Abstract
Several species of owls and seed-eating birds breeding in boreal forests may occur irruptively in areas south of their regular wintering range. One common explanation is that they may be forced to move south if food supplies have crashed (push hypothesis). Little attention has been devoted to the alternative idea that the birds are drawn towards abundant food further south (pull hypothesis), even if they could overwinter further north. I evaluate these two hypotheses (push or pull) using data on winter irruptions of the Pine Grosbeak (Pinicola enucleator) and one main type of winter food, seeds in berries of Rowan (Sorbus aucuparia). Grosbeak numbers during winter in southern Norway and Sweden usually peaked at 2–3 year intervals during 1959–2020, and Rowanberries in Norway peaked at 2–3 year intervals during 1980–2020. Pine Grosbeak peaks coincided with Rowanberry peaks in most years, and annual number of grosbeaks was positively correlated with the Rowanberry index. However, even in irruption years grosbeaks moved north already in January–February, inconsistent with the push hypothesis. Furthermore, Rowan masting in Norway and Finland usually had synchronous cycles, implying that irruptions were triggered even when Rowans had large crops close to the most likely origin of irruptions, opposite to what is expected by the push hypothesis. Thus, available evidence is most consistent with the pull hypothesis: Pine Grosbeaks move south in winters with high Rowanberry abundance and exploit this resource until it is gone by mid-winter, and then return to northern areas.
Zusammenfassung
Masseneinflüge des Hakengimpels, die durch Mastjahre der Vogelbeere in südliche Regionen angezogen werden
Mehrere Arten von Eulen und samenfressenden Vögeln, die in borealen Wäldern brüten, können in Regionen südlich ihres regulären Überwinterungsgebietes in Massen auftauchen. Eine gängige Erklärung ist, dass diese Arten gezwungen sein könnten, nach Süden zu ziehen, wenn das Nahrungsangebot eingebrochen ist (engl. push hypothesis). Wenig Aufmerksamkeit wurde bisher der alternativen Hypothese gewidmet, dass die Vögel durch einem reichhaltigen Nahrungsangebot weiter südlich angezogen werden (engl. pull hypothesis), selbst wenn sie weiter nördlich überwintern könnten. Ich bewerte diese beiden Hypothesen (push- oder pull-Hypothese) anhand von Daten über Wintermasseneinflüge des Hakengimpels (Pinicola enucleator) und einer Hauptnahrungsart dieser Art im Winter, den Samen der Vogelbeere (Sorbus aucuparia). Die Zahl der Hakengimpel im Winter in südlichen Norwegen und Schweden erreichten im Zeitraum 1959–2020 in der Regel in Intervallen von zwei bis drei Jahren einen Höchststand. Mastjahre der Vogelbeere traten in Schweden im Zeitraum 1980–2020 in Abständen von zwei bis drei Jahren auf. Spitzenwerte von Hakengimpelzahlen fielen in den meisten Jahren mit der Mast der Vogelbeere zusammen und die jährliche Zahl von Hakengimpel korrelierte positiv mit dem Vogelbeeren-Index. Allerdings zogen die Hakengimpel auch in den Jahren mit starken Masseneinflügen bereits Januar–Februar nach Norden, was der push-Hypothese widerspricht. Darüber hinaus verlief die Vogelbeerenmast in Norwegen und Finnland in der Regel in synchronen Zyklen, was bedeutet, dass Masseneinflüge des Hakengimpels auch dann ausgelöst wurden, wenn die Vogelbeeren in der Nähe des wahrscheinlichen Ursprungs der Masseneinflüge große Fruchtbestände aufwiesen, was der push-Hypothese widerspricht. Die vorliegenden Belege stimmen also am ehesten mit der pull-Hypothese überein: In Wintern mit hohen Vogelbeerenaufkommen ziehen Hakengimpel nach Süden und nutzen diese Ressource, bis diese Mitte des Winters aufgebraucht ist, woraufhin sie wieder in ihre nördlichen Wintergebiete zurückkehren. Hhypotheseds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several species of owls and seed-eating birds breeding in boreal forests may occur irruptively in areas south of their regular wintering range in some years (Newton 2008, 2012). For example, rodent-eating Northern Hawk-owls (Surnia ulula) show cyclic irruptions to areas at the southern margin of their distribution range (Mikkola 1983; Cheveau et al. 2004). The most common explanation for this phenomenon has been that the birds may be forced to move south if food supplies have crashed, in particular if low food availability follows a peak breeding season that resulted in a large population size (Lack 1954; Svärdson 1957; Bock and Lepthien 1976; Koenig and Knops 2001; Newton 2006a). For example, among seed-eating birds, the Northern Bullfinch (Pyrrhula p. pyrrhula) occurs south of the regular wintering range when seed crops of Rowan (Sorbus aucuparia) are low (Fox et al. 2009). In this scenario, emigrating individuals can be viewed as having been pushed away from their regular range due to food shortage.
Little attention has been devoted to the alternative idea that the birds are drawn towards abundant food in southern areas. This pull scenario could occur if food resources show predictable spatial asynchrony, e.g. due to climatic dipoles (Strong et al. 2015; LaMontagne et al. 2020; Zuckerberg et al. 2020), so that emigrating individuals consistently move from areas with low food abundance towards areas with high food abundance. In this case, emigration results from both push and pull factors. On the other hand, if food resources peak synchronously over large areas, but are more plentiful in the south, southward emigration could be triggered by increased food abundance in the regular range. In this case, birds are not pushed away from the regular range because they could overwinter in the north, but are pulled to southern areas that have higher abundance of food. Thus, there are at least two alternative, but not necessarily mutually exclusive, hypotheses for bird irruptions in the context of food resources; one based on the idea that birds are pushed away from their regular range because of food shortage, the other that the birds are pulled towards areas outside their regular range because of abundant food. These ideas are similar to the push–pull paradigm for explaining migration and movements of humans and other mammals (Loe et al. 2009; Hoffmann et al. 2016; Byrne et al. 2019).
Strong et al. (2015) found that irruptions of Pine Siskins (Spinus pinus) conformed to both the push and pull scenarios, with birds leaving areas with low food availability, and moving to areas with high food availability. Movements of nomadic Common Crossbills (Loxia curvirostra) also fits the idea of both push and pull factors causing long-distance movements from areas where they have reproduced and exploited one cone crop to another area where a new cone crop is forming (Newton 2006b). Irruptive migration can be seen as an extreme form of facultative migration which in general involve species where the timing, extent and direction of migration is highly variable and determined in response to availability of food or weather (Newton 2008, 2012). There are also indications that even facultative migration may be influenced by photoperiod and endogenous regulation of both fat deposition and migratory activity (Watts et al. 2017; Cornelius et al. 2021). Although both push and pull factors may be involved in facultative migration (Newton 2008), there are few studies shedding light on the relative importance of push and pull factors for explaining bird irruptions and facultative migration.
