Abstract
Migratory behaviour in birds shows a remarkable variability at species, population and individual levels. Short-distance migrants often adopt a partial migratory strategy and tend to have a flexible migration schedule that allows a more effective response to extreme environmental variations. Weather seasonality and environmental heterogeneity have been reported as significant factors in the diversification of migratory behaviour for Mediterranean migrants, but relatively few studies investigated the migration patterns of non-passerine birds migrating within the Mediterranean basin. In this study, we investigated the migratory strategy of 40 Eurasian Stone-curlews Burhinus oedicnemus tagged with geolocators and GPS-GSM tags and belonging to continental and Mediterranean populations of the Italian peninsula. The proportion of migrants was higher in continental populations, but we observed a significant variability also within Mediterranean populations. All birds spent the winter within the Mediterranean basin. Continental Stone-curlews departed earlier in spring and later in autumn and covered longer distances than those from Mediterranean areas. The speed of migration did not change between seasons for continental birds, while Mediterranean individuals migrated faster in spring. The likelihood of departure for autumn migration of GPS-tagged birds increased when temperatures were near or below 0 °C suggesting that Stone-curlews tend to delay departure until weather conditions worsen abruptly. As a consequence of global warming in the Mediterranean, the frequency of migratory birds in the considered populations might decrease in the near future. This could affect the distribution of species throughout the year and should be taken into account when targeting conservation measures.
Zusammenfassung
Unterschiede im Zugverhalten eines Kurzstreckenziehers innerhalb von und zwischen Populationen: der Triel ( Burhinus oedicnemus , Charadriiformes, Burhinidae)
Das Zugverhalten von Vögeln zeigt bemerkenswerte Unterschiede auf Art-, Populations- und Individuen-Ebene. Kurzstreckenzieher verfolgen häufig eine partielle Zugstrategie und haben in der Regel eine flexible Zeitplanung, die eine effektivere Reaktion auf extreme Umweltveränderungen ermöglicht. Die jahreszeitlichen Wetterschwankungen und die unterschiedlichen Umgebungsbedingungen werden als wichtige Faktoren für die Differenzierung des Zugverhaltens von Zugvögeln im Mittelmeerraum genannt, aber nur relativ wenige Studien haben sich mit den Zugmustern von Nichtsperlingsvögeln, die innerhalb des Mittelmeerraums wandern, befasst. In dieser Studie untersuchten wir die Zugstrategien von 40 mit Ortungs- und GPS-GSM-Sendern versehenen Trielen (Burhinus oedicnemus) von kontinentalen und mediterranen Populationen auf der italienischen Halbinsel. Der Anteil der Zieher war in den kontinentalen Populationen höher, aber auch innerhalb der mediterranen Populationen wurde eine erhebliche Bandbreite festgestellt. Alle Vögel überwinterten im Mittelmeerraum, wobei die kontinentalen Triele im Frühjahr früher und im Herbst später abflogen und größere Entfernungen zurücklegten als die Vögel aus den Mittelmeergebieten. Die kontinentalen Vögel zogen unabhängig von der Jahreszeit mit gleicher Geschwindigkeit, während die Vögel des Mittelmeerraums im Frühjahr schneller zogen. Die mit GPS-Sendern versehenen Vögel brachen bei Temperaturen nahe oder unter 0 °C mit höherer Wahrscheinlichkeit zum Herbstzug auf, was darauf schließen lässt, dass Triele dazu neigen, den Aufbruch hinauszuschieben, bis sich die Wetterbedingungen abrupt verschlechtern. Als Folge der globalen Erwärmung im Mittelmeerraum könnte die Anzahl der ziehenden Vögel in den hier untersuchten Populationen in der nahen Zukunft abnehmen. Dies könnte sich auf die Verteilung der Arten über ein gesamtes Jahr hinweg auswirken und sollte bei der Planung von Bestandserhaltungsmaßnahmen berücksichtigt werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The extent and the pattern of migration may vary strongly among different taxa and even within the same taxon (Dingle and Drake 2007). Migratory behaviour in birds, in particular, shows a remarkable variability at species (Schmaljohann 2018; Anderson et al. 2020), population (Monti et al. 2018a, 2018b; Phipps et al. 2019) and individual levels (Shamoun-Baranes et al. 2017a; Tedeschi et