Abstract
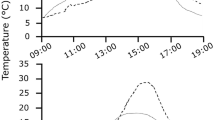
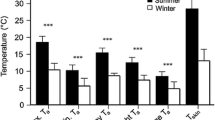
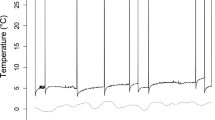
Australian owlet-nightjars (Aegotheles cristatus; ∼50 g) are one of only a few avian species that roost in cavities year-round and regularly enter torpor. Cavity roosts act as thermal buffers, and roost type likely affects energy expenditure of small birds. We used radiotelemetry to locate diurnal winter roost sites of owlet-nightjars in central Australia and to measure body (T b) and skin (T skin) temperature. We also recorded ambient temperature inside (T IN) and outside roosts. Individual owlet-nightjars used one to seven different roosts (tracking time 3–10 weeks), selecting either rock crevices (four birds) or tree hollows (four birds), or switching between the two roost types (seven birds). Rock crevices (T IN +9°C to +33°C) were warmer and thermally more stable than tree hollows (T IN −4.0°C to +37°C). Torpor, often expressed by a reduction of T skin/T b by >10°C for 3–4 h at dawn, was influenced by roost selection; torpor use in tree hollows was almost twice that in rock crevices. Despite the potential energy savings accrued from roosting in well-insulated cavities, owlet-nightjars roosted in tree hollows more often (65% bird days, n = 398) than in rock crevices (35% bird days, n = 211). Lower costs of arousal from torpor via passive rewarming and basking and decreased risk of predation are two possible explanations for the preference to roost in tree hollows. We provide the first evidence for the influence of cavity roost selection on torpor use in a free-ranging bird and show that roost selection and thermal biology are strongly interrelated in determining energy expenditure.






Similar content being viewed by others
References
Audet D, Fenton MB (1988) Heterothermy and the use of torpor by the bat Eptesicus fuscus (chiroptera: verspertilionidae): a field study. Physiol Zool 61:197–204
Bartholomew GA, Howell TR, Cade TJ (1957) Torpidity in the white-throated swift, Anna’s hummingbird, and poor-will. Condor 59:145–155
Brigham RM (1992) Daily torpor in a free-ranging goatsucker, the common poorwill (Phalaenoptilus nuttallii). Physiol Zool 65:457–472
Brigham RM, Geiser F (1997) Breeding biology of Australian owlet-nightjars Aegotheles cristatus in eucalypt woodland. Emu 97:316–321
Brigham RM, Gutsell RCA, Wiacek RS, Geiser F (1999) Foraging behaviour in relation to the lunar cycle by Australian owlet-nightjars Aegotheles cristatus. Emu 99:253–261
Brigham RM, Körtner G, Maddocks TA, Geiser F (2000) Seasonal use of torpor by free-ranging Australian owlet-nightjars (Aegotheles cristatus). Physiol Biochem Zool 73(5):613–620
Brigham RM, Woods CP, Lane JE, Fletcher QE, Geiser F (2006) Ecological correlates of torpor use among five caprimulgiform birds. Acta Zoolog Sin 52(Supplement):401–404
Broadbent K (1910) Birds of Cardwell and Herbert River Districts (NQ). Emu 10:233–245
Chruszcz BJ, Barclay RMR (2002) Thermoregulatory ecology of a solitary bat, Myotis evotis, roosting in rock crevices. Funct Ecol 16:18–26
Doucette LI (2007) Behavioural ecology and thermal physiology of the Australian owlet-nightjar (Aegotheles cristatus). PhD Thesis, University of New England
Doucette LI, Geiser F (2008) Seasonal variation in thermal energetics of the Australian owlet-nightjar (Aegotheles cristatus). Comp Biochem Physiol A 151:615–620
Geiser F, Drury RL (2003) Radiant heat affects thermoregulation and energy expenditure during rewarming from torpor. J Comp Physiol B 173(1):55–60
Geiser F, Holloway JC, Körtner G, Maddocks TA, Turbill C, Brigham RM (2000) Do patterns of torpor differ between free-ranging and captive mammals and birds? In: Heldmaier G, Klingenspor M (eds) Life in the cold: 11th International Hibernation Symposium. Springer Verlag, Berlin, pp 95–102
Geiser F, Goodship N, Pavey CR (2002) Was basking important in the evolution of mammalian endothermy? Naturwissenschaften 89(9):412–414
Geiser F, Drury RL, Körtner G, Turbill C, Pavey CR, Brigham RM (2004) Passive rewarming from torpor in mammals and birds: energetic, ecological and evolutionary implications. In: Barnes BM, Carey HV (eds) Life in the cold: evolution, mechanisms, adaptation, and application twelfth international hibernation symposium. Institute of Arctic Biology, University of Alaska, Fairbanks, pp 51–62
Holyoak DT (2001) Nightjars and their allies: the caprimulgiformes. Oxford University Press, Oxford
Kendeigh SC (1961) Energy of birds conserved by roosting in cavities. Wilson Bull 73:140–147
Kerth G, Weissmann K, König B (2001) Day roost selection in female Bechstein’s bats (Myotis bechsteinii): a field experiment to determine the influence of roost temperature. Oecologia 126:1–9
Knorr OA (1957) Communal roosting of the pygmy nuthatch. Condor 59:398