I test these two hypotheses (push or pull) using data on winter irruptions of the Pine Grosbeak (Pinicola enucleator) to southern parts of Norway and Sweden, and data on one main type of winter food, seeds in berries of Rowan (Sorbus aucuparia) in Norway and Finland. Previous observations in northern Europe have suggested that Pine Grosbeaks occur more commonly in winter when there is high abundance of Rowanberries, but Pine Grosbeaks become scarce when food has disappeared in mid-winter (Grenquist 1947; Markgren and Lundberg 1959; Cramp and Perrins 1994). However, quantitative analyses have not yet been conducted. From the pull hypothesis, I predicted that peak irruption years of Pine Grosbeaks should coincide with peak Rowanberry years in southern Norway. The pull hypothesis further predicts that birds should disappear from southern areas and move northwards when Rowanberries have been consumed, even if this takes place in mid-winter, because this hypothesis does not assume that food availability in northern areas is low during irruptions. However, the push hypothesis predicts that the birds must stay in southern areas through the winter because irruptions are assumed to occur during years with low food availability in northern areas, and no new food is produced in the northern range until spring. Futhermore, I analyse synchrony in Rowanberry crop size between Norway and Finland and evaluate the likely origin of Pine Grosbeak irruptions to assess whether irruptions may have been pushed by low Rowanberry abundance in the areas from where irruptions originated. The push hypothesis predicts that irruptions should take place when Rowanberry crops in northern areas (such as northern Finland) are small.
Materials and methods
Study area and study species
Pine Grosbeaks may occur irruptively to all parts of western and southern Scandinavia (Online Resource 1, Fig. S1), and data were collected from western and southern parts of Norway and from southern Sweden. In Norway, the most northern county (Troms and Finnmark) is less affected by irruptions, being also the main breeding area for the species (Frantzen 1994; Online Resource 1, Fig. S2). Furthermore, there are few birders in the northernmost parts of Norway, in particular during the dark winter months, so there is little data available from Troms and Finnmark. Thus, data on irruptions were collected for counties south of Troms and Finnmark. The data were also divided into eastern and western regions (Online Resource 1, Fig. S3). The eastern region consisted of the counties Innlandet, Viken, Oslo, Vestfold and Telemark, and Agder (ca. 58–62.5˚N). This region has a small breeding population (in particular in Innlandet county; Frantzen 1994; Shimmings and Øien 2015; Online Resource 1, Fig. S2), but numbers are too small to have any significant impact on Pine Grosbeak numbers in peak irruption years. The western region consisted of the counties Rogaland, Vestland, Møre and Romsdal, Trøndelag, and Nordland (ca. 58–69˚N). Although Nordland is quite far to the north (ca. 65–69˚N), the county has no breeding population of Pine Grosbeaks, but is reached by irruptions with a more westerly direction.
In Sweden, data were collected for landscapes north to and including Dalarne and Hälsingland (ca. 62˚N), approximately the same northern limit as for eastern Norway (Online Resource 1, Fig. S3). Landscapes further north in Sweden have breeding populations of Pine Grosbeaks (Svensson et al. 1999; Online Resource 1, Fig. S2). The breeding distribution continues north to Troms and Finnmark in Norway (ca. 69–70˚N), and the latitudinal breeding distribution range in Finland is ca. 66–70˚N (Keller et al. 2020; Online Resource 1, Fig. S2).
Most parts of Norway have cold winters with snow cover, but coastal areas in the western region and in the far south of the eastern region have January temperatures just above zero with little snow cover (Statens kartverk 1996). The growth season in the breeding areas of the Pine Grosbeak (Troms and Finnmark, and higher elevations of Innlandet) starts in the last half of May (Frantzen 1994; Statens kartverk 1996). In Sweden, areas north of ca. 60˚N also have cold winters with snow cover (boreal zone), whereas areas to the south, especially along the coasts, have less frequent snow cover (nemoral zone).
During irruptions to Norway and Sweden, Pine Grosbeaks are well known for eating primarily Rowanberries (Haftorn 1971; Cramp and Perrins 1994; Bekken 2006). During the 2019 invasion, Pine Grosbeaks were observed to eat Rowanberries in 89% of records where food was reported in one part of southeastern Norway (Dale 2019). Other winter food may include other berries and shoots or buds of various trees and shrubs. Rowans are distributed over most parts of Fennoscandia, including the breeding range of Pine Grosbeaks, but are most common in mixed forests in southern areas (Räty et al. 2016). Rowan is a typical masting tree and produces large crops at 2–3 year intervals (Kobro et al. 2003).
Pine Grosbeak irruptions
To obtain data on the number of Pine Grosbeaks observed in each year, I searched the websites of the National Biodiversity Information Centre in Norway (www.artsobservasjoner.no) and the Swedish Species Observation System (www.artportalen.se) and extracted all Pine Grosbeak records. These websites are online portals for reporting observations of species, and they are open to the public. Most reports of birds are submitted by members of BirdLife Norway and BirdLife Sweden. Both websites are built on almost identical systems for handling species observations. The unit used for analyses was a 'record', i.e. one or more individuals observed in one place at one time (at www.artsobservasjoner.no termed 'funn', at www.artportalen.se termed 'fynd'). The seasonal or yearly number of records was strongly correlated with the number of individuals recorded (see Online Resource 1 for details).
For each year, the number of Pine Grosbeak records included records from September–December in one year and records from January–March in the following year. For example, 2019 included records from the period September 2019—March 2020. Thus, years refer to when irruptions started. Breeding season observations (April–August) were not included. Data were collected for the period September 1959—March 2021. The number of Pine Grosbeak observations in years before this period was limited. To evaluate the temporal pattern of occurrence, peak years were defined as years that had more records than both the year before and the year after. Low years were defined as years where there were more records in both the year before and the year after. Other years were defined as intermediate. The 2020–2021 season was defined as a low year because 2021–2022 had large numbers of Pine Grosbeak again (and also large Rowanberry crop), but total numbers were not yet ready for analysis at the time of writing.
The number of Pine Grosbeak records in Norway and Sweden increased over time (log-transformed number of records; r = 0.73, n = 62 years, p < 0.0001; Online Resource 1, Fig. S4). This was likely due to increased observation activity or reporting (Online Resource 1, Fig. S5). The increase in number of records was far too large to represent population increase [number of records during the last 20 years (n = 30,203) was 20.4 times higher than that of the first 20 years (n = 1480)], and there is no evidence for population increase [no significant change in Swedish breeding population during the last 20 years (Green et al. 2021); some range contraction and possible population decline in northern Europe during the last decades (Keller et al. 2020)]. The increase in number of records is also unlikely to be related to other natural processes such as climate change or changes in Rowan abundance. The larger number of records in later parts of the time series allowed more fine-scaled analyses. The whole period 1959–2020 was used to analyse the combined data from Norway and Sweden, the period 1972–2020 allowed comparisons between Norway and Sweden, and the period 1992–2020 also allowed comparisons between western and eastern Norway (Table 1).
Rowanberry indices
Rowanberry indices for southern Norway were taken from the VIPS project (see https://www.vips-landbruk.no/applefruitmoth/) run by the Norwegian Institute of Bioeconomy Research (NIBIO) and Norsk Landbruksrådgivning (the Norwegian Agricultural Extension Office). VIPS (Varsling innen PlanteSkadegjørere) monitors the risk of pest species attacks on a number of crop species. To forecast risk of attack by Apple Fruit Moths (Argyresthia conjugella) on apple, the moth's alternative host Rowan has been monitored at 59 sites in both eastern and western Norway from 1979 (Online Resource 1, Fig. S2). The number of flower clusters was counted on reference Rowan trees in the vicinity of apple orchards in May each year.
