes with other local males to gain a new territory. Therefore, it would be expected that being philopatric is more advantageous for males than for females (Greenwood 1980; Ward and Weatherhead 2005). However, dispersal is assumed to entail certain costs in terms of time, energy and risk from predators devoted by dispersing individuals to finding a new breeding site (Forero et al. 1999; Danchin and Cam 2002; Hansson et al. 2004). In other words, under selection pressure, there might be a trade-off between the benefits and costs for dispersal and philopatric behavior (Calabuig et al. 2008). Despite this importance, little is known about breeding dispersal, particularly in relationships between breeding success and dispersal of a reintroduced population, mainly because gathering longitudinal information on individuals is difficult due to limited population size.
After nearly four decades of protection, a great success both in in situ and ex situ conservation makes it possible to release the captive-bred ibises to balance the risk of extinction. The Crested Ibis (Nipponia nippon) is now being reintroduced subsequently to protected sites in Ningshan (Yu et al. 2009, 2015), Tongchuan (Wang 2016), and Qianyang counties (Yu et al. unpublished data), Shaanxi Province, with a goal of establishing self-sustaining populations. One decade after release, progresses towards self-sustaining populations have been made under some interventions (Wang 2016; Wang et al. 2017). However, as one of the important processes in population dynamics, the breeding dispersal and its relations to breeding success, sex and age remain unclear. In this study, we examined the process of breeding dispersal at both the individual and population levels. By doing this, we aimed to quantify the probability and distance of ibises’ breeding dispersal and their relationships to age, sex, and breeding success.
Methods
Study area
The study area is located in Ningshan County (33°07′–33°50′N, 108°02′–108°56′E), a mountainous region on the south slope of the Qinling Mountains in Shaanxi Province, China. The study area within approximately 15 km in diameter is in a warm, temperate and moderately humid zone, where the elevation ranges from 700 to 1100 m. This area is often developed for rice paddies interspersed with dry farmlands in the valley and forest stands on the hillsides. Numerous shallow rivers and streams run southward through this area into the Hanjiang River, the longest tributary of the Yangtze River. The specific geographical location and natural conditions have been reported in details by Yu et al. (2015).
Study species and field observations
Twenty-six captive-bred Crested Ibises (13 females, 13 males) were released in the study area firstly in 2007, and followed up in 2008 (4 females, 2 males), 2009 (6 females, 8 males), and 2011 (3 females, 7 males) (Yu et al. 2015). All captive-bred individuals prior to release and nestlings at 23–24 days of age were marked individually with numbered metal bands and colored alphanumeric plastic bands (provided by the National Bird Banding Center of China) on their legs to allow identification of age and fates in the field (Yu et al. 2010). By the end of 2016, a total of 168 individuals (56 captive-bred ibises and 112 wild-born fledglings) were banded and monitored in the study area.
From February to July each year in the study period, an intensive survey was carried out in search of ibis adults and their nests within the study area. All found nests were located and revisited two to four times per week to determine the chronology of nesting, egg laying, hatching of young, and subsequent fledging success. We investigated each clutch to document nest failure if it occurred. The causes of nesting failure, if identified, were also recorded. Each pairs’ breeding success was then calculated as the number of fledglings divided by clutch size in the given year.
Breeding dispersal
The breeding dispersal of Crested Ibises was characterized with dispersal probability and dispersal distance. The dispersal probability is binary (0/1) in which ‘1’ represents the occurrence of dispersal and ‘0’ means non-dispersal. It is considered that dispersal events occur once a pair disperses more than 50 m from the previous territory between successive breeding seasons. The Crested Ibis usually establishes a small breeding territory of no more than 50 m in diameter (Shi and Cao 2001), in accordance with the average nearest neighbor distance (approximately 50 m) for active ibis’s nest sites in our study area, supporting the criteria for dispersal (Catlin et al. 2005). The dispersal distance was measured as the Euclidean distance between the nests in two consecutive years for each breeder (Greenwood and Harvey 1982). We treated dispersal probability and subsequent dispersal distance as separate events, since they may be affected by different factors (Catlin et al. 2005). Based on age groups classification of the wild population (Yu et al. 2007) and specific age structure of our focal population (Li et al. 2018), all monitored individuals were reclassified into three age groups: 1‒3 years old; 4‒6 years old; and > 7 years old.
