Abstract
Offspring desertion by a single parent, mainly the female, occurs in many charadriiform birds. According to the differential parental capacity hypothesis (DPCH), females are more likely to desert, since they may be in poorer body condition than males as a consequence of egg-laying costs. This study investigated the body condition of 122 male and 118 female Whiskered Terns. In this species half of females desert during the chick-rearing period, the remainder during the post-fledging period. Body condition was investigated during the late incubation and early chick-rearing phases during the biparental care period in: (1) females that deserted during the chick-rearing period, (2) females that did not desert during this time, (3) males deserted by females, and (4) males not deserted by females. Among females that stayed, body condition during the pre-hatching period did not vary with relative catching date (clutch/brood age), whereas females that later deserted showed a very poor body condition when caught around seven days prior to hatching. After hatching, body condition was lower in both deserting and non-deserting females caught later. Among males whose females stayed, body condition remained nearly stable, whereas the body condition of males whose females deserted was lower the later they were caught. These results tally only partially with the DPCH: the pattern of parental body condition changes during breeding is apparently more complex than assumed by the DPCH and the cost of egg laying may be lower than suggested. Desertion by Whiskered Tern females is better explained by the sex role differences in parental care. The male’s ability to compensate for the missing female may be playing a role in the evolution of desertion.
Zusammenfassung
Physische Verfassung von Männchen und Weibchen der Weißbart-Seeschwalbe Chlidonias hybrida, einer Art, bei der die Weibchen ihre Brut im Stich lassen: ein Test der Hypothese der unterschiedlichen elterlichen Belastbarkeit
Bei vielen Regenpfeifervögeln kommt es vor, dass ein Elternteil, in der Regel das Weibchen, seine Nachkommen verlässt. Nach der Hypothese der unterschiedlichen elterlichen Belastbarkeit (DPCH) ist es eher wahrscheinlich, dass die Weibchen ihre Brut verlassen, da sie wegen der Belastungen durch die Eiablage möglicherweise in einer schlechteren körperlichen Verfassung als die Männchen sind. In dieser Studie wurde die körperliche Verfassung von 122 männlichen und 118 weiblichen Weißbart-Seeschwalben untersucht. Bei dieser Art verlässt die Hälfte der Weibchen ihre Küken während der Aufzucht, die anderen in der Zeit nach dem Ausfliegen. Die physische Verfassung wurde in der fortgeschrittenen Brutzeit und in der frühen Aufzuchtphase der Küken während der Betreuung noch durch beide Elterntiere untersucht: (1) Weibchen, die während der Aufzucht ihre Küken verrließen, (2) Weibchen, die während dieser Zeit bei ihrer Brut blieben, (3) Männchen, die von den Weibchen verlassen wurden, und (4) Männchen, die nicht von den Weibchen verlassen wurden. Bei den Weibchen, die blieben, variierte ihre physische Verfassung während der Zeit vor dem Schlüpfen nicht mit dem relativen Fangdatum (Gelege-/Brutalter), während die Weibchen, die ihre Brut später verließen, zum Zeitpunkt ihres Fangs, etwa sieben Tage vor dem Schlüpfen, einen sehr schlechten Körperzustand aufwiesen. Nach dem Schlüpfen war die körperliche Verfassung sowohl der „desertierten “ als auch der gebliebenen Weibchen schlechter, als sie anschließend gefangen wurden. Bei den Männchen, deren Weibchen geblieben waren, blieb die körperliche Verfassung nahezu unverändert, während der Körperzustand derjenigen Männchen, deren Weibchen „desertiert “ waren, umso schlechter war, je später sie gefangen wurden. Diese Ergebnisse stimmen nur teilweise mit der DPCH überein: das den Veränderungen der körperlichen Verfassung der Elterntiere während der Aufzucht zugrunde liegende Muster ist offenbar komplexer als von der DPCH angenommen, und die Kosten des Eierlegens sind möglicherweise geringer als bisher gedacht. Das „Desertieren “ von Weibchen der Weißbart-Seeschwalben lässt sich besser mit geschlechtsspezifischen Unterschieden in der elterlichen Brutpflege erklären. Die Fähigkeit der Männchen, fehlende Weibchen zu ersetzen, spielte möglicherweise bei der Evolution des Desertierens ein Rolle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural offspring desertion, not caused by harsh environmental conditions or predation pressure, is a reproductive strategy where one parent deserts its offspring, shifting on to the other the burden of raising the offspring until independence (Székely et al. 1996; Parker et al. 2002; Webb et al. 2002; Cockburn 2006; Lessells 2012). The deserter may primarily increase its fitness via extra offspring which it may invest in shortly after desertion (Beissinger 1987; Fujioka 1989; Székely and Williams 1995; Pilastro et al. 2001; Morton et al. 2010; Béziers and Roulin 2016). Leaving the partner could also conserve the deserter’s energy and enhance its survival probability (Székely et al. 1996; Lessells 2012). Furthermore, the deserter could benefit by departing on migration earlier (e.g. Gratto-Trevor 1991; Currie et al. 2001; Nisbet et al. 2011a, 2011b; Byholm et al. 2022) or moulting earlier (e.g. Ezaki 1988; Urano 1992; Kloskowski 2003; Hemborg 2004; Mumme 2018). It has been shown that the mate continuing parental care increases provisioning rates, in this way compensating the lack of the mate. Therefore, desertion does not usually have a negative influence on breeding success (Beissinger 1987; Fujioka 1989; Osorno and Székely 2004; Ledwoń and Neubauer 2017; Harrod and Mumme 2021; but see Székely and Williams 1995).