Some monitoring sites had shorter time series or several years with missing data. First, only six sites were monitored in 1979 compared to ≥ 27 sites during 1980–2020. Thus, 1979 was excluded from analyses. Furthermore, 14 sites that had < 30 years of data (mean 18.9) during the period 1980–2020 were excluded. The remaining data used for analyses were from 45 sites [14 from eastern Norway (ca. 58–60˚N), 31 from western Norway (ca. 59–62˚N)] and had data for 32–41 years (mean 38.0). For both eastern and western Norway the yearly Rowanberry index was calculated as the median number of flower clusters across sites. The national index for 1980–2020 was calculated as the mean of the indices from eastern and western Norway so that the two regions were weighted equally. Peak Rowanberry years were defined as years that had higher number of flower clusters than both the year before and the year after.
To match the period for which data on Pine Grosbeak could be separated into eastern and western Norway (1992–2020, Table 1), separate Rowanberry indices for eastern and western Norway, as well as a national index (eastern and western Norway combined), were calculated specific for this period. This data set included sites that lacked at most two years of data during the period 1992–2020, and consisted of 44 sites (13 from eastern Norway, 31 from western Norway). For western Norway the sites included were identical to those used for the 1980–2020 index, but for eastern Norway 11 sites were identical whereas three sites used for the 1980–2020 index were not included and two additional sites were included in the 1992–2020 index.
In addition to Rowanberry indices from Norway, I used data from Finland (Gallego Zamorano et al. 2018, their Fig. 2). They presented Rowanberry indices from 248 sites, mostly in southern Finland, but also some northern sites overlapping with the breeding distribution of Pine Grosbeaks (overall, Rowanberry sites were located at ca. 60–69˚N; Online Resource 1, Fig. S2). Finnish Rowanberry sites were ca. 500–1,500 km from Rowan sites in southern Norway. During 1986–2014, the number of Rowanberries was estimated in early autumn, and classified on a categorical abundance scale. Peak Rowanberry years were defined as years that had a higher index than both the year before and the year after.
Timing of irruptions and northward migration
The seasonal pattern of irruptions was analysed by summarizing the number of Pine Grosbeak records for each month during the year during the period September 1992–August 2021 (Table 1). Monthly numbers were recorded for Norway and Sweden separately. A more detailed analysis of timing of irruptions was made with data from Norway, using 14 irruption seasons with at least 50 records during September—March (the 14 years included had 5,986 records, whereas the 15 years that were excluded had 299 records). Date of first record, and date of 50th, 75th and 95th percentile of all records were calculated for each year.
To assess when northward movements of Pine Grosbeaks started, I compared the proportion of the Pine Grosbeak records for each month during October–March (there were too few observations in September) that were in northern versus southern counties (Norway) or landscapes (Sweden). I divided both the eastern and the western regions of Norway into southern and northern parts (Online Resource 1, Fig. S3). In the eastern region I compared Innlandet county (north, ca. 60–62.5˚N) versus the other counties (south: Viken, Oslo, Vestfold and Telemark, and Agder, ca. 58–61˚N). In the western region I compared Nordland and Trøndelag counties (north, ca. 62.5–69˚N) versus Møre and Romsdal, Vestland, and Rogaland counties (south, ca. 58–63˚N). Swedish landscapes were also divided into northern and southern parts (Online Resource 1, Fig. S3). The southern landscapes (all landscapes north to and including Dalarne and Hälsingland, ca. 55.5–62˚N), above used to calculate number of records during the winter, were compared to all landscapes further north (ca. 62–69˚N) which also include breeding areas. The pull hypothesis predicted that a larger proportion of the Pine Grosbeak records should be in the south during late autumn and early winter, but an increasing proportion should be from northern regions in late winter due to northwards movements when food supplies in the south have been consumed. Northward migration was analysed for the period 1992–2020 (Table 1).
Statistical analyses
Pearson correlations were used to analyse the relationships between annual number of Pine Grosbeak records (log10-transformed) between different regions, Rowanberry indices (log10-transformed) between different regions, and relationships between number of Pine Grosbeak records and Rowanberry indices (Table 1). As stated earlier, the number of records of Pine Grosbeaks per year in Norway and Sweden increased over time. This was most likely due to increased observation effort or an increased reporting frequency over time (see above). To control for this temporal trend in observation and reporting effort, analyses were conducted using detrended Pine Grosbeak data (i.e. using residuals from a regression of number of records (log10-transformed) on year). Detrending was done separately for the time periods 1972–2020, 1980–2020 (used also for 1986–2014) and 1992–2020 (Table 1). Due to the steady increase in number of Pine Grosbeak records over time (Online Resource, Fig. S4), the slopes of the original relationships between time and number of records (log-transformed) were quite similar for each time period (0.03, 0.03 and 0.02, respectively). However, separate detrending was done because data quality increased over time so that detrending based on more recent time periods would allow more accurate analyses.
The Rowanberry index in Norway showed a negative temporal trend during 1980–2020 (log10-transformed index; r = -0.38, n = 41 years, p = 0.014, Online Resource 1, Fig. S6) which could be due to either natural factors or temporal changes in study sites, including habitat changes and changes in methodological procedures. Analyses were therefore based on a detrended Rowanberry index (i.e. using residuals from a regression of number of flower clusters (log10-transformed) on year). Detrending was done separately for the time periods 1980–2020 (also used for 1986–2014) and 1992–2020 (Table 1). The Rowanberry index from Finland was not detrended because there was no temporal trend (no log-transformation of data; r = 0.05, n = 29 years, p = 0.81).
Alternative models without detrending of Pine Grosbeak numbers or Norwegian Rowanberry indices were also conducted. First, Pine Grosbeak numbers were expressed as the proportion of all bird observations of any bird species submitted to the online websites for reporting bird observations that were observations of Pine Grosbeaks (Online Resource 1). This also represents another way of controlling for observer effort. Second, Pine Grosbeak irruption size was measured as the ratio between numbers in year x relative to numbers in year x + 1 (Online Resource 1). The alternative models showed quantitatively similar results as the analyses using detrended data (Online Resource 1, Table S1). Thus, the main text reports the results of analyses based on detrending. All statistical analyses were conducted in JMP Pro version 15.
Results
Pine Grosbeak irruptions
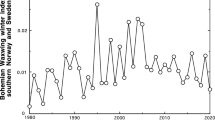
During the period 1959–2020, the total number of Pine Grosbeak records in southern Norway and Sweden had peaks in 24 years, lows in 24 years, and were at intermediate levels in 13 years (Fig. 1). Peaks generally occurred at 2–3 year intervals (two-year intervals: n = 13, three-year intervals: n = 8, four-year interval: n = 1, five-year interval: n = 1). In peak years there was an average number of 1,529.5 Pine Grosbeak records, whereas intermediate years had 121.9 records and low years 58.4 records on average (numbers not corrected for the temporally increasing trend in number of records). The ratio between number of records in a peak year and the following low year was on average 25.4 (median 11.0, range 1.5–238.5, n = 24). The ratio was > 30 in six cases. The largest ratio was between the 2019–2020 season (18,366 records) and the following year (2020–2021 season, 77 records).