Körtner G, Geiser F (1998) Ecology of natural hibernation in the marsupial mountain pygmy-possum (Burramys parvus). Oecologia 113:170–178
Kurta A (1985) External insulation available to a non-nesting mammal, the little brown bat (Myotis lucifugus). Comp Biochem Physiol 82A:413–420
Lane JE, Brigham RM, Swanson DL (2004) Daily torpor in free-ranging whip-poor-wills (Caprimulgus vociferus). Physiol Biochem Zool 77(2):297–304
Lausen CL, Barclay RMR (2003) Thermoregulation and roost selection by reproductive female big brown bats (Eptesicus fuscus) roosting in rock crevices. J Zool Lond 260:235–244
Lausen CL, Barclay RMR (2006) Benefits of living in a building: big brown bats (Eptesicus fuscus) in rocks versus buildings. J Mammal 87(2):362–370
Lovegrove BG, Körtner G, Geiser F (1999) The energetic cost of arousal from torpor in the marsupial Sminthopsis macroura: benefits of summer ambient temperature cycles. J Comp Physiol B 169:11–18
McKechnie AE, Lovegrove BG (2002) Avian facultative hypothermic responses: a review. Condor 104:705–724
McKechnie AE, Ashdown RAM, Christian MB, Brigham RM (2007) Torpor in an African caprimulgid, the Freckled nightjar Caprimulgus tristigma. J Avian Biol 38:261–266
Nilsson SG (1984) The evolution of nest-site selection among hole-nesting birds: the importance of nest predation and competition. Ornis Scand 15:167–175
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Rendell WB, Robertson RJ (1989) Nest-site characteristics, reproductive success and cavity availability for tree swallows breeding in natural cavities. Condor 91:875–885
Reynolds PS, Lee RM (1996) Phylogenetic analysis of avian energetics: passerines and nonpasserines do not differ. Am Nat 147:735–759
Schleucher E (2004) Torpor in birds: taxonomy, energetics, and ecology. Physiol Biochem Zool 77:942–949
Sedgeley JA (2001) Quality of cavity microclimate as a factor influencing selection of maternity roosts by a tree-dwelling bat, Chalinolobus tuberculatus, in New Zealand. J Appl Ecol 38:425–438
Smith PG, Racey PA (2005) The itinerant natterer: physical and thermal characteristics of summer roosts of Myotis nattereri (mammalia: chiroptera). J Zool 266:171–180
Solick DI, Barclay RMR (2006) Thermoregulation and roosting behaviour of reproductive and nonreproductive female western long-eared bats (Myotis evotis) in the Rocky Mountains of Alberta. Can J Zool 84(4):589–599
Speakman JR, Thomas DW (2003) Physiological ecology and energetics of bats. In: Kunz TH, Fenton MB (eds) Bat ecology. University of Chicago Press, Chicago, pp 430–490
Stawski C, Geiser F (2010) Fat and fed: frequent use of summer torpor in a subtropical bat. Naturwissenschaften 97:29–35
Turbill C, Körtner G, Geiser F (2003) Natural use of heterothermy by a small, tree-roosting bat during summer. Physiol Biochem Zool 76:868–876
Walsberg GE (1985) Physiological consequences of microhabitat selection. In: Cody M (ed) Habitat selection in birds. Academic Press, Inc, Orlando, pp 389–453
Walsberg GE (1986) Thermal consequences of roost-site selection: the relative importance of three modes of heat conservation. Auk 103:1–7
White FN, Bartholomew GA, Howell TR (1975) The thermal significance of the nest of the sociable weaver Philetairus socius: winter observations. Ibis 117:171–179
White FN, Bartholomew GA, Kinney JL (1978) Physiological and ecological correlates of tunnel nesting in the European bee-eater, Merops apiaster. Physiol Zool 51:140–154
Willis CKR, Brigham RM (2007) Social thermoregulation exerts more influence than microclimate on forest roost preferences by a cavity-dwelling bat. Behav Ecol Sociobiol 62:97–108
Woods CP (2002) Ecological aspects of torpor use and inactivity during winter by common poorwills. PhD Thesis, University of Regina
Zar JH (1998) Biostatistical analysis. Prentice Hall, Upper Saddle River
Acknowledgements
Thanks to Fred Harvey for extensive field assistance. This study was facilitated by funding from the University of New England, the National Science and Engineering Council of Canada and the Australian Research Council. Small grants were provided by Birds Australia, the Royal Zoological Society of New South Wales and the Australian Bird Study Association. Permits for this research were issued by the University of New England Animal Ethics Committee, the Northern Territory Parks and Wildlife Service and the Australia Bird and Bat Banding Scheme. Accommodation at Ormiston was provided by the NT Parks and Wildlife Service.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Rights and permissions
About this article
Cite this article
Doucette, L.I., Brigham, R.M., Pavey, C.R. et al. Roost type influences torpor use by Australian owlet-nightjars. Naturwissenschaften 98, 845 (2011). https://doi.org/10.1007/s00114-011-0835-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-011-0835-7