al. 2020). One of the most extreme forms of intra-population variation in migratory behaviour is partial migration, where only a fraction of individuals are migrants (Chapman et al. 2011, 2014). This seems to be the most common strategy in birds (Chapman et al. 2011), especially among short-distance migrants (Pulido et al. 1996; Newton 2008). Besides intra-population heterogeneity in migratory strategy, short-distance migrants also show considerable variability in migratory behaviour, mainly due to the effect of environmental factors in modulating the phenology of migration (Berthold 1996; Pulido and Widmer 2005; Both et al. 2010; Newton 2012). This is especially true for post-breeding migration as birds do not have the urgency to get to wintering grounds first and in time (McNamara et al. 1998; Nilsson et al. 2013). Due to the relative proximity between breeding and wintering grounds, short-distance migrants can also optimize their departure schedules by selecting weather conditions that allow them to minimize energy expenditure throughout the trip (Alerstam and Lindström 1990; Lehikoinen et al. 2004; Rubolini et al. 2007; Knudsen et al. 2011; Nilsson et al. 2013). This leads to more flexible migration schedules for short-distance than for long-distance migrants (Pulido and Widmer 2005; Newton 2008; Pulido and Berthold 2010; Monti et al. 2018b) and allows the former to respond more effectively to extreme environmental variations, like those due to climate change (Jenni and Kéry 2003; Gordo 2007).
Weather seasonality and environmental heterogeneity at regional and local scales are significant factors in the diversification of migratory behaviour at inter and intra-population levels for some species migrating within the Mediterranean region (Pérez-Tris and Tellería 2002; García de la Morena et al. 2015). Moreover, the ongoing increase in temperature recorded in this area due to global warming (Mariotti et al. 2015) can affect migration timing of short distance migrants (e.g., Birtsas et al. 2013) and might even lead to the complete suppression of their migratory behaviour (Gordo 2007; Morganti 2015). Relatively few studies, however, have investigated migration patterns and the role of environmental factors in modulating migratory behaviour of non-passerine birds migrating within the Mediterranean basin (but see Birtsas et al. 2013; García de la Morena et al. 2015; Monti et al. 2018a; Alonso et al. 2019).
The Eurasian Stone-curlew Burhinus oedicnemus (Charadriiformes, Burhinidae, hereafter, Stone-curlew) is a steppe bird whose European populations are mainly concentrated in the Mediterranean area (Vaughan and Vaughan-Jennings 2005; Delany et al. 2009; Keller et al. 2020). In Italy the species is relatively widespread in Sardinia, Sicily and in the Southern part of the peninsula, while it is scarce, although locally common, along the riverbanks and in farmlands in the Centre and the North of the peninsula (Brichetti and Fracasso 2004; Biondi and Pietrelli 2015).
The species is of European conservation concern (BirdLife International 2021) but information on the status of its populations is limited (Gaget et al. 2019). Available information suggests that the migratory behaviour of the Stone-curlew should be rather variable at intra- and inter-population level throughout its range (Cramp and Simmons 1983; Green et al. 1997; Vaughan and Vaughan-Jennings 2005; Giunchi et al. 2015), but relatively little is known about its movements. Investigating the migratory strategies of different populations, the geographical range of their movements and the degree of migratory connectivity is an important contribution for understanding the factors affecting population trends (Webster et al. 2002; Taylor and Norris 2010; Finch et al. 2017). Furthermore, understanding how migration phenology is influenced by proximate meteorological factors can help to understand how a species would react to the ongoing climate change (Gill et al. 2014; Haest et al. 2018, 2019; Burnside et al. 2021; Linek et al. 2021).
In this study we aimed at: (i) investigating the spring and autumn migratory strategies of Stone-curlews belonging to four Italian populations, two from the North and two from the center of the peninsula and (ii) performing a preliminary evaluation of the possible role of exogenous factors in modulating the phenology of autumn migration.