Detrended annual variation in number of Pine Grosbeak (Pinicola enucleator) records from southern Norway and Sweden during the period 1959–2020. For each year, the number includes records from September–December and from January–March the following year. Thus, e.g. 2019 includes records from the period September 2019—March 2020. Detrended values are residuals of log-transformed number of Pine Grosbeak records regressed on year
During the period 1972–2020, the number of Pine Grosbeak records peaked in 20 years in southern Norway and in 19 years in southern Sweden. Eighteen peaks in each area were in identical years, whereas one peak in Sweden occurred one year earlier than in Norway, and one peak in Norway was absent in the Swedish data. Annual numbers of Pine Grosbeak records in Norway and Sweden were strongly and positively correlated (log-transformed and detrended data: r = 0.76, n = 49, p < 0.0001; Fig. 2a).
Correlations between different regions in Scandinavia in detrended annual number of Pine Grosbeak (Pinicola enucleator) records: a numbers in southern Norway in relation to numbers in southern Sweden during 1972–2020, and b numbers in western Norway in relation to numbers in eastern Norway during 1992–2020. Detrended values are residuals of log-transformed number of Pine Grosbeak records regressed on year
During the period 1992–2020, the number of Pine Grosbeak records peaked in 12 years in eastern Norway and in 13 years in western Norway. All peak years in eastern Norway were also peak years in western Norway, whereas one peak in western Norway was absent in eastern Norway. Annual numbers of Pine Grosbeak records in eastern and western Norway were strongly and positively correlated (log-transformed and detrended data: r = 0.80, n = 29, p < 0.0001; Fig. 2b). Annual numbers of records were also correlated with numbers in southern Sweden (log-transformed and detrended data: eastern Norway versus Sweden: r = 0.81, n = 29, p < 0.0001; western Norway versus Sweden: r = 0.77, n = 29, p < 0.0001).
Rowanberry fluctuations
During the period 1980–2020, the Rowanberry index in Norway (eastern and western Norway combined) showed peaks in 16 years, lows in 16 years, and were at intermediate levels in 8 years (Fig. 3). Peaks generally occurred at 2–3 year intervals (two-year intervals: n = 9, three-year intervals: n = 4, four-year interval: n = 2). In peak years the index was on average 267.2, whereas intermediate years had an index of 103.9 and low years 38.7 on average (numbers not corrected for the temporally decreasing trend of the Rowanberry index). The ratio between the index in a peak year and in the following low year was on average 21.6 (median 6.7, range 1.6–154.0, n = 16). The ratio was > 20 in six cases. The largest ratio for the Rowanberry index coincided with the largest ratio for Pine Grosbeak records.
Detrended annual variation in Rowanberry (Sorbus aucuparia) index from southern Norway during the period 1980–2020. Detrended values are residuals of log-transformed number of Rowanberry flower clusters regressed on year. Peak years of the Rowanberry index that coincided with peak years of Pine Grosbeak (Pinicola enucleator) records are marked with asterisks
During the period 1980–2020, the Rowanberry index peaked in 15 years in eastern Norway and in 17 years in western Norway. Thirteen peaks were in identical years. Eastern and western Norway were out of phase during six years (the periods 1985–1987 and 1991–1993). Annual Rowanberry indices in eastern and western Norway were strongly and positively correlated (log-transformed and detrended data; r = 0.87, n = 41, p < 0.0001).
During the period 1986–2014, the Rowanberry index peaked in 10 years in Finland and in 11 years in Norway. Seven peaks were in identical years, all of these were in the period 1989–2006. Indices were out of phase with each other during 2008–2013 (three years had peaks in Finland and lows in Norway, three years had lows in Finland and peaks in Norway). Norway had one peak in 1987 which was an intermediate year in Finland. Despite the six years with opposite patterns, the annual Rowanberry indices in Finland and Norway were positively correlated (log-transformed and detrended data for Norway only; r = 0.66, n = 29, p < 0.0001; Fig. 4).
Pine Grosbeak irruptions in relation to Rowanberry indices
During the period 1980–2020, both the total number of Pine Grosbeak records in Norway and Sweden and the Rowanberry index in Norway peaked in 16 years. Eleven of these peaks were in identical years (Fig. 3). Four Pine Grosbeak peaks occurred when the Rowanberry index was at a low level and one at an intermediate level of Rowanberries. The observed number of Pine Grosbeak peaks matching a Rowanberry peak (n = 11) and the number of Pine Grosbeak peaks not matching a Rowanberry peak (intermediate plus low Rowanberry years; n = 5) differed significantly from a random expected distribution based on the number of peak (n = 16; see above) and intermediate plus low (n = 24) Rowanberry years (χ2 = 5.51, df = 1, p = 0.019). Pine Grosbeaks were generally in phase with Rowanberry peaks during the period 1987–2006, out of phase with Rowanberry peaks in particular during 2008–2013, and in phase again during 2014–2019 (Fig. 3).
During the period 1980–2020, the total number of Pine Grosbeak records in southern Norway and Sweden was positively correlated with the Rowanberry index in Norway (log-transformed and detrended data; r = 0.46, n = 41, p = 0.0024; Fig. 5a). The relationship between the number of Pine Grosbeak records and the Rowanberry index was also significant for Norwegian Pine Grosbeak data (log-transformed and detrended data; r = 0.43, n = 41, p = 0.0046) and Swedish Pine Grosbeak data separately (log-transformed and detrended data; r = 0.46, n = 41, p = 0.0024). Analyses using only peak Pine Grosbeak years (n = 16) in Norway and Sweden indicated that the size of irruptions was positively and marginally non-significantly related to the size of the Rowanberry crop in Norway (log-transformed and detrended data; r = 0.43, p = 0.098).
Detrended annual number of Pine Grosbeak (Pinicola enucleator) records from southern Norway and Sweden in relation to Rowanberry (Sorbus aucuparia) index a from southern Norway during 1980–2020 (detrended), and b from Finland during 1986–2014. Detrended values are residuals of log-transformed numbers of Pine Grosbeak records and Rowanberry flower clusters regressed on year
During the period 1986–2014, the total number of Pine Grosbeak records in Norway and Sweden peaked in 12 years and the Rowanberry index in Finland peaked in 10 years. All Rowanberry peaks in Finland were also peak years for Pine Grosbeaks in Norway and Sweden. The two other Pine Grosbeak peaks had intermediate Rowanberry index in Finland. The observed number of Pine Grosbeak peaks matching Rowanberry peaks or not differed significantly from a random expected distribution (χ2 = 11.05, df = 1, p = 0.0009). Notably, during the period 2008–2013, when Pine Grosbeaks and Rowan in Norway were out of phase with each other (see Fig. 3), Pine Grosbeaks had all three peaks identical with Rowanberry peaks in Finland. The number of Pine Grosbeak records in Norway and Sweden during the period 1986–2014 was positively correlated with the Rowanberry index in Finland (log-transformed and detrended data for Pine Grosbeak only; r = 0.60, n = 29, p = 0.0007; Fig. 5b).