Statistical analyses
We analyzed potential effects of age, sex, and breeding success on breeding dispersal of Crested Ibises via a generalized linear mixed modelling (GLMM) procedure, which is a flexible approach to power analysis by accounting for random effects, overdispersion and diverse response distributions (Breslow and Clayton 1993). We formulated a GLMM with a Bernoulli error structure to test the relationship between dispersal probability (response variable) and age groups, sex (fixed factor) and breeding success (covariate). In some cases the same individual was recorded in two or more years, we therefore incorporated individual identity as a random factor in order to avoid the problem of pseudo-replication.
The relationships between the dispersal distance (response variable) and age, sex, and breeding success (predictor variables) were modelled using a GLMM with a Normal error structure. Both individual identity and breeding year were included as random factors with a consideration of breeding distance expected to vary between years and individuals.
For both GLMMs, we employed the second-order Akaike information criterion adjusted for small sample size (AICc; Burnham and Anderson 2002) to select the most parsimonious fixed-effects models. Initially, all variables and factors were fitted together. The model with the lowest AIC value was selected and the significance of the remaining variables was tested using Likelihood ratio (Alain et al. 2007). We considered models with differences of AICc (ΔAICc) values < 2 from the best-fit model equally parsimonious (Burnham and Anderson 2002), thereby including variables from all such models for further mixed effects analysis. We then defined a 95% confidence set of models for inference, summing ΔAICc from best to worst model until the sum of ΔAICc exceeded 95%. Models not meeting a 95% confidence set were excluded. When there was no clear parsimonious model (ΔAICc < 0.90), we used the model.avg function in the R-package MuMln for model averaging to determine the direction and magnitude of the effect of each explanatory variable (Burnham and Anderson 2002). Instead of relying on the estimates of the best candidate model, we computed a weighted average of the estimate or a given parameter based on the Akaike weights (AICw). We also computed the relative importance of the fixed factors by summing the ΔAICc of the models that contained each factor. The model-averaged fixed factors coefficient estimation and 95% confidence interval were listed in the parentheses of report.
All GLMMs were constructed and performed using the package ‘lme4’ (Bates and Maechler 2009) in the statistical software R 3.2.0 (R Development Core Team 2015). The differences of yearly breeding success, dispersal distances within age groups were tested by ANOVA analysis, and sexual differences of dispersal distance were tested by Mann–Whitney U test or Student’s t test. We report mean ± SE unless otherwise noted and statistical tests were considered significant at p < 0.05.
Results
Breeding success
A total of 32 breeding pairs including 30 males and 32 females performed 65 breeding attempts from 2008 to 2016. There are 220 eggs in 65 clutches to be laid with an average clutch size of 3.38 ± 0.83 (n = 65). In total, 160 eggs hatched (2.46 ± 1.12, n = 65) and 112 nestlings successfully fledged, with an average of 1.73 ± 1.26 (n = 65) fledglings per breeding pair each year, during nine breeding seasons. First reproduction of wild-born females was significantly later than by males (females 3.63 ± 1.19 years, n = 8; males 2.56 ± 0.73 years, n = 9, t = 2.26, p = 0.03). The average breeding success varies among years with non-significant differences (ANOVA, F = 1.19, df = 8, p = 0.55, Fig. 1), indicating a stable breeding outcome for the reintroduced population.