Regarding the migratory strategies applied by Stone-curlews, we expected that: (i) the northern populations would have a higher proportion of migrants than the southern ones, as reported for other Palearctic migrants (Newton and Dale 1996; Newton 2008; Linek et al. 2021) and suggested for the Stone-curlew (Delany et al. 2009; Biondi and Pietrelli 2015); (ii) Stone-curlews should use a time-minimization strategy during spring migration and an energy-minimization strategy during autumn migration (Alerstam and Lindström 1990; Kokko 1999; Nilsson et al. 2013), with a spring migration faster than the autumn one in all populations, as reported for several bird species, including waders (Zhao et al. 2017; Schmaljohann 2018); (iii) the breeding latitude should affect the timing of departure, as already observed in long-distance migrants where northern populations tend to depart later in both autumn and spring than birds from southern populations (Conklin et al. 2010; Briedis et al. 2016; Neufeld et al. 2021).
When evaluating the possible effect of exogenous cues on the birds’ migratory choices, we hypothesised that the timing of autumn migration of tagged Stone-curlews was significantly affected by weather variables and expected that: (i) northern populations should anticipate their departure compared to southern populations, with a quite variable migration timing, such as other short-distance migrants that usually show a quite flexible migratory schedule (Pulido and Widmer 2005; Newton 2008; Pulido and Berthold 2010; Monti et al. 2018b); (ii) this variability in migration timing should be particularly high in autumn, when there is no compelling need to reach the wintering sites (Shamoun‐Baranes et al. 2006; Nilsson et al. 2013); (iii) meteorological variables, such as wind conditions (Liechti 2006; Grönroos et al. 2012), temperature (Burnside et al. 2021), and atmospheric pressure (Dänhardt and Lindström 2001) would significantly affect migratory departures. Low temperatures, in particular, should significantly increase the likelihood of migratory departure, given (1) their negative effect on the availability of invertebrates (Eggleton et al. 2009; Abram et al., 2017) which are the main food supply for the Stone-curlew (Amat 1986; Green et al. 2000; Giannangeli et al. 2004; Giovacchini et al. 2017), and (2) the low resting/basal metabolic rate of the Stone-curlew (Duriez et al. 2010; Gutiérrez et al. 2012), which could make the thermoregulation process in cold conditions rather costly.
Methods
Study areas
The study was carried out on four Italian areas, two located in the continental biogeographical region (https://www.eea.europa.eu/data-and-maps/data/biogeographical-regions-europe-3), Taro river (Parma Province, Northern Italy, 44.74° N 10.17° E; n = 9) and Piave river (Treviso Province, Northern Italy, 45.80° N 12.28° E; n = 12), and two in the Mediterranean biogeographical region, Grosseto Province (Southern Tuscany, Central Italy, 42.60° N 11.22° E; n = 10) and Viterbo Province (Northern Latium, Central Italy, 42.35° N 11.83° E; n = 10) (Fig. 1). According to the Köppen-Geiger climate classification, which is based on the relationship between climate and vegetation (Köppen 2011; Chen and Chen 2013), Taro and Piave areas are within a mild temperate, fully humid zone with warm summer (warmest month average temperature < 22 °C and at least four months with temperatures ≥ 10 °C), while Grosseto and Viterbo areas are within a mild temperate zone with dry and hot summer (warmest month average temperature ≥ 22 °C) (data obtained using the R-package ‘kgc’ 1.0.02, Bryant et al. 2017). Birds were captured during the breeding period (March-July) from 2013 to 2019 using different methods (i.e., mist nets, fall traps, dip nets). All birds were measured according to standard ringing procedures (Busse and Meissner 2015) and molecularly sexed according to Griffiths et al. (1998). Biometric measures and sex were not available for birds belonging to Viterbo population.