The decision by one of the mates to desert depends on several factors: the availability of food, the number of eggs/nestlings, the probability of remating, the ability of the mate to compensate for the missing partner, the deserter’s sex, not to mention the costs and benefits accruing as a result of desertion (reviewed in Székely et al. 1996; Lessells 2012). One important factor which could influence desertion is body condition (Erckmann 1983; McNamara and Houston 1996; Webb et al. 2002; Houston et al. 2005). However, the relationship between natural desertion and body condition has rarely been studied. The principal hypothesis explaining the relationship between natural parental desertion and body condition is the differential parental capacity hypothesis (hereafter DPCH), formulated for Charadriiformes by Erckmann (1983). In this group of birds, the offspring are more often deserted by females than by males, but in some species both males and females are known to desert (Warriner et al. 1986; Székely and Williams 1995; Székely et al. 1996; Amat et al. 1999; Jamieson 2012; Ledwoń and Neubauer 2017). Erckmann’s hypothesis (Erckmann 1983) assumes that the female of a charadriiform bird is the sex more vulnerable to body-reserve depletion during breeding, mostly because of her high investment in egg production. The DPCH suggests that egg laying may not stress females so much that they cannot participate in incubation, but may leave them less able to undertake the entire, long process of parental care. Therefore, if the condition of a female declines after laying to such an extent that her body reserves are no longer sufficient to ensure parental care, she will desert during incubation or brooding. Erckmann supported his hypothesis with the results of his own study (Erckmann 1981), along with those of Ashkenazie and Safriel (1979) and Maxson and Oring (1980). They showed that in Semipalmated Sandpipers Calidris pusilla, Western Sandpipers C. mauri and Spotted Sandpipers Actitis macularius, the maximum daily amount of energy expended by females during egg laying was considerably greater (approximately 40% or more – depending on species) than by males in territory defence. Furthermore, Erckmann (1981) experimentally mimicked desertions during the incubation period of both male and female Western Sandpipers – a species with bi-parental incubation and female offspring desertion. He found that in response to such experimental desertion, mates of both sexes abandoned their nests before the eggs hatched. The mean time from experimental desertion to nest abandonment was significantly shorter in females, which suggested that they were subjected to greater stress than males. Furthermore, body condition (the ratio of body weight to bill length) in this species was a much better predictor of females rather than males remaining with the clutch. In addition, deserted females lost weight more rapidly than deserted males, which again suggests that females were under greater stress than males. To date, the DPCH has been tested in three charadriiform species: Little Auks (Dovekie) Alle alle (Wojczulanis-Jakubas et al. 2012), among which only females naturally desert; Kentish Plovers Charadrius alexandrinus (Amat et al. 1999, 2000); and Dunlins Calidris alpina (Jamieson 2012), among which it is the females that mainly desert. But this hypothesis was not confirmed in any of these species, since the body conditions of males and females did not differ.
One of the species from the order Charadriiformes in which females almost exclusively desert is the Whiskered Tern Chlidonias hybrida: 97% of females do so (Ledwoń and Neubauer 2017). Desertions start when the chicks are ~ 5 days old and no longer require intensive brooding, but the vast majority of desertions occur after the first week of the chicks' lives. Slightly more than half of the desertion events (52%) take place during the chick-rearing period, the remainder during the post-fledging period. Two weeks after fledging, which falls around the age of 21 days, there are no families with female care. Males care for the juveniles after they have fledged for several more weeks at least: they feed them and teach them to forage (ML unpublished data). As in other Charadriiformes, female Whiskered Terns incur considerable egg-laying costs (Rahn et al. 1975). The weight of a clutch (usually three eggs) represents about one-third of the female body mass (ML unpublished data). Moreover, it has been predicted for Charadriiformes that the energetic cost of egg production is high and represents approximately 80–130% of the Basal Metabolic Rate (Carey 1996; but see Williams 2005). Female Whiskered Terns are less resistant to acute stress than males: female desertion after induced stress (when temporarily held in captivity) was found to be more likely if the body condition was poor, whereas the desertion rate in males was low, irrespective of their body condition (Ledwoń et al. 2019, 2022). This suggests that natural desertion by female Whiskered Terns may be associated with the level of their body condition.
If, in line with the DPCH, brood desertion by Whiskered Tern females is indeed induced by body reserve depletion, we should expect females to be in worse body condition than males during both the incubation and chick-rearing periods. Furthermore, desertion by Whiskered Tern females can happen almost any time after hatching: during both the chick-rearing and post-fledgling periods. Males, by contrast, invariably continue parental care after the female has deserted (Ledwoń and Neubauer 2017). Hence, the DPCH leads us to expect earlier-deserting females to be in poorer body condition (chick-rearing period) than those that stay and desert later, during the post-fledging period. The main aim of the present study was to test the DPCH by comparing body condition (measured as scaled body mass) in relation to clutch/brood age in four groups of birds: females that (1) deserted during the chick-rearing period and (2) remained at the nest during this time (but deserted later, see Ledwoń and Neubauer 2017); males, whose females (3) deserted during the chick-rearing period or (4) stayed.
Methods
Study species
The Whiskered Tern is a semi-precocial, colonial waterbird species (Gochfeld et al. 2020). Both males and females invest heavily in parental care. Males feed females before and during egg laying (Spina 1982; Betleja 2003; Paillisson et al. 2007; Ledwoń 2010). The rates of extra-pair paternity and intra-specific brood parasitism are low and involve less than 8 and 6% of offspring, respectively (Minias et al. 2013; Ledwoń and Szczys 2022). Both parents incubate the eggs and brood the chicks (Ledwoń 2010). Provisioning rates between pair members differ: females supply ca 25% less food than males (Ledwoń and Neubauer 2017). The timing of brood desertion varies between females, ranging from ~ 5 to 40 days after hatching. Each year, 31–61% of nests are deserted by females during the chick-rearing period (Ledwoń and Neubauer 2017; Ledwoń et al. 2023). Following female desertion, males increase their provisioning rates, but in bigger (2- and 3-chick) broods they can only partly compensate for the loss of female care; only in single-chick broods is total compensation possible. Despite this, termination of parental care by females has no effect on fledging success (Ledwoń and Neubauer 2017). Female provisioning rates are significantly correlated with the chances of nest desertion: daily desertion rates are lower when females supply more food (Ledwoń and Neubauer 2017). After desertion, only ca 5% of females remate and renest (Ledwoń et al. 2023). As a result of desertion, females probably start migrating earlier than males (ML unpublished data).
