Abstract
Torpor, a controlled reduction in metabolism and body temperature, reduces energy expenditure substantially. However, torpor expression in wild passerines is currently understudied. We show that skin temperature (Ts) of resting White-throated Treecreepers (N = 4) fell by ~ 5 °C on average in both summer and winter, independent of ambient temperature, but we could not confirm torpor use (Ts reduction > 5 °C). It is possible that roosting in tree hollows provides sufficient insulation to minimise energy loss, or torpor is used only during extreme conditions. Further studies are needed to characterise the physiological flexibilities of species and, therefore, their capability to cope with changing environmental conditions.
Zusammenfassung
Ausgeprägte tägliche Heterothermie beim Weißkehl-Baumrutscher ( Cormobates leucophaea)
Torpor ist eine kontrollierte Verringerung des Stoffwechsels und der Körpertemperatur und reduziert den Energieverbrauch erheblich. Wir konnten zeigen, dass die Hauttemperatur (Ts) ruhender Weißkehl-Baumrutscher (N = 4) sowohl im Sommer als auch im Winter unabhängig von der Umgebungstemperatur im Durchschnitt um ~5°C abnahm, aber den Einsatz von Torpor (Ts-Reduktion > 5 °C) konnten wir nicht bestätigen. Möglicherweise bieten die Schlafplätze in Baumhöhlen eine ausreichende Thermoisolierung, um den Energieverlust zu minimieren, oder aber der Torpor wird nur unter extremen Bedingungen eingesetzt. Weitere Untersuchungen sind erforderlich, um die physiologische Flexibilität von Arten und damit ihre Fähigkeit, mit veränderten Umgebungsbedingungen zurechtzukommen, zu ermitteln.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Birds may use physiological and/or behavioural approaches to deal with energetically challenging periods (e.g. cold temperatures or food shortages) and conserve energy. Behavioural approaches may for example include roosting in tree hollows or huddling (Doucette et al. 2011). Physiological approaches include nocturnal heterothermy, during which metabolic rates and body temperature (Tb) are reduced during the rest phase to save energy (McKechnie and Lovegrove 2002). While some potential costs may occur at low Tbs on a cellular level (Nowack et al. 2019), many species use torpor, a substantial reduction in both metabolism, often by ~ 50–95% below the resting metabolic rate, and in Tb, typically by 5–35 °C (Ruf and Geiser 2015). Torpor in birds is mainly correlated with low Ta and food availability, likely as most are diurnal and do not have large endogenous reserves. Most studies have, therefore, focussed on the colder months, and evidence for torpor in summer is less commonly reported (Geiser 2021).
While this heterothermic response could save energy in small birds (Ruf and Geiser 2015), only few passerine species are known to express torpor in the wild (Noisy Miners Manorina melanocephala (Geiser 2019), Superb Fairy Wrens Malurus cyaneus (Romano et al. 2019) and Eastern Yellow Robin Eopsaltria australis (Aharon-Rotman et al. 2021). Other studies were done on captive birds and typically in controlled environments, and therefore, do not represent the thermal conditions and ecological complexity, especially food availability, experienced in the wild (e.g. Nord et al. 2009).
The aim of our study was to test whether free-living White-throated Treecreepers (Cormobates leucophaea; hereafter “treecreeper”) express torpor during winter and summer at a cool temperate climate site in Eucalyptus woodland in the Australian Northern Tablelands (elevation range 980–1050 m). The treecreeper is a small (~ 22 g) passerine bird, common in dry, open eucalypt forests of south-eastern Australia. They breed in tree hollows and often roost alone, primarily in partially exposed tree fork crevices or under bark (Gibbons and Lindenmayer 2002). To this end, we measured the magnitude of skin temperature Ts reduction and tested whether this reduction was related to ambient temperature (Ta).
Methods
We studied the treecreepers in Imbota Nature Reserve, New South Wales, Australia (30.58°S, 151.72°E), a 218 ha open Eucalyptus and Acacia woodland. We captured three treecreepers (2 males, 1 female; body mass 24.4, 22.8 and 20.8 g, respectively) during the southern hemispheric winter in 2020 (17th–21st July) and 2021 (13th–22nd June) and one treecreeper (male, body mass 18.3 g) during summer in 2020 (27th October–4th November). Capturing birds, transmitter calibrations and attachment to birds are described in Aharon-Rotman et al. (2021). Briefly, after capture, we recorded biometrics, banded birds and attached a calibrated temperature-sensitive radio transmitter (LB-2XT, 0.33 g, Holohil Systems Ltd, Canada) directly to the skin. Birds were then released at the site of capture. Ts of each bird was calculated from the interval between two pulses and recorded automatically in 10-min intervals with receiver/loggers placed near the roost site. Transmitters remained attached to the birds for 6–9 days, and we recorded a total of 21 measurement nights for 4 individuals (7, 8, 3 and 3 nights/individual) and a total of 3388 Ts data points. Birds were active during the day and mostly remained within range of the logger.