Breeding dispersal probability
From 2008 to 2016, we caught 102 individuals including 50 males and 52 females as breeders in two consecutive breeding seasons (n = 65 breeding attempts). Of the total dispersal events, 48 individuals (47%) dispersed from their previous breeding territories, while 54 individuals (53%) were philopatric. Fifty-five percent (27/50) males and 51% (27/52) females were observed to maintain their previous territories following nesting success. In response to failed breeding cases, 15 out of 23 (65%) for males and 16 out of 25 (64%) for females, moved into new breeding territories.
For dispersal probability, the 95% confidence set of models contained three models, revealing some model uncertainty (Additional file 1: Table S1). The mean relative importance of the breeding success variables was 1.00, and it was 0.33 for the sex variables and 0.17 for age groups variables based on model-averaged predictions. The model-averaged coefficients clearly indicated that the breeding success was negatively associated with dispersal probability of the Crested Ibis (β = − 1.74 ± 0.41, 95% CI = − 2.55 to − 0.93, p < 0.001) (Table 1). There was very limited evidence to suggest that males were less likely to disperse than females (males: β = − 0.40 ± 0.51, 95% CI = − 1.49 to 0.59, p = 0.39) (Table 1).
Breeding dispersal distance
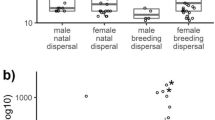
Model selection resulted in four models used for inference on the factors underlying dispersal distance of the Crested Ibis (Additional file 1: Table S2). A common feature of these models, however, was that they all contained breeding success, age and sex. The mean relative importance of breeding success and age was 0.93 and 0.56 respectively, based on model-averaged predictions. The model-averaged coefficients for breeding success (β = − 0.15 ± 0.05, 95% CI = − 0.26 to − 0.04, p < 0.001), sex (male) were negative, whereas model-averaged coefficients for age was positive (β = 0.16 ± 0.08, 95% CI = − 0.02 to 0.35, p = 0.08) (Table 2). The dispersal distances increased with age (group 1: 172 ± 41 m, n = 6; group 2: 300 ± 205 m, n = 30; group 3: 916 ± 1384 m, n = 12) and there were significant differences within the three age groups (ANOVA: F = 4.32, df = 2, p = 0.01) (Fig. 2). The mean breeding dispersal distances of males and females were 294 ± 172 m (n = 23) and 435 ± 234 m (n = 25) respectively, with a maximum of 4378 m for one female. There was a significant difference between both sexes in breeding dispersal distance (t = − 2.33, p = 0.02) irrespective of breeding success. However, the failed breeders regardless of females and males, dispersed greater distances than successful breeders (females: 825 ± 216 m vs 196 ± 101 m, U = 35, p = 0.03; males: 372 ± 164 m vs 210 ± 127 m, U = 29, p = 0.01).
Discussion
There might be a trade-off between costs and benefits of dispersal and philopatric decisions (Danchin and Cam 2002; Hansson et al. 2004; Calabuig et al. 2008). At the proximate level, breeding dispersal involves many factors such as breeding success that may affect individual decisions. Many studies have found breeding dispersal in birds to be associated with lower reproductive success in the year before dispersal (Wiklund 1996; Forero et al. 1999; Sedgwick 2004; Pasinelli et al. 2007; Calabuig et al. 2008; Włodarczyk et al. 2013). It is generally thought that breeding dispersal allows individuals to gain higher quality territories or mates and improve future breeding performance (Greenwood and Harvey 1982; Payne and Payne 1993; Blakesley et al. 2006). However, for the Crested Ibis, few studies have been so far conducted on breeding dispersal for either wild or reintroduced populations. Our focal population exhibited a similar dispersal pattern because approximately fifty percent dispersal events occurred following poor reproductive performance. Most of the breeding failures of either wild or reintroduced ibis population were mainly contributable to predators such as the King Rat Snakes (Elaphe carinata), the Northern Goshawk (Accipiter gentilis) and human disturbance from local inhabitants and researchers (Yu et al. 2006, 2015). More to the point, the banding process of one nest usually lasting for more than one hour every year may be the most frequent disturbance leading to replacement of nest site. On the contrary, earlier studies on wild populations described that ibis pairs usually reuse the same breeding site for up to five years without any inferences (Shi et al. 1989; Yu et al. 2006). As the movement from one breeding site to another, breeding dispersal may be caused by species’ adaptive utilization of resources or environmental interferences (Clobert et al. 2001). Therefore, we speculate that Crested Ibis individuals (50% of breeding events) may disperse to gain higher quality territories after breeding failure due to predators and frequent human disturbance.