Field work
The study took place in the northern part of the Upper Vistula Valley (49°59′31.5"N, 19°26′12.6"E), southern Poland (for a detailed description of the study area, see Ledwoń et al. 2013, 2014, 2023; Gwiazda and Ledwoń 2015). The fieldwork, carried out in 16 colonies during eight breeding seasons (May – August; 2006–2007, 2012–2017), embraced the whole of the Whiskered Tern's breeding season. The clutches in all the monitored nests were initiated between late May and late July. Monitoring of most nests began during the early stages of incubation (up to about the 10th day after egg laying; for a detailed description of the field methods, see Ledwoń and Neubauer 2017). Each colony was entered 5–12 times per season at 2–4-day intervals. Plastic mesh fences were erected around the monitored nests with eggs to prevent the chicks from escaping until they were able to fly (for a detailed description of the enclosure, see Ledwoń et al. 2015). The enclosure had no adverse effect on either breeding success or bird behaviour (Ledwoń 2010; Ledwoń et al. 2015; 2016) and facilitated trapping and behavioural observations of the birds.
Hatching dates were deduced on the basis of nest visits (see above) and from observations using spotting scopes from the pond shore (see below; for a detailed description of the methods, see Ledwoń and Neubauer 2017). The presence of chicks or eggs in a monitored nest was easy to record during the observations as the nests were plainly visible. The hatching date was determined mainly from the presence of freshly hatched chicks. These could be accurately aged as they were still wet, having just emerged from the eggshell, or from the presence of a hatching star on the egg, which indicates that the chick has started hatching and will emerge within 24 h. The hatching date of chicks was hereafter coded as day 0. Hatching dates were also determined by backward calculation based on a chick’s wing length (Paillisson et al. 2008; Banach et al. 2021) or the presence of freshly laid eggs (see also Ledwoń and Neubauer 2017). The exact hatching date was known for 82% of nests; for the remaining 18%, the accuracy was still high at ± 1 day, the middle day being taken as the hatching date in these nests (see Ledwoń and Neubauer 2017). In all these cases, the first-egg laying date was calculated on the assumption that incubation lasts for 21 days (Betleja 2003).
Trapping and measurements of adult birds
Nests for trapping adults were selected at random. Trapping took place between the 8th day before hatching and the 8th day after hatching with the aid of a roof trap (for a detailed description of the trapping method, see Ledwoń et al. 2015, 2016). In all the nests examined, both partners were always present after any capture (of one or both birds), which was confirmed during behavioural observations after trapping. Only 3% of females deserted during the first week of a chick’s life, (Ledwoń and Neubauer 2017). So taking both conditions into account, it can reasonably be assumed that female trapping during the chick-rearing period was not biased by female desertion. No birds were caught during the late-chick or post-fledging periods, as adults did not enter the trap during this time.
Total head length was measured from the tip of the bill to the back of the skull to the nearest 0.1 mm with callipers (Ledwoń 2011). Body mass was measured to the nearest 1 g with a Pesola spring balance. All the measurements were taken by one person. Each trapped bird was uniquely marked with hair dye applied to small areas of the wing and tail feathers so that it could be identified from a distance (Ledwoń and Neubauer 2017). Blood was taken from 208 birds (87% of trapped individuals) for molecular sexing (Ledwoń et al. 2015; Goławski et al. 2016). The remaining birds were sexed on the basis of sexual size dimorphism (Ledwoń 2011).
Behavioural observations
On the first observation day after trapping, both parents were present in all the nests (see Ledwoń and Neubauer 2017). Thereafter, all nests with trapped birds were observed in order to determine the occurrence of natural desertion during the chick-rearing period (up to the 21st day of a chick’s life). The focal nests were monitored during observation sessions in each colony with marked individuals from the pond shore at distances from several tens of metres to ca 100 m using binoculars (10 × 42, 15 × 56) and spotting scopes (× 30, × 50), every 1–4 days in the morning, for an average of 190 min per observation session (Ledwoń and Neubauer 2017). The nests were clearly visible because they had been built on the floating leaves of aquatic plants. During each observation session, the number of chicks and the presence or absence of each parent were noted. The female desertion event could thus be established precisely for each individual nest. Because adult birds were never again recorded at nests following their absence during two consecutive observation sessions on different days, desertion was defined as having occurred when one parent did not attend the chicks on two consecutive days of behavioural observations, i.e. it was assumed to have taken place midway between the dates on which the bird was seen for the last time and the next observation session (Székely and Lessells 1993). Because the majority of fledglings disperse, observation of most nests with marked birds was not possible during the post-fledging period. For a further description of the field observation methods and the determination of desertion, see Ledwoń and Neubauer 2017.
The data set
As only 7% of the birds were caught in two or more years, only one, randomly selected trapping event per bird was included; hence, each adult involved in the analysis was considered just once. In total, 240 birds from 188 nests were trapped in 16 colonies during 8 years. In 136 nests only one parent was trapped. Among the 52 nests where both parents were trapped, both were trapped on the same day in 40 (78%) nests, while the difference in the other nests between the dates of trapping the mates varied between 2 and 8 days. Observations of the marked birds, where at least one mate was caught and uniquely marked with hair dye, enabled each individual to be assigned to one of the following groups: (1) females that deserted during the chick-rearing period (n = 53), (2) females that did not desert during the chick-rearing period (n = 65), (3) males whose females deserted (n = 58) and (4) males whose females did not desert (n = 64).
Statistical analysis
Body condition was estimated as residuals of body mass regressed on total head length. The body condition calculated in this way was then treated as a normally distributed response in the analysis. Body condition and mass were highly correlated (Pearson r = 0.807, df = 238, p < 0.001) but body condition did not correlate with total head length (Pearson r = − 0.001, df = 238, p > 0.05).