Air temperature (Ta) was recorded every 10 min using four temperature loggers (iButton DS1922L, 0.06 °C resolution, Maxim Integrated Products, Inc. Sunnyvale, USA) placed ~ 1 m above ground on the southern (shady) side of trees near the roost locations.
We fitted two separate linear mixed effect models with bird identity as a random effect to explain (1) minimum Ts as a function of body mass, mean Ta and minimum Ta as explanatory variables and (2) average Ts range (maximum Ts–minimum Ts), again as a function of body mass and mean and minimum Ta. The torpor threshold was defined as a reduction by > 5 °C below the resting Ts (Ruf and Geiser 2015). Resting Ts was calculated as the average minimum Ts during daytime, between 10:00 and 14:00, the most active time of day for the studied individuals.
Results
Average daytime Ta (between sunrise and sunset) at our study site was 8.3 ± 2.8 °C (range 0.5 to 19.5 °C) in winter, while in summer it was 18.1 ± 3.2 °C (range 4.2–30.0 °C). The average night-time Ta was 5.4 ± 1.6 °C (range − 0.5 to 11.5 °C) in winter and 9.3 ± 1.9 °C (range 4.2–14.0 °C) in summer.
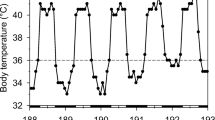
In winter, the Ts of three treecreepers decreased from an average maximum daytime Ts of 39.3 ± 0.9 °C to an average minimum night-time Ts of 34.2 ± 1.1 °C, and the average individual daily fluctuation of Ts was 5.4 ± 0.5 °C (range 3.9 to 6.0 °C; Fig. 1, Table S1). The lowest Ts was 32.6 °C recorded at 00:40 and 4:20 am when Ta was 4.6 and 2.6 °C, respectively. Ts started to rise at 04:50 on average (range 04:00–05:30), about 2 h before sunrise (Fig. 2). In summer, the Ts of the one individual decreased from an average maximum Ts of 38.3 ± 0.7 °C to an average minimum Ts of 33.5 ± 1.0 °C and the average daily fluctuation of Ts was 4.9 ± 0.7 °C (range 4.1–6.0 °C; Fig. 1, Table S1). The lowest summer Ts was 31.3 °C recorded on at 05:50, when Ta was 9.5. Ts started to rise at 05:10 on average (range 04:30–05:50 h), about an hour before sunrise (06:00; Fig. 2).
Examples of skin temperature (Ts) fluctuation of two individual White-throated Treecreepers (red line) in summer (upper panel) and winter (lower panel) and the corresponding ambient temperature (Ta) measured near the roosting sites (blue line) over a seven and eight-day period. Red dashed lines depict the torpor thresholds (summer: 32.1 °C, winter: 34.4 °C), based on 5 °C reduction from average resting Ts of 37.1 °C and 39.4 °C. Two other individuals were measured in winter and their torpor thresholds were 32.4 °C and 33.6 °C, based on 5 °C reduction from average resting Ts of 37.4 °C and 38.6 °C, respectively.
Body mass, average daily Ta, minimum daily Ta or season did not significantly affect the average minimum Ts (all T < 1.09, df = 1.14, all p > 0.35; Table S2), and the average Ts range (all T < 1.59, df = 1.12, all p > 0.36; Table S2) of the treecreepers.
Discussion
Our study did not record torpor in treecreepers, rather a rest-phase heterothermy (Ts reduction larger than the 1–2 °C variation ascribed to the usual normothermic “circadian rhythm”; Reinertsen and Haftorn 1986), with night-time Ts dropping by as much as 6 °C from resting Ts in both summer and winter. While technically we defined Ts decrease by > 5 °C as torpor expression, Ts in our study dropped only briefly below the torpor threshold only on the last days of measurement (Fig. 1), where possibly the transmitters became loose, and therefore may be affected by Ta.
While Ts measurements with external transmitters are less accurate than measuring core Tb, we argue that the patterns recorded were a valid drop in Ts. First, if Ta was influencing the measurement Ts significantly we would expect to see Ts fluctuate closely with Ta. Instead, the rewarming to active Ts was completed before sunrise, while Ta was still low (about 4–6 °C on average; Fig. 2). Second, Ts is very close to core Tb in small birds, variation being less than 2 °C in the 50 g common poorwill (Brigham 1992). Moreover, a reduction of Tb by > 5 °C is a conservative measure to define torpor in comparison to metabolism. For example, MR can fall by 60% with a Tb reduction by only 5 °C (See examples in Geiser 2021, chapter 2).
The magnitude of nocturnal Ts drop did not increase with decreasing mean and minimum Ta, and seems to be similar in summer and winter. Although previous studies suggested a strong link between torpor use and periods of high energetic costs for thermoregulation (Reinertsen and Haftorn 1984), other factors, such as food predictability and availability (Nord et al. 2009) and fat reserves (Nord et al. 2011) may be stronger predictors of the use of heterothermy in treecreepers.