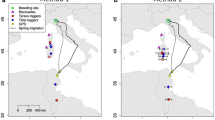
Capture areas (dots), migration tracks and winter locations (triangles) of Stone-curlews equipped with GPS in Italy in the period 2012–2019. A Autumn migration (n = 14 individuals); B spring migration (n = 19 individuals). Darker symbols: continental populations; lighter symbols: Mediterranean populations. For the Taro population, autumn migration was not available due to a temporary failure of the tags
Tracking data
Two types of tracking devices were used throughout the study period: geolocators and solar powered GPS tags. Geolocator data were collected in the years 2010–2011 only for the Taro population and were already published (see Giunchi et al. 2015 for details). These data were taken into account solely for comparing the proportion of migrants and residents from the four populations. GPS devices (GPS-UHF and GPS-GSM) were used in all populations in the period 2012–2019 (see Supplementary Table S1 for details). Part of the tracks considered in this study were already published in Giunchi et al. (2015) and in Cerritelli et al. (2020). The GPS were fitted on birds using a Teflon ribbon leg-loop (Rappole and Tipton 1991) or backpack harness (Viterbo population only). The weight of the tags plus the harness corresponded to 3–4% of the bird's body weight (average bird weight = 475.4 g ± 37.3 SD, n = 23; average tag weight = 17.2 ± 2.3 g, n = 24). The weight of birds tagged in the Viterbo province was not available. The weight of the tags was less than recommended 5% of the body weight (Barron et al. 2010), but slightly above the recently suggested 3% limit (Geen et al. 2019). We have not had the opportunity to adequately test the impact of tags and cannot rule out the possibility that weight of the devices might have an effect on the migratory behaviour of tagged animals (Geen et al. 2019). However, we can assume that the impact was similar in the different populations, and therefore should not have affected most of the results reported in the paper.
Since the number of birds in each population followed for more than one year was relatively small, we randomly selected a single track for each season whenever more than two tracks were available for a given individual to avoid pseudoreplication (Hulbert 1984) and simplify the analysis.
To minimize possible bias inherent in using subjective criteria (Cerritelli et al. 2020), we objectively identified departure and arrival dates in both breeding and wintering areas and stopovers through the segmentation method implemented in the package ‘segclust2d’ 0.2.0 (Patin et al. 2020) in R 4.0.2 (R Core Team 2020). This method allows to find similar segments in function of the speed and tortuosity (i.e., low variability for limited periods), corresponding to stationary phases, and cluster them in a common class, corresponding to a given state. Two parameters had to be set to carry out the analysis (Patin et al. 2020): a minimum segment length corresponding to the minimum time that a certain behavior could be considered stationary (Lmin) and the maximum number of states (M). The optimal number of segments (K) and of states (M) are then chosen by means of model selection according to the Bayesian information criterion (BIC). Track segmentation was performed in two steps. In the first step, aimed at identifying the onset and the end of migration, we considered for each individual a tracking period ranging from 20 days before the presumed departure to 20 days after the presumed arrival, as inferred by the visualization of the tracks. The track was regularized (Patin et al. 2020) using ‘adehabitatLT’ 0.3.25 R-package (Calenge 2006) at 1 fix every 60 or 180 min to obtain the best trade-off between the maximum number of tracks available for the analyses and the minimum time lag between fixes (Supplementary Table S1). The tracks were segmented by setting the Lmin so that the minimum time of stationarity was 12 h, the maximum number of states M = 3 (residency in breeding and wintering areas and migration states).
In the second step of the segmentation process, we used the migratory tracks identified in the previous step to identify the stopover periods. This analysis was performed on the tracks of 14 individuals which had at least 1 fix/90 min. The tracks were regularized according to the original duty cycle (Supplementary Table S1) and then segmented by considering a minimum time of stationarity of 6 h with three states (rest at stopover, foraging at stopover, migration). Stopovers were not identified using the segmentation method for six birds (Supplementary Table S1, individuals marked with an asterisk), as the duty cycles of their tags included switching off the instrument for 8 h during the day. For these birds, we considered as stopovers the group of fixes collected during at least 12 h and spaced < 4 km apart, i.e., the third quartile of the distribution of the distances calculated among locations identified as stopovers for the remaining birds using the clustering-segmentation method (median = 2.1 km, first-third quartile = 0.7–4.0 km, min–max = 0–17.7 km).