During the period 1992–2020, the number of Pine Grosbeak records in southern Norway was positively correlated with the Rowanberry index in Norway (log-transformed and detrended data; r = 0.56, n = 29, p = 0.0017). The relationships between the number of Pine Grosbeak records and Rowanberry indices were also significant for eastern Norway (log-transformed and detrended data; r = 0.56, n = 29, p = 0.0015) and western Norway separately (log-transformed and detrended data; r = 0.40, n = 29, p = 0.031). Annual number of Pine Grosbeak records in Sweden was also correlated with both the Rowanberry index from eastern Norway (log-transformed and detrended data; r = 0.53, n = 29, p = 0.0030) and western Norway (log-transformed and detrended data; r = 0.53, n = 29, p = 0.0029).
Seasonal timing of irruptions
Irruptions to southern Norway usually started in September–October (yearly first observations: median: 27 September, range: 3 September—25 October, n = 14 years), peaked in November, and numbers declined rapidly during January–February (Fig. 6a). The median date of records was 6 December (range 11 November—10 January, n = 14 years). The 75th percentile of records was reached at 11 January (range: 28 November—8 February) and the 95th percentile at 23 February (range: 28 January—17 March). In Sweden, irruptions also peaked in November, but remained fairly high through December and into January, but thereafter declined rapidly (Fig. 6b).
Northward migration
The decline in the number of Pine Grosbeak records during January–March (Fig. 6) was related to a northward shift in Pine Grosbeak records (Fig. 7). In eastern Norway, the monthly distribution of the records in the most northern county (Innlandet; n = 790 records) was significantly different from the distribution of records in southern counties (n = 2,880 records; χ2 = 294.59, df = 5, p < 0.0001; Fig. 7). Similarly, in western Norway, the monthly distribution of the records also differed between northern counties (Trøndelag, Nordland; n = 2,132 records) and southern counties (n = 503 records; χ2 = 115.16, df = 5, p < 0.0001; Fig. 7). The same pattern was also present in data from Sweden (northern landscapes: n = 12,437 records; southern landscapes: n = 31,165 records; χ2 = 3129.65, df = 5, p < 0.0001; Fig. 7).
Proportion of monthly number of Pine Grosbeak (Pinicola enucleator) records during 1992–2020 that were from the northern part of each region of Norway (eastern region: Innlandet county; western region: Nordland and Trøndelag counties), and Sweden (all landscapes north of Dalarne and Hälsingland). The total included southern counties (eastern Norway: Viken, Oslo, Vestfold and Telemark, and Agder counties; western Norway: Møre and Romsdal, Vestland, and Rogaland counties) and landscapes (Sweden: all landscapes north to and including Dalarne and Hälsingland). September is excluded due to few Pine Grosbeak records
Discussion
Pine Grosbeaks and Rowanberries
Pine Grosbeak winter numbers in southern Norway and Sweden showed large fluctuations with peaks at 2–3 year intervals, although peak years varied substantially in absolute numbers. The fluctuations were synchronous across southern Scandinavia. Rowanberries also peaked at 2–3 year intervals, and the fluctuations were synchronous between eastern and western Norway. In most cases Pine Grosbeak peaks coincided with Rowanberry peaks (11 out of 16 peaks during 1980–2020, 69%). However, Pine Grosbeak peaks were out of phase with Rowanberry peaks in Norway during 2008–2013. This was related to a temporary disruption of large-scale synchrony in Rowan masting because the Rowanberry indices for Norway and Finland, although being synchronous during 1989–2006, were asynchronous during 2008–2013 (see also further in the paragraph 'Spatial extent of synchrony of Rowan masting'). Because of this, Pine Grosbeak peaks in southern Norway and Sweden actually matched Finnish Rowanberry peaks better (10 out of 12 peaks during 1986–2014, 83%). The finding that the Rowanberry indices in Norway and Finland were strongly correlated despite one period of asynchrony is notable because Norwegian Rowanberry indices were based on flower clusters in May which may not always be directly related to berries (e.g. if frosts damage some flowers), whereas the Finnish indices were based on actual counts of berries in early autumn. This suggests that flower clusters predict Rowanberry abundance well, and that Rowan masting is normally highly synchronous across Fennoscandia as also noted earlier (Kobro et al. 2003).
Despite the six-year period when Pine Grosbeak peaks did not coincide with Rowanberry peaks in Norway, there was a positive correlation (r = 0.43) across all years between the number of Pine Grosbeak records and the Rowanberry index in Norway. There was an even stronger correlation (r = 0.60) between the number of Pine Grosbeak records and the Rowanberry index in Finland. Furthermore, there was also a marginally non-significant trend that irruption size within only peak years was correlated with the Rowanberry index. The correlations between the number of Pine Grosbeak records and the Rowanberry index in Norway were also significant for three separate regions (western and eastern Norway, and Sweden). Thus, these results support the pull hypothesis: in general, Pine Grosbeaks migrate to southern Scandinavia during autumn in years when the Rowanberry crop is large.
Timing of irruptions
A main prediction from the push hypothesis in the context of food resources was that irruptions should be triggered by low food availability in the regular (northern) wintering areas. Findings relevant to this prediction are discussed below (see further in the paragraph 'Spatial extent of synchrony of Rowan masting'). However, the push and pull hypotheses also differed in their predictions regarding the timing of irruptions, specifically that the hypotheses differed regarding the amount of food available to the Pine Grosbeaks in the regular wintering areas. The push hypothesis assumes that irruptions are triggered by low food availability in the northern areas so that they must remain in southern areas until spring when new food becomes available, whereas according to the pull hypothesis irruptions may occur even when there is enough food to winter in the north, and, hence, birds may return north as soon as the food in southern areas is depleted. I found that irruptions peaked in November and that numbers decreased as early as January–February. There was also evidence that northward movements started already in January–February. However, note that there was considerable variation in when Pine Grosbeak numbers started to decrease; e.g. the 75th percentile of records varied from 28 November—8 February, suggesting that northward migration was temporally flexible, likely related to when food was depleted.
The timing of irruptions was based on analyses of how far south the bulk of the Pine Grosbeaks were observed (Fig. 7). The southern parts of the study area included the southernmost parts of Norway and Sweden. Pine Grosbeaks occur only exceptionally south of the study area with very few records in Great Britain, Denmark and Germany (Cramp and Perrins 1994). Thus, lower numbers in southern Norway and Sweden from January–February clearly indicate northward migration, and not that they have moved further south.
The finding that northward migration started already in January—February is in line with the prediction from the pull hypothesis that Pine Grosbeaks stay in southern Fennoscandia until Rowanberries are depleted. Rowanberries are eaten by several other bird species (notably Bohemian Waxwing Bombycilla garrulus, thrushes Turdus spp., Common Starling Sturnus vulgaris, and Eurasian Bullfinch), and in Finland crops may, at least locally, be depleted as early as November–December (Suhonen and Jokimäki 2015; Suhonen et al. 2017). Rowanberries in southern Norway have also generally been consumed by birds by late autumn or early winter (S. Dale, personal observations; Dale 2019).