The breeding dispersal of the Crested Ibis follows the general pattern of female-biased dispersal observed in most bird species (Greenwood and Harvey 1982; Clarke et al. 1997). The sex-biased dispersal has been generally explained as a consequence of asymmetrical investment in the acquisition and defense of resources between the sexes. Because dispersing males must usually compete with other local males to gain a new territory. Therefore, it would be expected that being philopatric is more advantageous for males than for females (Greenwood 1980; Ward and Weatherhead 2005). In fact, both sexes of the Crested Ibis invest almost equally in the reproductive process except territory defense by males which occasionally occurred between two adjacent nests (Yu et al. 2006, 2015). The pair formation of the monogamous birds occurs often following the flocking period in late winter and early spring (Huo et al. 2014). They became territorial in the breeding season, and each pair occupies a small nest-centric territory less than 50 m in diameter. The males usually display ritualistic territorial behaviors such as imitation of nesting when other individuals, especially males, intrude their territory (Shi and Cao 2001).
The fact that older individuals dispersed farther distances is contrast with other studies on many species (Greenwood and Harvey 1982; Dow and Fredga 1983; Hoover 2003; Fisher and Wiebe 2006). It is generally thought that age negatively influenced the probability and distance of dispersal due to having less breeding experiences for younger individuals (Serrano et al. 2001; Calabuig et al. 2008). For the Crested Ibis, the behavioral difference between spouses of some pairs may be the real cause of the opposite result. It might be, at least partially, attributable to the coexistence of released and wild-born individuals. There are three pair-bond types including ‘captive-captive’, ‘captive-wild’ and ‘wild-wild’ during the study period in the initial stage of establishment. With current sample size, it’s hard to examine whether captive birds are responsible for observed increase of dispersal distance with age.
Reintroduction projects aim to establish a self-sustaining population by releasing captive-bred or wild individuals (IUCN/SSC 1998, 2013). Since the first reintroduction of the Crested Ibis on the south slope of Qinling Mountains in 2007 (Yu et al. 2015; Wang et al. 2017), further releases have been subsequently conducted in Yaozhou District in Tongchuan City (35°02′N, 108°49′E) (Wang 2016) and Qianhu National Wetland Park (34°37′N, 107°09′E) (Yu et al. Unpublished data) on the loess plateau in the north of Qinling Mountains. The two reintroduced populations were considered to be at initial stage of establishment. In addition, feasibility assessments for another two subsequent releases in Malan river valley of Xunyi County (35°15′N, 108°36′E) and Louguantai of Zhouzhi County (34°04′N, 108°19′E) have been also developed (Yu et al. Unpublished data). Under these circumstances, a conservation framework, on meta-population levels, should be taken into consideration. Our focal population has become a satellite population when two wild females from wild population mated with the released individuals (Yu et al. 2015). As Armstrong and Seddon (2007) proposed, multiple releases should be conducted among different sites away from the core (wild) population in order to establish a meta-population, as traditionally defined as networks of semi-isolated populations connected by natural dispersal (Hanski 1998). There is increasing evidence that the exchange of individuals between populations has a strong effect on local population dynamics and persistence (Schaub and von Hirschheydt 2009; Schaub et al. 2010) and enhances meta-population functionality (Macdonald and Johnson 2001; Kenward et al. 2002). Simply speaking, one of the meta-populations would be established once as individuals from reintroduced and wild population (s) mated each other through natal/breeding dispersal. For this purpose, the landscape connectivity, namely dispersal corridors, such as rivers and streams among these populations should be taken into account in response to the distance of both natal (Yu et al. 2010) and breeding dispersal (present study). For instance, studies on some mammals, birds and other species provided evidence for an influence of landscape connectivity on dispersal (Bar-David et al. 2005; Dzialak et al. 2005; Baguette and Dyck 2007). Therefore, the analysis of landscape suitability and the simulation of dispersal of released individuals could demonstrate the actual process of population spreading (Morgia et al. 2011).