Generalized additive linear mixed models (GAMMs, Zuur et al. 2014) were used to capture the (possible) nonlinear patterns of body condition over time. Models were fitted in the gamm4 library (Wood and Scheipl 2014) in R (R Core Team 2020). Body condition was treated as a response, modelled as a function of the following factors in the global model: a ‘category’ factor with four levels, identifying females that deserted their broods during the chick-rearing period and those that stayed during this period, and also males which were or were not deserted by females, included as interaction terms with smoothing functions to obtain four separate smoothers illustrating body condition changes over time; brood size (number of hatched chicks, factor with three levels, 1, 2 or 3); clutch initiation date (measured in days numbered from 1st May each year, with 1st May being day 1); clutch/brood age – age (date) of the clutch or of the chicks whose parents were trapped and body condition measured (the hatching date was coded as day 0, negative values were assigned to the incubation period (range − 8 to − 1) and positive ones to the chick-rearing period (range 1 to 8). The number of hatched chicks, date of clutch initiation and clutch/brood age were treated as interacting with the ‘category’ factor in the global model. The data were structured hierarchically, with nests, colonies, fish pond complexes and years having multiple observations: because of their spatiotemporal correlation, these could not be treated as independent. To account for this non-independence, the random part of the models always included four random effects: year (8 levels), fish pond complex (7 levels in total, variable across years, range 1–4), colony id (16 levels, variable across years, 2–3 colonies per year) and nest id (188 levels, 6–45 nests per year). The last three of these effects were nested, whereas year was treated separately because the colony locations changed between ponds annually: as none of these locations were permanent, the year effect could not be included in the nested structure. The global model as well as the nested, reduced model versions were fitted with the MuMIn library (Bartoń 2015), resulting in a total of 26 fitted models, including the null one. Model ranking was based on AIC. Only the top few models received some support, all of which had nearly identical smooth functions, so the inferences were based on the two top-supported models with Δ AIC ≤ 2 (Table 1). The model-averaged parameters were calculated with the MuMIn library (Bartoń 2015) and the model-averaged predictions were obtained manually with appropriate model weights. Both top-supported models were nearly identical with respect to the significance of effects and predicted response values.
Results
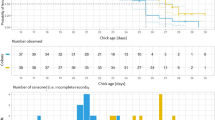
There were two well supported models (Δ AIC ≤ 2) among the 26 fitted to explain the variation in body condition of males and females in relation to whether a female did or did not desert (Table 1). No covariates other than group (‘category’ factor) were found to affect mean body condition (deserting and non-deserting females and males deserted and not deserted by females) except for clutch/brood age (Table 2). During the biparental care period studied here, the variation in body condition and clutch/brood age differed between the groups (sexes and the occurrence of desertion, Fig. 1A, B). The body condition of females that did not desert did not vary with relative catching date, but was lower in females caught later during the chick-rearing phase (Fig. 1A). The body condition of males not deserted by females was only slightly negatively correlated with relative catching date across the whole period considered, i.e. both prior to and after hatching (Fig. 1A). Variation in the body condition of females that deserted and of males deserted by females was strikingly different: the body condition of females was the poorest of all the groups, on average, when they were caught around the seventh day prior to hatching (Fig. 1A, B). When caught around hatching, however, the condition of deserting females was similar to that of males and non-deserting females. After hatching, deserting females were of lower condition when caught later. The condition of deserted males was more strongly negatively correlated with relative catching date, but, importantly, these males were in much better condition than females at the earliest relative catching date. There was no such difference between the sexes in the ‘non-desertion’ group.
Predicted model-averaged relationships between body condition and clutch age (in days, hatching date coded as day 0) in nests with biparental care: no female desertion until fledging A, with female desertion prior to fledging B. The lines show the relationships predicted by the top-supported Generalized linear additive mixed models (see Table 1; both top-supported models produced nearly identical relationships), solid lines–females, dashed lines–males, grey areas– ± SE
Discussion
The relationship between Whiskered Tern body condition and brood age was found to vary in relation to parental sex and to the occurrence of female desertion. It therefore appears to be more complex than assumed by Erckmann 1983, see Introduction). A potential drawback of the present study is the fact that just a single trapping per bird was used in the analysis; hence, it was not possible to study the changes in body condition at the individual level (like Jamieson 2012). Trapping the same birds multiple times during the breeding period and both mates on the same day was avoided so as to minimize disturbance. The present approach – comparing the condition of randomly trapped males and females during the incubation and chick-rearing periods – is identical to the one reported by Wojczulanis-Jakubas et al. (2012) in Little Auks, a species in which females desert. The conclusions remain valid so long as the assumption that randomly trapped individuals with a single measurement of condition are representative for the entire population is met.
The DPCH (Erckmann 1983, see also Introduction) attempts to explain offspring desertion by charadriiform females as being the result of the energetic limitations imposed on females by the cost of reproduction, primarily egg production. The DPCH assumes that females should be in a worse condition than males and that the condition of the former should decline after laying. This has been shown to occur in Cooper's Hawks Accipiter cooperii (Kelly and Kennedy 1993). But the present results only partially support this hypothesis. The initial level of body condition of deserting females was indeed lower than in males, but higher shortly before hatching (Fig. 1B). Furthermore, during the chick-rearing period, the body condition of deserting and non-deserting females and deserted males declined with relative catching date, while only in males that were not deserted by females did the body condition remain almost stable. The DPCH failed to explain female desertion in terms of body condition in three other charadriiform species (Amat et al. 2000; Wojczulanis-Jakubas et al. 2012; Jamieson 2012, see Introduction). The costs of egg production in species studied so far may not be as high as Erckmann (1983) suggested, so that they do not cause any substantial deterioration of a female’s body condition. It has been suggested recently that the commonly assumed high cost of egg production incurred by female birds is actually more complex than previously thought and deserves further attention (Williams 2005; Wojczulanis-Jakubas et al. 2014). Research on Little Auks, a species in which only females desert, suggests that when food is unlimited during the egg-laying period, the energetic costs of egg production may simply be negligible. In such cases, female body condition might be affected by other costs unrelated to food (Wojczulanis-Jakubas et al. 2014). As in other larids, egg production in Whiskered Terns relies mainly on exogenous resources, in other words, the “income” breeding strategy is employed (sensu Jönsson 1997). In this species, males deliver food to their mates during the courtship period (both before and during egg laying) and to a lesser extent during incubation as well (Ledwoń 2010; Ledwoń and Neubauer 2018). Females spend about 90% of their time on the nesting platform during incubation, limiting their energy expenditure on foraging (Ledwoń 2010; Ledwoń and Neubauer 2017). Furthermore, the Whiskered Tern population studied here breeds on carp ponds where food is plentiful. It has been suggested with regard to the Dunlin, a species with mainly female desertion, that the high availability of prey on the breeding grounds ensures that the body condition of females is superior to that of males (Jamieson 2012). The exact costs of egg production by Whiskered Tern females remains unknown, but the results obtained in this study suggest that they are probably not high enough to bring about a significant and permanent difference in body condition between females and males.