After the identification of migratory and stopover periods, we calculated: the total migration distance as the sum of distances between all fixes, excluding movements in stopover areas, the straightness index, i.e., the ratio between the straight distance between wintering and breeding site and the total distance travelled by the birds (Batschelet 1981), the number of stopovers, the time spent in stopovers, the total migration length (including the stopover period) and the time spent flying. All distance measurements were performed by means of the orthodromic Vincenty ellipsoid method using the R-package ‘geosphere’ 1.5–10 (Hijmans 2019). Every location was classified as nocturnal (from sunset to sunrise) or diurnal considering nautical crepuscule, by means of the R-package ‘amt’ 0.1.2 (Signer et al. 2019) in order to verify whether this species migrates mostly during the night (Vaughan and Vaughan-Jennings 2005).
Regional weather data
The centroids of breeding areas for each individual were calculated and the weather data recorded in each location were extracted from the European Centre for Medium-Range Weather Forecasts European Reanalysis v5-Land data (ECMWF ERA5-Land; spatial resolution 0.1° × 0.1°; temporal resolution 1 h). As the sample size of tracked birds was relatively small, we considered a small set of variables which are known to affect the likelihood of departure (see e.g., Dänhardt and Lindström 2001; Liechti 2006; Conklin and Battley 2011; Grönroos et al. 2012; Duijns et al. 2017; Burnside et al. 2021) to avoid data dredging. We considered the period starting about 25 days before the departure of the first bird, i.e., from 1 October until the departure of all birds from the breeding areas. The variables included in the analysis were (Supplementary Table S2): (1) the daily average of the northward component of the wind speed (10 m above the ground; Vwind, m/s), (2) the daily minimum temperature (2 m above the ground; Tmin, °C), (3) the difference between the minimum temperature of the day and that of the previous one (ΔTmin, °C) and (4) the difference between the daily average of the sea surface atmospheric pressure of the day and that of the previous one (ΔPatm, hPa). The correlation among these variables, assessed with the Spearman correlation coefficients was always well below 0.5. Photoperiod was not included in the model because its effect on migration timing is well known (Gwinner 1990; Berthold 1996; Gwinner and Helm 2003) and the aim of our analysis was to assess the effect of proximate meteorological variables in modulating the start of migration in a time window when day length is permissive for migration.
Statistical analyses
We initially tested if the proportion of migrants and residents differed in the four populations including in the analysis both birds tagged with geolocators and those tagged with GPS. We used a Firth's bias-reduced penalized-likelihood logistic regression model as implemented in the R-package ‘logistf’ 1.24 (Heinze et al. 2020), with migration strategy (presence or absence of migration) as binary dependent variable and population (four-levels factor: Taro, Piave, Grosseto, Viterbo) as independent variable. Model significance was assessed using the likelihood ratio (LR) test. We considered three planned contrasts, i.e., continental versus Mediterranean populations, Taro vs Piave population and Grosseto vs Viterbo population.
The analysis of the migratory behaviour was performed by comparing birds from the Mediterranean region (one from Grosseto population and eight from Viterbo population), with birds from the Piave (n = 9) and Taro (n = 2) populations, as representative of the continental region. We used standard linear models (LM) with departure or arrival date (Julian day, 1 = 1st of January) as dependent variable and region as predictor to investigate the relationship between timing of migration, season and area. Data from the two migratory seasons were analysed separately. To verify if this species is a nocturnal migrant and if this behaviour changes between seasons and regions, the proportion of migratory fixes recorded during the night was compared between seasons (two-levels factor: autumn vs spring) and regions (two-levels factor: continental region vs Mediterranean region) using a Generalized Linear Mixed Model (GLMM) with binomial error distribution and bird ID as random intercept. A Linear Mixed Model (LMM) was used to test whether the distance travelled was affected by the migratory season, the breeding region and by their interaction and to investigate the relationship between speed of migration, season and area of origin. To take into account the effect of different duty cycles in LMM, we included a weight variable in LMM of distance and speed: a weight of 1 was assigned to the animals with a duty cycle of 60 min during migration, whereas a weight equal to 0.66 was assigned to the two animals with a duty cycle of 90 min. In all LMM models, bird ID was included as a random intercept.