Conclusions
Higher reproductive outcomes might indicate that our focused population is now at persistence phase without any interventions. The failed breeders irrespective of male and female ibises would disperse to gain high quality territories and improve future breeding performance. The female-biased dispersal follows the general pattern in most other birds which explained as a consequence of asymmetrical investment in defense of territory by males. From the view of conservation, the dispersal corridors, such as rivers and streams among different reintroduced populations may be fundamental to establish a meta-population in response to the distance of both natal and breeding dispersal.
References
Alain FZ, Elena NI, Graham MS. Analysing ecological data. Berlin: Springer; 2007.
Andreu J, Barba E. Breeding dispersal of great tits Parus major in a homogeneous habitat: effects of sex, age, and mating status. Ardea. 2006;94:45–58.
Armstrong DP, Seddon PJ. Directions in reintroduction biology. Trends Ecol Evol. 2007;23:20–5.
Baguette M, Dyck HV. Landscape connectivity and animal behavior: functional grain as a key determinant for dispersal. Landscape Ecol. 2007;22:1117–29.
Bar-David S, Saltz D, Dayan T. Predicting the spatial dynamics of a reintroduced population: the Persian fallow deer. Ecol Appl. 2005;15:1833–46.
Bates D, Maechler M. lme4: linear mixed-effects models using S4 classes. R package version 0.999375-31. 2009. http://CRAN.R-project.org/package=lme4. Accessed 20 Jan 2018.
Blakesley JA, Anderson DR, Noon BR. Breeding dispersal in the California spotted owl. Condor. 2006;108:71–81.
Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88:9.
Bötsch Y, Arlettaz R, Schaub M. Breeding dispersal of Eurasian Hoopoes Upupa epops within and between years in relation to reproductive success, sex, and age. Auk. 2012;129:283–95.
Burnham KP, Anderson DR. Model selection and multi model inference: a practical information-theoretic approach. 2nd ed. New York: Springer; 2002.
Calabuig G, Ortego J, Cordero PJ, Aparicio JM. Causes, consequences and mechanisms of breeding dispersal in the colonial lesser kestrel, Falco naumanni. Anim Behav. 2008;76:1989–96.
Catlin DH, Rosenberg DK, Haley KL. The effects of nesting success and mate fidelity on breeding dispersal in burrowing owls. Can J Zool. 2005;83:1574–80.
Clarke AL, Saether BE, Roskaft E. Sex biases in avian dispersal: a reappraisal. Oikos. 1997;79:429–38.
Clobert J, Danchin E, Dhondt AA, Nichols JD. Dispersal. Oxford: Oxford University Press; 2001.
Danchin E, Cam E. Can non-breeding be a cost of breeding dispersal? Behav Ecol Sociobiol. 2002;51:153–63.
Dow H, Fredga S. Breeding and natal dispersal of the goldeneye Bucephala clangula. J Anim Ecol. 1983;52:681–95.
Dzialak MR, Lacki MJ, Larkin JL, Carter KM, Vorisek S. Corridors affect dispersal initiation in reintroduced peregrine falcons. Anim Conserv. 2005;8:421–30.