The DPCH focuses mainly on the energy costs incurred by the female when laying eggs, yet ignores those of the males. This is likely to be one of the reasons why the present results do not support the DPCH. Male Whiskered Terns have high energy costs prior to incubation, as they spend most of their time foraging and bringing food to their females, not to mention supplying nesting material (Ledwoń and Neubauer 2018). Therefore, in the Whiskered Tern, the differences in energy expenditure between males and females prior to incubation might not be as great as the DPCH assumes. In the Dunlin, by contrast, the high energetic costs of the males’ aerial displays during the courtship period could be responsible for their poor body condition at the beginning of incubation (Jamieson 2012).
This study found that during the biparental care period, the body condition of deserting females, non-deserting females and deserted males decreased during the chick-rearing period, and that only in males whose females did not desert was the body condition almost independent of relative catching date. One might expect mass loss during the chick feeding period to be adaptive because flight costs are reduced (Ricklefs 1974; Freed 1981; Norberg 1981, 2015; Jones 1994; Cavitt and Thompson 1997; Cichoń 2001). Whiskered Terns forage while flying, catching their prey items mainly by plunge diving or picking them off plants and the water surface (Gwiazda and Ledwoń 2015, 2016). Therefore, the loss of mass and thus body condition in adults of this species during the chick-rearing period can reduce flight costs. Furthermore, it is assumed in larids that first part of the chick-rearing period is the most demanding energetically, because the adult energy expenditure per hour spent off the nest is the highest in this period (Moe et al. 2002). Increased parental care (food provisioning, brooding) may therefore decrease body condition. This could differ between the sexes according to their level of investment (Ricklefs 1983; Nur 1984; Salamolard and Weimerskirch 1993; Wendeln and Becker 1996; Moe et al. 2002; Lormée et al. 2003; Williams 2012). However, neither of these predictions are mutually exclusive (Moreno 1989).
The body condition of males not deserted by females did not vary with relative catching date significantly as it did in other birds during the chick-rearing period. This probably indicates that males, whose females stayed longer, worked less, whereas the body condition of males that were deserted decreased throughout the period of biparental care. This strong decrease in the condition of deserted males during both the incubation and chick-rearing periods suggests a high investment in parental care on their part. The relatively small contribution to parental care by these females is confirmed by the probability of female desertion increasing with her declining provisioning rate (Ledwoń and Neubauer 2017). During biparental care in this species, both mates feed the chicks, but males deliver a quarter more food in terms of energy (kJ/h) than females, irrespective of the clutch size (Ledwoń and Neubauer 2017, see Introduction). The present results suggest that even though males bring more food (see above), the cost of parental care (feeding chicks) is higher for females that did not desert. It is difficult to state exactly why these females do not desert prior to fledging but do so later, during the post-fledging period. The desertion of a female can be affected not only by her own condition, but also by her mate’s condition, his investment in parental care and the cost to her mate or the brood that has been deserted (McNamara et al. 1999; Lessells 2012).
The brood desertion phenomenon in Charadriiformes appears to be much more complex (Jamieson 2012; Amat et al. 2000; Wojczulanis-Jakubas et al. 2012) than Erckmann (1983) realised. The roles of both sexes in parental care during the chick-rearing and post-fledging periods, as well as other conditions, may offer a better explanation of offspring desertion by female Whiskered Terns than the DPCH. Male Whiskered Terns deliver more food than females, irrespective of brood size. The considerable sexual size dimorphism in Whiskered Terns (Ledwoń 2011) could be responsible for the male’s superior feeding ability (Gwiazda and Ledwoń 2015). In the present Whiskered Tern population, female desertion did not lead to additional chick mortality because the males, having been deserted, delivered larger amounts of food to the brood (Ledwoń and Neubauer 2017). In this situation, the costs for a brood suffering female desertion, while not seeming high in terms of breeding success, can still be so for males. Furthermore, males of this species are more resistant to stressors (Ledwoń et al. 2019, 2022) and, if deserted, are more than capable of raising the brood successfully on their own (Ledwoń and Neubauer 2017). It seems likely that the female is probably not necessary to maintain a level of parental care that ensures breeding success (Lessells 2012), but the costs to the male remain to be studied.
To conclude: this study has shown that the relationship between Whiskered Tern body condition and brood age differed between sexes, and also varied according to whether the female deserted. The results offer only partial support to the DPCH; the body condition of females that deserted was indeed poorer than that of males, but shortly before hatching was higher than the males’ levels. Furthermore, soon after hatching, body condition decreased in both deserting and non-deserting females, and also in males deserted by females. In males whose females did not desert, the decline in body condition was weak, suggesting an important role of the female in raising the offspring, despite their contribution to food provisioning being smaller than that of the males. Therefore, the pattern of parental body condition changes during breeding is apparently more complex than assumed by the DPCH. It is likely that the costs incurred by females to produce and lay eggs are not as high as the DPCH assumes and do not result in significant and permanent differences in body condition between males and females during the period under scrutiny. Female desertion is most likely better explained by the sex role differences in parental care during the chick-rearing and post-fledging periods. The male’s ability to compensate for the missing female and, in consequence, the absence of any detectable costs of female desertion in terms of chick survival, may be playing a role in the evolution of desertion. However, the costs incurred by the male remain unexplored.