(G)LMM were fitted using the package ‘lme4’ 1.1–26 (Bates et al. 2015). Significance of fixed effects was tested by means of the likelihood ratio (LR) test. Model fit, overdispersion and collinearity were checked by means of the packages ‘performance’ 0.6.1 (Lüdecke et al. 2021) and ‘DHARMa’ 0.3.3.0 (Hartig 2020). 95% confidence intervals were estimated using the package ‘parameters’ 0.6.1 (Lüdecke et al. 2021). Adjusted R2 was reported for every linear model. Conditional and marginal R2 for the linear mixed models was calculated according to Nakagawa et al. (2017) using the package ‘MuMIn’ 1.43.17 (Bartoń 2020).
The possible effect of the considered meteorological variables on the start of migration of the Stone-curlew was investigated by means of the non-parametric Cox proportional hazard model (Moore 2016). This model describes the probability per unit of time that an event occurs as a function of the baseline hazard and a set of covariates (fixed or time dependent). The models were fitted using the R-package ‘survival’ 3.2–11 (Therneau 2020; Therneau and Grambsch 2000). Data were clustered by bird ID and robust variance was estimated using a jackknife approach (Therneau 2020; Therneau and Grambsch 2000). The predictors included in the two models were the breeding region (two-levels factor: continental region vs Mediterranean region) and four time-dependent covariates (Vwind, ΔPatm, Tmin and ΔTmin; Supplementary Table S2). The breeding region was included in the model to take into account the difference in migratory length among the populations belonging to the two areas (see Results). Model selection was performed according to the corrected Akaike Information Criterion (AICc; Burnham and Anderson 2002) using the ‘MuMIn’ package (Bartoń 2020). We considered all combinations of the three time-dependent predictors while keeping the stratum covariate fixed. Given the relatively small available sample size, we reduced model complexity by not considering any interaction and by limiting the maximum number of terms to three (intercept included). The model with the lowest AICc value was used for inferences, provided that the null model was not included in the set of models within two units from the best one (Burnham and Anderson 2002). For each model, we calculated the Akaike weight wi, representing the probability of the model given the data (Burnham and Anderson 2002). Model significance was tested using the Robust Score (RS) test while goodness-of-fit was evaluated by means of model concordance (Therneau 2020; Therneau and Grambsch 2000). RS test, model concordance, covariate coefficients and estimated hazard ratios of departure after 1 SD increase of the variables included in the model were reported for all models within two units from the best one (Burnham and Anderson 2002). Model assumptions were checked according to Therneau and Grambsch (2000) using the package ‘survminer’ 0.4.9 (Kassambara et al. 2021).
All statistical analyses were performed with R (version 4.0.2) (R Core Team 2020). All geographical analyses and plots were carried out using QGIS 3.4 (QGIS Development Team 2020).
Results
Migration strategy
The proportion of migrants differed significantly among populations (χ2 = 18.9, df = 3, P = 0.0003, Firth’s bias-reduced penalized-likelihood logistic regression model, LR test; Supplementary Fig. S1), with migratory birds being more common in continental than in Mediterranean populations (χ2 = 7.4, P = 0.007). The proportion of migrants was not different between the two continental populations (Taro vs Piave: χ2 = 2.1, df = 1, P = 0.1), while it was unexpectedly different among the two Mediterranean populations, being significantly higher in Viterbo than in Grosseto population (χ2 = 9.8, df = 1, P = 0.002).