The push hypothesis predicted that Pine Grosbeaks should stay in southern areas until spring when new food may become available again in northern areas. Spring migration could then potentially start in March–April when snowmelt has begun, although the growth season in the breeding areas of the Pine Grosbeak starts in the last half of May (Frantzen 1994; Statens kartverk 1996). Thus, the timing of irruptions and northward migration of Pine Grosbeaks did not fit the push hypothesis.
An important question given the finding that Pine Grosbeaks moved north already during mid-winter, is what kind of food they utilize when they have returned to northern areas. In addition, the 2–3 year intervals in irruptions indicate that most Pine Grosbeaks stay the whole winter in northern areas more than every second year. Pulliainen (1974) found that the winter food of Pine Grosbeaks during February–April in northeastern Lapland in Finland at almost 68˚N was to a large degree buds from Norway Spruce (Picea abies). Pulliainen (1974) did not specify whether the buds were for vegetative shoots or for reproductive shoots (male and female flowers), but chemical analyses indicated that the nutrient content of the buds was higher than that of spruce seeds in that year. Newton (1972) stated that buds from several different trees forms a large part of the winter diet in years when fruits and berries are scarce. Thus, use of buds from spruce and other trees indicates that Pine Grosbeaks may have a large food supply available in every winter, although Rowanberries may be preferred if available.
In conclusion, the results indicated that Pine Grosbeaks moved south to take advantage of large Rowanberry crops, but returned north already during mid-winter. Returning north during mid-winter implies that northern areas have had food available through the whole winter, likely in particular in the form of spruce buds. This is in contrast to the push hypothesis which assumes that irruptions occur when food is scarce. However, a full evaluation of the push hypothesis also needs to take into account the origin of irruptive Pine Grosbeaks, and the extent of spatial synchrony of Rowan masting (see following paragraphs).
Origin of Pine Grosbeak irruptions
Cramp and Perrins (1994) argued that the number of Pine Grosbeaks involved in irruptions to southern Fennoscandia was too large to be accounted for by the breeding population in northern Fennoscandia. They therefore assumed that irruptions come from Russia although no long-distance ringing recoveries were known. The assumption that irruptions come from the east, i.e. Russia, has been repeated many times later (e.g. Ullman 1999).
However, Sundberg (2001) argued that several irruptions have affected Sweden without affecting Finland (which is between Sweden and Russia), and therefore point to a possible origin in northern Fennoscandia. Nilsson (2019) analysed Pine Grosbeak occurrence and movements during the large irruption in 2019, and noted that the irruption was large in Norway and Sweden, but not in Finland, and suggested that the Pine Grosbeaks came from northern Fennoscandia. This interpretation was supported by observations in southern Norway during 2019 of four Pine Grosbeaks ringed in northern Sweden and Finland during previous breeding seasons (Winnem 2021). There have been similar disagreements regarding the origin of other boreal bird irruptions. Northern Hawk-owl irruptions have been claimed to come from Russia, but analyses have indicated that a northern Fennoscandian origin is more likely, at least during the irruptions investigated in detail (Hagen 1956; Dale 2017).
The breeding population size of Pine Grosbeaks in Fennoscandia may amount to ca. 20,000–30,000 pairs [Norway: 500–1,000 pairs (Shimmings and Øien 2015); Sweden: 10,000 pairs (Ottosson et al. 2012); Finland: 10,000–20,000 pairs (Valkama et al. 2011)]. Assuming two surviving young per breeding pair, the total autumn population size of the Fennoscandian population may be ca. 100,000 individuals. There are few estimates of the number of individuals involved in irruptions, but Svensson (2001) suggested a crude estimate of at least 100,000 individuals during the large irruption in 1976–1977 (the largest among irruptions analysed in the present study, see Fig. 1). Thus, total population size in Fennoscandia may be large enough to account for many irruptions. In conclusion, the origin of many irruptions may therefore have been northern Fennoscandia, and not necessarily Russia.
Spatial extent of synchrony of Rowan masting
The main prediction from the push hypothesis is that irruptions occur when there is crop failure in the regular (northern) wintering areas. Rowanberries are a main type of food in winter for Pine Grosbeaks (Cramp and Perrins 1994), and also the main type of food that shows large fluctuations from year to year. Thus, Pine Grosbeak irruptions would be expected to occur when Rowan trees have small crops in northern areas, and, as argued above, specifically in northern Fennoscandia. Given that irruptions generally occurred when Rowanberries were abundant in southern Fennoscandia, the push hypothesis implies an asynchrony between Rowanberry numbers in northern and southern Fennoscandia.
However, available evidence suggest that Rowan masting is synchronous across large parts of Fennoscandia. Kobro et al. (2003) found that masting was spatially synchronous in Norway (between western and eastern Norway using an early part of the same data set as used in the present study), and based on identical years of peak crops and crop failures in Norway and Finland they argued that masting of Rowan is synchronous across Fennoscandia. Following the results of Kobro et al. (2003), Fox et al. (2009) based their analyses of bullfinch irruptions on a high level of masting synchrony of Rowan throughout the Nordic countries. The results of the present study confirmed that masting was synchronous between Norway and Finland in most years, although there was a period of asynchrony during 2008–2013.
Koenig and Knops (2000) found that in general both masting in multiple tree species in the Northern Hemisphere and climatic variables were spatially synchronous over distances up to ca. 1,000 km (see also Koenig and Knops 1998). Gallego Zamorano et al. (2018) found that annual crop size in Rowan (and other tree species as well) in Finland was also spatially synchronous at scales up to 1,000 km. Furthermore, they found that the main driver of synchrony was spring and summer temperatures, and temperature was spatially autocorrelated across most parts of Fennoscandia, the Baltic countries and western Russia (Gallego Zamorano et al. 2018; see their Supplementary Fig. A1). Thus, available evidence suggests that Pine Grosbeak irruptions occur when Rowanberries have masting years in both the southern wintering areas of Pine Grosbeaks and the most likely northern breeding areas of Pine Grosbeaks, contrary to the push hypothesis. Specifically, irruptions occurred in years when Rowanberry crops also peaked in large parts of Finland, close to the main breeding areas of Pine Grosbeaks in Fennoscandia. The observation that irruptions continued south to southern Norway and Sweden even during the 6-year period of asynchrony, when Pine Grosbeaks therefore arrived in years with low Rowanberry abundance in southern Norway, strongly suggests that irruptions were triggered by Rowanberry masting in northern areas. This is opposite of what is expected from the push hypothesis for Pine Grosbeak irruptions.
The main remaining possibility for the push hypothesis to be relevant for explaining Pine Grosbeak irruptions in southern Scandinavia is that irruptions originate from areas further east in Russia where Rowan seed production may be asynchronous with seed production in Fennoscandia. However, as reported above, there is no direct evidence that irruptions come from that far away. In conclusion, there is little evidence that Pine Grosbeak irruptions can be explained by the push hypothesis, whereas there was strong evidence that irruptions are linked to Rowanberry peaks, thereby supporting the pull hypothesis.