Other species studied at our study site expressed torpor with Ts drop to as low as 29 °C, with relation to Ta (Eastern Yellow-Robin (Aharon-Rotman et al. 2021); noisy miner (Geiser 2019)). These differences are likely a result of behavioural approaches to solving problems (high energetic costs for thermoregulation in our case), rather than a physiological approach (use of torpor). Yellow robins and noisy miners roost in the open and therefore experience much colder temperatures overnight, while treecreepers generally roost in tree hollows or under bark. Although we could not confirm the exact roosting location of individuals at our study site (i.e. inside tree hollow/under bark/in the open), the temperature in a tree hollow where we believe one treecreeper was roosting one night in July (winter) 2020 was on average 3 °C higher than outside temperatures, with a maximum difference of 12 °C. The use of insulated cover for energy conservation may therefore explain the small nocturnal Tb reduction.
While our study did not confirm torpor use in treecreepers in the northern tablelands of eastern Australia, we do not suggest that treecreepers are not capable to express torpor. They possibly express torpor only under extreme conditions, not experienced during our study, to avoid the potential costs associated with torpor use. Other passerine species show similar thermal physiology with Tb reduced to 33–37 °C during rest phase in winter in the wild, likely as a response to food availability and Ta (e.g. Willow Tit Parus montanus (Reinertsen and Haftorn 1984); Blue Tit Cyanistes caeruleus (Nord et al. 2009)).
Although torpor is becoming widely accepted as a common avian strategy to conserve energy, it must be balanced with associated costs. Further studies are therefore needed for a better understanding of the use of heterothermy in wild passerines. Our data are important for conservation prioritisation, as species with highly flexible energetic capabilities may have an advantage over species that depend on behavioural strategies, such as our treecreepers, to deal with the current increasing frequency of extreme and unpredictable weather events, driven by changing climate.
References
Aharon-Rotman Y, Mcevoy JF, Beckmann C, Geiser F (2021) Heterothermy in a small passerine: Eastern yellow robins use nocturnal torpor in winter. Front Ecol Evol 9:759726
Brigham RM (1992) Daily torpor in a free-ranging goatsucker, the common poorwill (Phalaenoptilus nuttallii). Physiol Zool 65:457–472
Burton R, Reichman O (1999) Does immune challenge affect torpor duration? Funct Ecol 13:232–237
Doucette LI, Brigham RM, Pavey CR, Geiser F (2011) Roost type influences torpor use by Australian owlet-nightjars. Naturwissenschaften 98:845–854
Geiser F (2019) Frequent nocturnal torpor in a free-ranging Australian honeyeater, the noisy miner. The Science of Nature 106:28
Geiser F (2021). Ecological Physiology of Daily Torpor and Hibernation. Switzerland: Springer Nature.
Gibbons P, Lindenmayer D (2002). Tree hollows and wildlife conservation in Australia. CSIRO publishing.
Mckechnie AE, Lovegrove BG (2002) Avian facultative hypothermic responses: a review. The Condor 104:705–724
Nord A, Nilsson JF, Sandell MI, Nilsson J-Å (2009) Patterns and dynamics of rest-phase hypothermia in wild and captive blue tits during winter. J Comp Physiol B 179:737–745
Nord A, Nilsson JF, Nilsson J-Å (2011) Nocturnal body temperature in wintering blue tits is affected by roost-site temperature and body reserves. Oecologia 167:21–25
Nowack J, Tarmann I, Hoelzl F, Smith S, Giroud S, Ruf T (2019) Always a price to pay: hibernation at low temperatures comes with a trade-off between energy savings and telomere damage. Biol Let 15:20190466
Reinertsen RE, Haftorn S (1984) The effect of short-time fasting on metabolism and nocturnal hypothermia in the Willow Tit Parus montanus. J Comp Physiol B 154:23–28
Reinertsen RE, Haftorn S (1986) Different metabolic strategies of northern birds for nocturnal survival. J Comp Physiol B 156:655–663
Romano AB, Hunt A, Welbergen JA, Turbill C (2019) Nocturnal torpor by superb fairy-wrens: a key mechanism for reducing winter daily energy expenditure. Biol Let 15:20190211
Ruf T, Geiser F (2015) Daily torpor and hibernation in birds and mammals. Biol Rev 90:891–926
Acknowledgements
We thank Gerhard Körtner for programming the loggers and advice throughout the study. We also thank Steve Debus for his help and great efforts in capturing and banding birds, and to Tessa Stewart, Dirk Browning and Lllyan Luyt for help in the field.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The study was funded by the University of New England-PDF program awarded to YAR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics statement
The animal study was reviewed and approved by the University of New England Animal Ethics Committee (Authority No. AEC20-009 and AEC21-017).
Additional information
Communicated by I. Moore.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aharon-Rotman, Y., McEvoy, J.F., Beckmann, C. et al. Pronounced daily heterothermy in the White-throated Treecreeper Cormobates leucophaea. J Ornithol 165, 241–245 (2024). https://doi.org/10.1007/s10336-023-02092-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02092-6