Migration routes, speed and timing
From the 20 GPS-tagged Stone-curlews identified as migrants, we reconstructed a total of 33 migratory tracks: 14 in autumn and 19 in spring. Migration turned out to be rather short in both seasons (on average less than 1000 km covered in less than 5 days; Table 1). Migrant Stone-curlews spent their wintering period within the Mediterranean basin (Fig. 1), i.e., in Sardinia (n = 4), Sicily (n = 3), Tunisia (n = 12) and Libya (n = 1).
All birds followed a straight and direct migratory route (most straightness indexes > 0.80) both in autumn and in spring (Table 1). The only exceptions to this pattern were TK2343 (Piave population) that showed a clear detour during autumn migration leading to a relatively low straightness index (0.59; Supplementary Fig. S2A) and GR06 (Grosseto population) which showed a moderate detour during spring migration just before arriving at the breeding site (0.67; Supplementary Fig. S2B).
The departure and arrival dates showed a high degree of variability in both migratory seasons, ranging from the last week of October to the end of December/early January in autumn, and from early February to early April in spring (Table 1). The continental populations departed on average 25 days earlier for autumn migration than the Mediterranean ones and delayed by about 10 days the onset of spring migration when compared with the Mediterranean populations, leading to significant differences in departure and arrival dates between the two areas (autumn departure: F1,12 = 9.3, P = 0.01, adjusted R2 = 0.39; autumn arrival: F1,12 = 8.3, P = 0.014, adjusted R2 = 0.36; spring departure: F1,17 = 5.2, P = 0.035, adjusted R2 = 0.19; spring arrival: F1,17 = 7.4, P = 0.015, adjusted R2 = 0.26; LM, Fig. 2A). As expected, most of the migratory movements took place at night, especially during autumn migration when the proportion of nocturnal locations tended to be higher than in spring (χ2 = 7.2, df = 1, P = 0.007; GLMM, LR test; Supplementary Fig. S3). No differences were observed in the proportion of nocturnal locations between regions (χ2 = 0.099, df = 1, P = 0.8), with all tracked birds showing comparable nocturnal migratory movement.
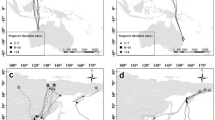
Plot of effects of the breeding region and migratory season on the main migratory parameters. A Departure and arrival of autumn (upper left panel) and spring (upper right panel) migration of Stone-curlews equipped with GPS in Italy in the period 2012–2019. Results from the linear models with the departure or arrival date (Julian day, 1 = 1st of January) as dependent variable and region as predictor. Departure and arrival dates were significantly different between the two regions both in autumn and in spring, with the continental populations departing earlier than the Mediterranean ones in autumn and delaying departure in spring. B Migration distance recorded in Stone-curlews equipped with GPS in Italy in the period 2012–2019. Birds from the Mediterranean populations migrated longer distances in the spring than the autumn. Results from a linear mixed model with season (two-levels factor: autumn vs spring), region (two-levels factor: continental region vs Mediterranean region) and their interaction as predictors and bird ID as random intercept. SD of the random intercept = 239.9, conditional R2 = 0.88, marginal R2 = 0.42, number of observations = 33, number of birds = 20. C Speed of migration recorded in Stone-curlews equipped with GPS in Italy in the period 2012–2019. Birds from the Mediterranean populations migrated faster in the spring than the autumn, but this was not the case for continental populations where migration speeds were similar between seasons. Results from a linear mixed model with season (two-levels factor: autumn vs spring), region (two-levels factor: continental region vs Mediterranean region) and their interaction as predictors and bird ID as random intercept. SD of the random intercept = 201.6, conditional R2 = 0.66, marginal R2 = 0.18, number of observations = 33, number of birds = 20
Migration length only differed between regions of origin (χ2 = 12.3, df = 1, P = 0.0004; LMM, LR test), with individuals from continental populations covering longer distances than the Mediterranean ones (Fig. 2B). No differences in migration lengths were observed between the two migratory seasons (χ2 = 0.42, df = 1, P = 0.5) and individuals from different populations travelled similar distances during both migrations (χ2 = 0.30, df = 1, P = 0.6).