Eeva T, Ahola M, Laaksonen T, Lehikoinen E. The effects of sex, age and breeding success on breeding dispersal of pied flycatchers along a pollution gradient. Oecologia. 2008;157:231–8.
Fisher RJ, Wiebe KL. Breeding dispersal of Northern Flickers Colaptes auratus in relation to natural nest predation and experimentally increased perception of predation risk. Ibis. 2006;148:772–81.
Forero MG, Donázar JA, Blas J, Hiraldo F. Causes and consequences of territory change and breeding dispersal distance in the black kite. Ecology. 1999;80:1298–310.
Greenwood PJ. Mating systems, philopatry and dispersal in birds and mammals. Anim Behav. 1980;28:1140–62.
Greenwood PJ, Harvey PH. The natal and breeding dispersal of birds. Ann Rev Ecol Syst. 1982;13:1–21.
Hanski I. Metapopulation dynamics. Nature. 1998;396:41–9.
Hanski I, Gaggiotti OE. Ecology, genetics, and evolution of metapopulations. Amsterdam: Academic Press; 2004.
Hansson B, Bensch S, Hasselquist D. Lifetime fitness of short- and long-distance dispersing great reed warblers. Evolution. 2004;58:2546–57.
Hoover JP. Decision rules for site fidelity in a migratory bird, the prothonotary warbler. Ecology. 2003;84:416–30.
Huo ZP, Guo JF, Li X, Yu XP. Post-fledging dispersal and habitat use of a reintroduced population of the Crested Ibis Nipponia nippon. Avian Res. 2014;5:7.
IUCN/SSC. Guidelines for reintroductions. Gland: IUCN Species Survival Commission; 1998.
IUCN/SSC. Guidelines for reintroductions and other conservation translocations. Version 1.0. Gland: IUCN Species Survival Commission; 2013.
Kenward RE, Rushton SP, Perrins CM, MacDonald DW, South AB. From marking to modeling: dispersal study techniques for land vertebrates. In: Bullock JM, Kenward RE, Hails RS, editors. Dispersal ecology. Massachusetts: Blackwell; 2002. p. 50–71.
Li YF, Ye XP, Wang M, Li X, Dong R, Huo ZP, Yu XP. Survival rates of a reintroduced population of the Crested Ibis Nipponia nippon in Ningshan County (Shaanxi, China). Bird Conserv Int. 2018;28:145–56.
Macdonald DW, Johnson DDP. Dispersal in theory and practice: consequences for conservation biology. In: Clobert J, Danchin E, Dhondt AA, Nichols JD, editors. Dispersal. Oxford: Oxford University Press; 2001. p. 358–72.
Morgia VL, Malenotti E, Badino G, Bona F. Where do we go from here? Dispersal simulations shed light on the role of landscape structure in determining animal redistribution after reintroduction. Landscape Ecol. 2011;26:969–81.
Pasinelli G, Müller M, Schaub M, Jenni L. Possible causes and consequences of philopatry and breeding dispersal in Red-backed Shrikes Lanius collurio. Behav Ecol Sociobiol. 2007;61:1061–74.
Payne RB, Payne LL. Breeding dispersal in Indigo Buntings: circumstances and consequence for breeding success and population structure. Condor. 1993;95:1–24.
Pearson WJ, Colwell MA. Effects of nest success and mate fidelity on breeding dispersal in a population of Snowy Plovers Charadrius nivosus. Bird Conserv Int. 2014;24:342–53.
R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2015. http://www.r-project.org/. Accessed 10 Sept 2017.
Schaub M, Aebischer A, Gimenez O, Berger S, Arlettaz R. Massive immigration balances high antropogenic mortality in a stable eagle owl population: lessons for conservation. Biol Conserv. 2010;143:1911–8.
Schaub M, von Hirschheydt J. Effect of current reproduction on apparent survival, breeding dispersal, and future reproduction in Barn Swallows assessed by multistate capture–recapture models. J Anim Ecol. 2009;78:625–35.