Data availability
Data are available on the Zenodo repository at DOI: https://doi.org/10.5281/zenodo.8144234.
References
Amat JA, Fraga RM, Arroyo GM (1999) Brood desertion and polygamous breeding in the Kentish Plover Charadrius alexandrinus. Ibis 141:596–607. https://doi.org/10.1111/j.1474-919X.1999.tb07367.x
Amat JA, Visser GH, Pèrez-Hurtado A, Arroyo GM (2000) Brood desertion by female shorebirds: a test of the differential parental capacity hypothesis on Kentish Plovers. P Roy Soc B 267:2171–2176. https://doi.org/10.1098/rspb.2000.1265
Ashkenazie S, Safriel UN (1979) Breeding cycle and behavior of the Semipalmated Sandpiper at Barrow, Alaska. Auk 96:56–67. https://doi.org/10.1093/auk/96.1.56
Banach A, Neubauer G, Flis A, Ledwoń M (2021) Sex-specific growth of nestlings of the Whiskered Tern Chlidonias hybrida, a species with sexual size dimorphism and female brood desertion. J Ornithol 162:1035–1047. https://doi.org/10.1007/s10336-021-01911-y
Bartoń K (2015) Package ‘MuMIn’. https://cran.rproject.org/web/packages/MuMIn/MuMIn.pdf
Beissinger SR (1987) Anisogamy overcome: female strategies in snail kites. Am Nat 129:486–500. https://doi.org/10.1086/284653
Betleja J (2003) Ecological conditions of the expansion of Whiskered Tern Chlidonias hybrida. Ph.D. dissertation, University of Wrocław, in Polish.
Béziers P, Roulin A (2016) Double brooding and offspring desertion in the barn owl (Tyto alba). J Avian Biol 47:235–244. https://doi.org/10.1111/jav.00800
Byholm M, Beal M, Isaksson N, Lötberg U, Åkesson S (2022) Paternal transmission of migration knowledge in a long-distance bird migrant. Nat Commun 13:1–7. https://doi.org/10.1038/s41467-022-29300-w
Carey C (1996) Avian energetics and nutritional ecology. Chapman and Hall, New York
Cavitt JE, Thompson CF (1997) Mass loss in breeding House Wrens: Effects of food supplements. Ecology 78:2512–2523
Cichoń M (2001) Body-mass changes in female Collared Flycatchers: state-dependent strategy. Auk 118:550–552. https://doi.org/10.2307/4089820
Cockburn A (2006) Prevalence of different modes of parental care in birds. Proc R Soc B 273:1375–1383. https://doi.org/10.1098/rspb.2005.3458
Currie D, Valkama J, Berg Å, Boschert M, Norrdahl K, Hänninen M, Korpimäki E, Pöyri V, Hemminki O (2001) Sex roles, parental effort and offspring desertion in the monogamous Eurasian Curlew Numenius arquata. Ibis 143:642–650. https://doi.org/10.1111/j.1474-919X.2001.tb04892.x
Erckmann WJ (1981) The evolution of sex-role reversal and monogamy in shorebirds. Ph.D. dissertation, University of Washington
Erckmann WJ (1983) The evolution of polyandry in shorebirds: an evaluation of hypothesis. In Social behaviour of female vertebrates (S. K. Waser, Editor). New York Academic Press, pp. 113–168.
Ezaki Y (1988) Mate desertion by male great reed warblers Acrocephalus arundinaceus at the end of the breeding season. Ibis 130:427–437. https://doi.org/10.1111/j.1474-919X.1988.tb08817.x
Freed LA (1981) Loss of mass in breeding wrens: the patterns of body-mass dynamics should be stress or adaptation? Ecology 62:1179–1186. https://doi.org/10.2307/1937282
Fujioka M (1989) Mate and nest desertion in colonial little egrets. Auk 106:292–302. https://doi.org/10.1093/auk/106.2.292
Gochfeld MJ, Burger G, Kirwan M, Garcia EFJ (2020) Whiskered Tern (Chlidonias hybrida), version 10. In: del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E (eds) Birds of the World. Cornell Lab of Ornithology, Ithaca, NY, USA
Goławski A, Kasprzykowski Z, Ledwoń M, Mróz E, Morelli F (2016) Brood sex-ratio in expansive and non-expansive tern species in east-central Poland. Bird Study 63:31–36. https://doi.org/10.1080/00063657.2015.1122738
Gratto-Trevor CL (1991) Parental care in Semipalmated Sandpipers Calidris pusilla: brood desertion by females. Ibis 133:394–399. https://doi.org/10.1111/j.1474-919X.1991.tb04587.x
Gwiazda R, Ledwoń M (2015) Sex-specific foraging behaviour of the whiskered tern Chlidonias hybrida during the breeding season. Ornis Fenn 92:15–22
Gwiazda R, Ledwoń M (2016) Sex-specific food choices in Whiskered Terns Chlidonias hybrida during chick rearing. Ardea 104:95–98
Harrod W, Mumme R (2021) Females compensate for moult-associated male nest desertion in Hooded Warblers. Ibis 163:159–170. https://doi.org/10.1111/ibi.12850
Hemborg C (2004) Sexual differences in moult-breeding overlap and female reproductive costs in pied flycatchers, Ficedula hypoleuca. J Anim Ecol 68:429–436. https://doi.org/10.1046/j.1365-2656.1999.00295.x
Houston AI, Székely T, McNamara JM (2005) Conflict between parents over care. Trends Ecol Evol 20:33–38. https://doi.org/10.1016/j.tree.2004.10.008
Jamieson SE (2012) Body mass dynamics during incubation and duration of parental care in Pacific Dunlins Calidris alpine pacifica: a test of the differential parental capacity hypothesis. Ibis 154:825–837. https://doi.org/10.1111/j.1474-919X.2012.01255.x
Jones IL (1994) Mass changes of least auklets Aethia pusilla during the breeding season: evidence for programmed loss of mass. J Anim Ecol 63:71–78. https://doi.org/10.2307/5584
Jönsson KI (1997) Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78:57–66. https://doi.org/10.2307/3545800
Kelly J, Kennedy PL (1993) Dynamic State Variable Model of Mate Desertion in Cooper’s Hawks. Ecology 74:351–366. https://doi.org/10.2307/1939298
Kloskowski J (2003) Offspring desertion in Red-necked Grebes Podiceps grisegena. Ardea 91:25–34
Ledwoń M (2011) Sexual size dimorphism, assortative mating and sex identification in the Whiskered Tern Chlidonias hybrida. Ardea 99:191–198