General theories for bird irruptions are also based on the idea that high population density after good breeding seasons may contribute to triggering irruptions. However, in the case of the Pine Grosbeak, population size alone cannot explain irruptions because yearly winter numbers fluctuated with a factor of 11–25 (median and mean, respectively, of ratio between peak and low years). For a single-brooded species with a clutch size of usually four eggs (Cramp and Perrins 1994), yearly variation in breeding success cannot cause such large fluctuations in winter numbers. In conclusion, large numbers of Pine Grosbeaks in southern Scandinavia in some years appears unlikely to be related to population fluctuations of the breeding population in northern Fennoscandia, unlikely to be due to irruptions from populations further east in Russia, and occurred in years with high food availability, contrary to the push hypothesis.
Pine Grosbeak responses to Rowan masting
The most likely scenario that irruptions occur when the Rowan is masting across Fennoscandia raises the question why Pine Grosbeaks move far south in years when there is an abundance of Rowanberries everywhere, including in the regular wintering areas in the north. However, there is strong competition from other bird species for Rowanberries (see above), and Rowanberries may become depleted already during autumn. Suhonen and Jokimäki (2015) found that more than half of Rowanberries in three sites in Finland at latitudes of 60–66˚N had been taken by Fieldfares (Turdus pilaris) and Bohemian Waxwings already by mid-October, and most berries were gone by early November. Because Rowans are more common in mixed forests in southern Fennoscandia than in boreal coniferous forest in northern Fennoscandia (Räty et al. 2016), and because Rowan and related species are often planted close to people, the total abundance of Rowanberries is likely larger in southern than in northern Fennoscandia. Thus, a tactic of moving slowly southwards and deplete Rowanberries on the way may lead to Pine Grosbeaks constantly lagging behind in the competition for Rowanberries, and when finally arriving to southern areas late in the season, there may be only small amounts of berries left.
Because of competition for Rowanberries, I suggest that the best tactic for Pine Grosbeaks in order to gain access to a plentiful resource, is to move south quickly if they see that Rowans are masting in northern areas. This will enable them to reach areas with large supplies before other species and other Pine Grosbeaks have consumed all Rowanberries. The idea is that moving south slowly will result in poorer access to resources than moving south quickly and early. Rapid migration has been noted in several irruptions, with birds appearing in most of southern Scandinavia within 1–2 weeks after the first records (Sundberg 2001; Falkenberg 2020). The cost of migrating long distances in a short time may be compensated for by better access to food. There may be additional benefits of moving south such as lower thermoregulatory costs and longer daylength to meet energy demands which could improve survival, although predation risk could increase (McKinnon et al. 2010).
After migrating south, the Pine Grosbeaks should thereafter stay in southern areas until foraging profitability drops to a level similar to or lower than they may expect if returning north. The tactic of responding to Rowan masting in breeding areas with irruptions would work in most years due to the large-scale synchrony of Rowan masting. In the short period of asynchrony during 2008–2013 irruptive Pine Grosbeaks did not encounter peak Rowanberry numbers in southern Scandinavia, but note that low years in this period had more Rowanberries than in many other low years (see Fig. 3), so the Pine Grosbeaks may still have been able to find some Rowanberries. In conclusion, this study provided evidence that Pine Grosbeak irruptions are pulled by high Rowanberry abundance in southern areas, and not triggered by low food abundance in northern areas as proposed by the push hypothesis.
Data availability
Data are available from the author on request.
References
Bekken J (2006) Konglebit Pinicola enucleator. In: Svorkmo-Lundberg T, Bakken V, Helberg M, Mork K, Røer JE, Sæbø S (eds) Norsk VinterfuglAtlas. Fuglenes utbredelse, bestandsstørrelse og økologi vinterstid. Norsk Ornitologisk Forening, Trondheim, pp 414–415. (In Norwegian)
Bock CE, Lepthien LW (1976) Synchronous eruptions of boreal seed-eating birds. Am Nat 110:559–571. https://doi.org/10.1086/283091
Byrne AW, O’Keeffe J, Buesching CD, Newman C (2019) Push and pull factors driving movement in a social mammal: context dependent behavioral plasticity at the landscape scale. Curr Zool 65:517–525. https://doi.org/10.1093/cz/zoy081
Cheveau M, Drapeau P, Imbeau L, Bergeron Y (2004) Owl winter irruptions as an indicator of small mammal population cycles in the boreal forest of eastern North America. Oikos 107:190–198. https://doi.org/10.1111/j.0030-1299.2004.13285.x
Cornelius JM, Hahn TP, Robart AR, Vernasco BJ, Zahor DL, Glynn KJ, Navis CJ, Watts HE (2021) Seasonal patterns of fat deposits in relation to migratory strategy in facultative migrants. Front Ecol Evol 9:691808. https://doi.org/10.3389/fevo.2021.691808
Cramp S, Perrins CM (1994) The birds of the western palearctic, vol VIII. Crows to finches. Oxford University Press, Oxford
Dale S (2017) Density, numbers and probable origin of Northern hawk owls Surnia ulula in southern Norway during the 2016 irruption. Ornis Norv 40:1–13. https://doi.org/10.15845/on.v40i0.1079
Dale S (2019) Invasjonen av konglebit høsten 2019 i Oslo og Akershus: hvorfor, hvor mange og fra hvor? Toppdykker’n 42:212–222 (In Norwegian)
Falkenberg F (2020) Enorm invasjon av konglebit til Norge: Kjempefinker fra den nordlige taigaen på bærjakt i sør. Vår Fuglefauna 43:24–28 (In Norwegian)
Fox AD, Kobro S, Lehikoinen A, Lyngs P, Väisänen RA (2009) Northern Bullfinch Pyrrhula p. pyrrhula irruptive behaviour linked to rowanberry Sorbus aucuparia abundance. Ornis Fenn 86:51–60
Frantzen B (1994) Konglebit Pinicola enucleator. In: Gjershaug JO, Thingstad PG, Eldøy S, Byrkjeland S (eds) Norsk fugleatlas. Norsk Ornitologisk Forening, Klæbu, pp 486–487. (In Norwegian)
Gallego Zamorano J, Hokkanen T, Lehikoinen A (2018) Climate-driven synchrony in seed production of masting deciduous and conifer tree species. J Plant Ecol 11:180–188. https://doi.org/10.1093/jpe/rtw117
Green M. Haas F, Lindström Å, Nilsson L (2021) Monitoring population changes of birds in Sweden. Annual report for 2020. Department of Biology, Lund University
Grenquist P (1947) Über die Biologie des Hakengimpels. Ornis Fenn 24:1–10 (In Finnish with German summary)