Sedgwick JA. Site fidelity, territory fidelity, and natal philopatry in willow flycatchers Empidonax traillii. Auk. 2004;121:1103–12.
Serrano D, Tella JL, Forero MG, Donázar JA. Factors affecting breeding dispersal in the facultatively colonial lesser kestrel: individual experience vs. conspecific cues. J Anim Ecol. 2001;70:568–78.
Shi DC, Cao YH. The Crested Ibis in China. Beijing: China Forestry Publishing House; 2001 (in Chinese).
Shi DC, Yu XP, Chang XY, Lu BZ. The breeding habits of the Crested Ibis Nipponia nippon. Zool Res. 1989;10(4):327–32 (in Chinese).
Wang HQ. Reproduction of Reintroduced Nipponia nippon in Tongchuan, Shaanxi Province. Sichuan J Zool. 2016;35(3):471–4 (in Chinese).
Wang M, Ye XP, Li YF, Yu XP. On the sustainability of a reintroduced Crested Ibis population in Qinling Mountains, Shaanxi, Central China. Restor Ecol. 2017;25:261–8.
Ward MP, Weatherhead PJ. Sex-specific differences in site fidelity and the cost of dispersal in yellow-headed blackbirds. Behav Ecol Sociobiol. 2005;59:108–14.
Wiklund CG. Determinants of dispersal in breeding merlins Falco columbarius. Ecology. 1996;77:1920–7.
Włodarczyk R, Wieloch M, Czyż S, Dolata PT, Minias P. Natal and breeding dispersal in Mute Swans Cygnus olor: influence of sex, mate switching and reproductive success. Acta Ornithol. 2013;48:237–44.
Yu XP, Liu NF, Xi YM, Lu BZ. Reproductive success of the Crested Ibis Nipponia nippon. Bird Conserv Int. 2006;16:325–43.
Yu XP, Lu XR, Lu BZ, Liu NF. Influences of age on the reproductive success of the crested ibis Nipponia nippon. Curr Zool. 2007;53:812–8 (in Chinese).
Yu XP, Chang XY, Li X, Chen WG, Shi L. Return of the Crested Ibis Nipponia nippon: a reintroduction programme in Shaanxi province, China. BirdingASIA. 2009;11:80–2.
Yu XP, Xi YM, Lu BZ, Li X, Gong MH, Shi L, Dong R. Post-fledging and natal dispersal of Crested Ibis in the Qinling mountains, China. Wilson J Ornithol. 2010;122:228–35.
Yu XP, Li X, Huo ZP. Breeding ecology and success of a reintroduced population of the endangered Crested Ibis Nipponia nippon. Bird Conserv Int. 2015;25:207–19.
Authors’ contributions
RD and X. Yu conceived and designed the study. RD, LZ, ML, XL and HW conducted the field work. RD and X. Ye carried out the analyses. RD prepared the draft of the manuscript and X. Yu and X. Ye revised it. All authors read and approved the final manuscript.
Acknowledgements
We thank the staff of the Reintroduction Base of Crested Ibis in Ningshan County for providing logistical help and research aid. Special thanks to Professor Kees van Achterberg for his much improvement on the English of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used in the present study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was completely supported by the National Nature Science Foundation of China (Nos. 31572282 and 31172103).
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
Table S1. Models selected from the candidate model set receiving up to 95% of the total weight for breeding dispersal probability (2008‒2016). Table S2. Models selected from the candidate model set receiving up to 95% of the total weight for breeding dispersal distance (2008‒2016).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Dong, R., Ye, X., Zhong, L. et al. Effects of breeding success, age and sex on breeding dispersal of a reintroduced population of the Crested Ibis (Nipponia nippon) in Ningshan County, China. Avian Res 9, 40 (2018). https://doi.org/10.1186/s40657-018-0132-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40657-018-0132-7