Ledwoń M, Neubauer G (2017) Offspring desertion and parental care in the Whiskered Tern Chlidonias hybrida. Ibis 159:860–872. https://doi.org/10.1111/ibi.12496
Ledwoń M, Neubauer G (2018) True deception during extra-pair courtship feeding: cheating whiskered tern Chlidonias hybrida females perform better. J Avian Biol. https://doi.org/10.1111/jav.01503
Ledwoń M, Szczys P (2022) Extra-pair paternity in a species with frequent extra-pair courtship feedings, few extra-pair copulations, and male-biased parental care. J Ornithol 163:437–444. https://doi.org/10.1007/s10336-021-01954-1
Ledwoń M, Neubauer G, Betleja J (2013) Adult and pre-breeding survival estimates of the Whiskered Tern Chlidonias hybrida breeding in southern Poland. J Ornithol 154:633–643. https://doi.org/10.1007/s10336-012-0926-z
Ledwoń M, Betleja J, Stawarczyk T, Neubauer G (2014) The whiskered tern Chlidonias hybrida expansion in Poland: the role of immigration. J Ornithol 155:459–470. https://doi.org/10.1007/s10336-013-1027-3
Ledwoń M, Betleja J, Neubauer G (2015) An effective method for trapping both parents and chicks in whiskered terns (Chlidonias hybrida) and its impact on breeding success. Waterbirds 38:290–295. https://doi.org/10.1675/063.038.0309
Ledwoń M, Betleja J, Neubauer G (2016) Different trapping schemes and variable disturbance intensity do not affect hatching success of whiskered terns Chlidonias hybrida. Bird Study 63:136–140. https://doi.org/10.1080/00063657.2015.1136263
Ledwoń M, Neubauer G, Żmuda A, Flis A (2019) Interaction between parent body condition and sex affects offspring desertion in response to acute stress. J Ornithol 160:417–428. https://doi.org/10.1007/s10336-019-01637-y
Ledwoń M, Flis A, Banach A, Kusal B, Łożyńska H, Atamas N, Broński S, Betleja J (2023) Do females of Whiskered Tern Chlidonias hybrida renest after desertion? The European Zool Journal 90:237–247. https://doi.org/10.1080/24750263.2023.2184876
Ledwoń M (2010) Male and female partitioning in parental care in Whiskered Tern Chlidonias hybrida. Ph.D. dissertation, Polish Academy of Sciences, Kraków, in Polish.
Lessells CM (2012) Sexual conflict. In: Royle NJ, Smiseth PT, Kölliker M (eds) The evolution of parental care. Oxford University Press, pp 150–170
Lormée H, Jouventin P, Trouve C, Chastel O (2003) Sex-specific patterns in baseline corticosterone and body condition changes in breeding red-footed boobies Sula sula. Ibis 145:212–219. https://doi.org/10.1046/j.1474-919X.2003.00106.x
Maxson SJ, Oring LW (1980) Breeding season time and energy budgets of the polyandrous Spotted Sandpiper. Behaviour 74:200–263. https://doi.org/10.1163/156853980X00474
McNamara J, Houston A (1996) State-dependent life histories. Nature 380:215–221. https://doi.org/10.1038/380215a0
McNamara JM, Garson CE, Houston AI (1999) Incorporating rules for responding into evolutionary games. Nature 401:368–371. https://doi.org/10.1038/43869
Minias P, Minias A, Dziadek J (2013) Occurrence of extra-pair paternity and intraspecific brood parasitism in the whiskered tern Chlidonias hybrida. Bird Study 61:130–134. https://doi.org/10.1080/00063657.2013.860949
Moe B, Langseth I, Fyhn M, Gabrielsen GW, Bech C (2002) Changes in body condition in breeding kittiwakes Rissa tridactyla. J Avian Biol 33:225–234. https://doi.org/10.1034/j.1600-048X.2002.330304.x
Moreno J (1989) Strategies of mass change in breeding birds. Biol J Linn Soc 37:297–310. https://doi.org/10.1111/j.1095-8312.1989.tb01907.x
Morton ES, Stutchbury BJM, Chiver I (2010) Parental conflict and brood desertion by females in blue-headed vireos. Behav Ecol Sociobiol 64:947–954. https://doi.org/10.1007/s00265-010-0910-7
Mumme R (2018) The trade-off between molt and parental care in Hooded Warblers: Simultaneous rectrix molt and uniparental desertion of late-season young. Auk 135:427–438. https://doi.org/10.1642/AUK-17-240.1
Nisbet ICT, Mostello CS, Veit RR, Fox JW, Afanasyev V (2011a) Migrations and winter quarters of five common terns tracked using geolocators. Waterbirds 34:32–39. https://doi.org/10.1675/063.034.0104
Nisbet ICT, Szczys P, Mostello CS, Fox JW (2011b) Female common terns Sterna hirundo start autumn migration earlier than males. Seabird 24:103–106
Norberg AR (1981) Temporary weight decrease in breeding birds may result in more fledged young. Am Nat 118:838–850. https://doi.org/10.1086/283874
Norberg UM (1995) How a long tail and changes in mass and wing shape affect the cost for flight in animals. Funct Ecol 9:48–54. https://doi.org/10.2307/2390089
Nur N (1984) Feeding frequencies of nestling blue tits (Parus caeruleus): costs, benefits and a model of optimal feeding frequency. Oecologia 65:125–137. https://doi.org/10.1007/BF00384475
Osorno J, Székely T (2004) Sexual conflict and parental care in magnificent frigatebirds: full compensation by deserted females. Anim Behav 68:337–342. https://doi.org/10.1016/j.anbehav.2003.06.027
Paillisson JM, Reeber S, Carpentier A, Marion L (2007) Reproductive parameters in relation to food supply in the whiskered tern (Chlidonias hybrida). J Ornithol 148:69–77. https://doi.org/10.1007/s10336-006-0102-4
Paillisson JM, Latraube F, Reeber S (2008) Assessing growth and age of Whiskered Tern Chlidonias hybrida chicks using biometrics. Ardea 96:271–277
Parker GA, Royle NJ, Hartley IR (2002) Intra familial conflict and parental investment: a synthesis. Philos T R Soc B 357:295–307. https://doi.org/10.1098/rstb.2001.0950
Pilastro A, Biddau L, Marin G, Mingozzi T (2001) Female brood desertion increases with the number of available mates in the rock sparrow. J Avian Biol 32:68–72. https://doi.org/10.1034/j.1600-048X.2001.320109.x
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org.