Haftorn S (1971) Norges Fugler. Universitetsforlaget, Oslo (In Norwegian)
Hoffmann EM, Konerding V, Nautiyal S, Buerkert A (2016) Is the push-pull paradigm useful to explain rural-urban migration? A case study in Uttarakhand. India Plos ONE 14:e0214511. https://doi.org/10.1371/journal.pone.0214511
Hagen Y (1956) The irruption of Hawk-owls (Surnia ulula (L.)) in Fennoscandia 1950–51. Sterna 1 (24):3–22
Keller V, Herrando S, Voříšek P, Franch M, Kipson M, Milanesi P, Martí D, Anton M, Klvaňová A, Kalyakin MV, Bauer H-G, Foppen RPB (2020) European Breeding Bird Atlas 2: Distribution. Abundance and Change. European Bird Census Council & Lynx Edicions, Barcelona
Kobro S, Søreide L, Djønne E, Rafoss T, Jaastad G, Witzgall P (2003) Masting of rowan Sorbus aucuparia L. and consequences for the apple fruit moth Argyresthia conjugella Zeller. Pop Ecol 45:25–30. https://doi.org/10.1007/s10144-003-0136-x
Koenig WD, Knops JMH (1998) Scale of mast-seeding and tree-ring growth. Nature 396:225–226. https://doi.org/10.1038/24293
Koenig WD, Knops JMH (2000) Patterns of annual seed production by Northern Hemisphere trees: a global perspective. Am Nat 155:59–69. https://doi.org/10.1086/303302
Koenig WD, Knops JMH (2001) Seed-crop size and eruptions of North American boreal seed-eating birds. J Anim Ecol 70:609–620. https://doi.org/10.1046/j.1365-2656.2001.00516.x
Lack D (1954) The Natural Regulation of Animal Numbers. Clarendon Press, Oxford
LaMontagne JM, Pearse IS, Greene DF, Koenig WD (2020) Mast seeding patterns are asynchronous at a continental scale. Nature Plants 6:460–465. https://doi.org/10.1038/s41477-020-0647-x
Loe LE, Mysterud A, Veiberg V, Langvatn R (2009) Negative density-dependent emigration of males in an increasing red deer population. Proc R Soc B 276:2581–2587. https://doi.org/10.1098/rspb.2009.0224
Markgren G, Lundberg S (1959) Om tallbit (Pinicola enucleator) och bändelkorsnebb (Loxia leucoptera) i Sverige under invasionsperioden 1956–57. Vår Fågelvärld 18:185–205 (In Swedish with English summary)
McKinnon L, Smith PA, Nol E, Martin JL, Doyle FI, Abraham KF, Gilchrist HG, Morrison RIG, Bêty J (2010) Lower predation risk for migratory birds at high latitudes. Science 327:326–327. https://doi.org/10.1126/science.1183010
Mikkola H (1983) Owls of Europe. T & AD Poyser, Calton, UK
Newton I (1972) Finches. Collins, London
Newton I (2006a) Advances in the study of irruptive migration. Ardea 94:433–460
Newton I (2006b) Movement patterns of common crossbills Loxia curvirostra in Europe. Ibis 148:782–788. https://doi.org/10.1111/j.1474-919X.2006.00585.x
Newton I (2008) The Migration Ecology of Birds. Academic Press, London
Newton I (2012) Obligate and facultative migration in birds: ecological aspects. J Orn 153:S171–S180. https://doi.org/10.1007/s10336-011-0765-3
Nilsson J (2019) Var kommer tallbitarna från? https://www.artdatabanken.se/arter-och-natur/Dagens-natur/var-kommer-tallbitarna-fran/. (In Swedish)
Ottosson U, Ottvall R, Elmberg J, Green M, Gustafsson R, Haas F, Holmqvist N, Lindström Å, Nilsson L, Svensson M, Svensson S, Tjernberg M (2012) Fåglarna i Sverige – antal och förekomst. Swedish Ornithological Society, Halmstad, Sweden (In Swedish)
Pulliainen E (1974) Winter nutrition of the common crossbill (Loxia curvirostra) and the pine grosbeak (Pinicola enucleator) in northeastern Lapland in 1973. Ann Zool Fenn 11:204–206
Räty M, Caudullo G, de Rigo D (2016) Sorbus aucuparia in Europe: distribution, habitat, usage and threats. In: San-Miguel-Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A (eds) European Atlas of Forest Tree Species. Publications Office EU, Luxembourg. https://forest.jrc.ec.europa.eu/media/atlas/Sorbus_aucuparia.pdf
Shimmings P, Øien IJ (2015) Bestandsestimater for norske hekkefugler. Norsk Ornitologisk Forening, Norway (In Norwegian)
Statens kartverk (1996) Kunnskapsforlagets store Norgesatlas. Kunnskapsforlaget, Oslo, Norway. (In Norwegian)
Strong C, Zuckerberg B, Betancourt JL, Koenig WD (2015) Climatic dipoles drive two principal modes of North American boreal bird irruption. Proc Natl Acad Sci USA 112:E2795–E2802. https://doi.org/10.1073/pnas.1418414112
Suhonen J, Jokimäki J (2015) Fruit removal from rowanberry (Sorbus aucuparia) trees at urban and rural areas in Finland: A multi-scale study. Landsc Urban Plan 137:13–19. https://doi.org/10.1016/j.landurbplan.2014.12.012
Suhonen J, Jokimäki J, Lassila R, Kaisanlahti-Jokimäki M-L, Carbó-Ramírez P (2017) Effects of roads on fruit crop and removal rate from rowanberry trees (Sorbus aucuparia) by birds in urban areas of Finland. Urban for Urban Greening 27:148–154. https://doi.org/10.1016/j.ufug.2017.08.001
Sundberg J (2001) Tallbitsinvasionen vintern 2000–2001. Vår Fågelvärld 60(3):27–29 (In Swedish)
Svärdson G (1957) The “invasion” type of bird migration. Brit Birds 50:314–343
Svensson S (2001) Tallbitinvasionerna kommer tätare, men ingen slår rekordet 1976/77. Vår Fågelvärld 60(8):19–21 (In Swedish)
Svensson S, Svensson M, Tjernberg M (1999) Svensk Fågelatlas. Sveriges Ornitologiska Förening, Stockholm, Sweden. (In Swedish)
Ullman M (1999) Tallbit. Vår Fågelvärld 58(1):49 (In Swedish)
Valkama J, Vepsäläinen V, Lehikoinen A (2011) The Third Finnish Breeding Bird Atlas. Finnish Museum of Natural History and Ministry of Environment, Finland. http://atlas3.lintuatlas.fi/english
Watts HE, Robart AR, Chopra JK, Asinas CE, Hahn TP, Ramenofsky M (2017) Seasonal expression of migratory behaviour in a facultative migrant, the pine siskin. Behav Ecol Sociobiol 71:9. https://doi.org/10.1007/s00265-016-2248-2
Winnem A (2021) Fugler i Norge 2019. Rapport fra Norsk faunakomité for fugl (NFKF). Fugleåret 2019:60–139 (In Norwegian)
Zuckerberg B, Strong C, LaMontagne JM, George SS, Betancourt JL, Koenig WD (2020) Climate dipoles as continental drivers of plant and animal populations. Trends Ecol Evol 35:440–453. https://doi.org/10.1016/j.tree.2020.01.010
Acknowledgements
I thank Gunnhild Jaastad and Geir Kjølberg Knudsen for providing Rowanberry data from the VIPS-project (Varsling innen PlanteSkadegjørere), developed by NIBIO—Norsk Institutt for Bioøkonomi and Norsk Landbruksrådgiving, Christian Solheim for help with producing maps, and Geir A. Sonerud and two anonymous reviewers for comments on the manuscript.
Funding
Open access funding provided by Norwegian University of Life Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflicts of interest to declare.
Additional information
Communicated by N. Chernetsov.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dale, S. Irruptions of Pine Grosbeaks pulled by Rowanberry peaks in southern areas. J Ornithol 164, 353–366 (2023). https://doi.org/10.1007/s10336-022-02032-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-022-02032-w