Rahn H, Paganelli CV, Ar A (1975) Relation of avian egg weight to body weight. Auk 92:750–762. https://doi.org/10.2307/4084786
Ricklefs RE (1974) Energetics of reproduction in birds. In: Paynter A (ed) Avian energetics (R. Massachusetts Nuttall Ornithological Club, Cambridge, pp 152–297
Ricklefs RE (1983) Some considerations on the reproductive energetics of pelagic seabirds. Stud Avian Biol-Ser 8:84–94
Salamolard M, Weimerskirch H (1993) Relationship between foraging effort and energy requirement through the breeding season in the wandering albatross. Funct Ecol 7:643–652. https://doi.org/10.2307/2390184
Spina F (1982) Contribution to the breeding biology of the whiskered tern Chlidonias hybrida in Val campotto (Northern Italy). Avocetta 6:23–33
Székely T, Webb JN, Housto AI, McNamara JM (1996) An evolutionary approach to offspring desertion in birds. In: V. Jr Nolan, and Ketterson, ED. Eds Current Ornithology. 6: 271–330
Székely T, Lessells CM (1993) Mate change by Kentish plovers Charadrius alexandrinus. Ornis Scand 24:317–322. https://doi.org/10.2307/3676794
Székely T, Williams TD (1995) Costs and benefits of brood desertion in female Kentish plovers, Charadrius alexandrinus. Behav Ecol and Sociobiol 37:155–161. https://doi.org/10.1007/BF00176712
Urano E (1992) Early settling the following spring: a long-term benefit of mate desertion by male Great Reed Warblers Acrocephalus arundinaceus. Ibis 134:83–86. https://doi.org/10.1111/j.1474-919X.1992.tb07235.x
Warriner JS, Warriner JC, Page GW, Stenzel LE (1986) Mating system and reproductive success of a small population of polygamous snowy plovers. Wilson Bulletin 98:15–37
Webb JN, Székely T, Houston AI, McNamara JM (2002) A theoretical analysis of the energetic costs and consequences of parental care decisions. Philos T R Soc B 1419:331–340. https://doi.org/10.1098/rstb.2001.0934
Wendeln H, Becker PH (1996) Body mass change in breeding common terns Sterna hirundo. Bird Study 43:85–95. https://doi.org/10.1080/00063659609460998
Williams TD (2005) Mechanisms underlaying the costs of egg production. Bioscience 55:39–48
Williams TD (2012) Physiological adaptations for breeding in birds. Princeton University Press
Wojczulanis-Jakubas K, Jakubas D, Kidawa D, Kośmicka A (2012) Is the transition from biparental to male-only care in a monogamous seabird related to changes in body mass and stress level? J Ornithol 153:793–800. https://doi.org/10.1007/s10336-011-0796-9
Wojczulanis-Jakubas K, Jakubas D, Kulaszewicz I, Kidawa D, Taylor JRE (2014) Influence of primary reproductive investments on blood biochemistry, leukocyte profile, and body mass in a small Arctic seabird. Auk 131:743–755. https://doi.org/10.1642/AUK-14-62.1
Wood S, Scheipl F (2014) gamm4: Generalized additive mixed models using mgcv and lme4. R package version 2014.
Zuur AF, Saveliev AA, Ieno EN (2014) A beginner’s guide to generalized additive mixed models with R. Highland Statistics Ltd.
Acknowledgements
We greatly appreciate the cooperation of the fish farmers concerned, in particular Jerzy Wojciech Adamek. We are grateful to Antonia Łobodzińska, Stanisław Broński and Nataly Atamas for their assistance in the field. We would like to thank Peter Senn for his linguistic guidance. We are also grateful to Piotr Minias for his constructive suggestions on a previous draft of the manuscript. This work was partially supported within a project from the National Science Centre (2014/15/B/NZ8/00214), the Polish Ministry of Science and Higher Education (30402731/0904) and the Institute of Systematics and Evolution of Animals, Polish Academy of Sciences (Small Grants). The fieldwork was carried out by permission of the Local Ethical Committee, the Ministry of the Environment and the General Directorate for Environmental Conservation.
Author information
Authors and Affiliations
Contributions
ML conceived the study, ML, AB and AF collected the data, ML coordinated the study, AB and ML molecularly sexed the birds, ML and GN analysed the data, ML and GN wrote the manuscript. All the authors commented on the draft version.
Corresponding author
Additional information
Communicated by S. Bouwhuis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ledwoń, M., Neubauer, G., Flis, A. et al. Female and male body condition in the Whiskered Tern Chlidonias hybrida, a species with female offspring desertion: a test of the differential parental capacity hypothesis. J Ornithol 165, 93–103 (2024). https://doi.org/10.1007/s10336-023-02099-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02099-z