The total duration of migration was rather short, often less than 5 days, and the median duration of active flight was about 1 day only (Table 1). The number of stopovers was rather variable in both seasons but usually it did not exceed two (Table 1); in two cases (birds VT42A, VT873), the individuals did not stop at all during spring migration, while we recorded seven stopovers for bird T25 for the same migration. Median stopover length was < 3 days, even though in some cases birds could stop for more than 1 week. The median of the total migratory speed was 300 km/day but active flight migration segments were often covered at more than 700 km/day (Table 1).
The migratory speed was not cohesive among populations and seasons (χ2 = 5.1, df = 1, P = 0.02; LMM, LR test): while individuals from continental populations kept a similar migratory speed rate between seasons, the Mediterranean population was generally migrating faster in spring than in autumn (Fig. 2C).
Effects of meteorological variables on the start of autumn migration
The analysis on the effects of meteorological factors on the start of autumn migration was performed on 14 birds (eight from the continental region and six from the Mediterranean region). The most supported model among the considered set included the daily minimum temperature (Tmin) and the daily averaged northward wind component (Vwind) (Table 2). The effect of temperature seems to be particularly strong, as this is the only covariate included in the second model, still within two units from the best one (Table 2). Both models were highly significant and have a high coefficient of concordance. As expected, the likelihood of departure increased when both Tmin and Vwind decreased, meaning that birds tended to depart when they were experiencing low temperatures and favourable wind conditions (wind blowing southward, i.e., tailwind conditions) (Fig. 3).
Plot of the effects of daily minimum temperature (Tmin) and daily averaged northward wind component (Vwind; negative values indicate North to South flows) on the likelihood of departure (departure hazard score) for autumn migration of Stone-curlews equipped with GPS in Italy in the period 2012–2019. Results from the best Cox proportional hazard models (see Table 2 for further details). Number of birds = 14
Discussion
This study reports one of the few detailed investigations of the migratory behaviour of a Mediterranean migrant and the first for the Stone-curlew. Most of the studied populations were partially migratory, with short distance movements within the Mediterranean basin. Interestingly, our results highlight the significant intra- and inter-population variability of the migratory behaviour, which is not fully explained by breeding latitude and suggest that temperature and wind conditions modulate the timing of autumn migration.
Migration strategy
Three of the four considered populations were found to be partially migratory. This result is not surprising as partial migration is a fairly common strategy among short-distance migrants (Pulido et al. 1996; Newton 2008). The analysis of the proportion of migrating individuals showed significant interpopulation variability, as recorded for other migratory species in the Mediterranean basin (García de la Morena et al. 2015; Monti et al. 2018a, b). Individuals belonging to continental populations (Taro and Piave) were more likely to undertake migration than populations belonging to the centre of the peninsula, in agreement with our expectations based on data from other Palaearctic migrants (Newton and Dale 1996; Newton 2008) and with what has been previously suggested for the Stone-curlew (Brichetti and Fracasso 2004; Biondi and Pietrelli 2015). The proportion of migrants was not different between the Piave and Taro populations, but the Taro population was the only one in which all tagged birds were found to be migrants. The relatively small number of birds tagged in the Taro could at least partly explain these results, as resident birds were probably rare, while an effect of the low accuracy of the geolocators used in the Taro population seems unlikely (see Giunchi et al. 2015 for details). Since individuals from the Taro population were tagged earlier than those from the Piave population, however, we cannot exclude that the small difference in the proportion of residents in the two populations could be interpreted as an ongoing change in the migratory strategies of the northern populations of the species, as recently observed for other birds migrating over short distances (Doswald et al. 2009; Pautasso 2012; Ambrosini et al. 2016; Tellería et al. 2016; Podhrázský et al. 2017). The difference in the proportion of migrants between the two southernmost populations (Viterbo and Grosseto) was unexpected, as their respective breeding areas are very close to each other (about 57 km) and subject to comparable climatic conditions. It should be noted, however, that of all the birds followed for at least 2 years (n = 6 for Viterbo and n = 7 for Grosseto and Piave populations), only two birds changed their migratory strategy (one from Viterbo and one from Grosseto). Although the sample size is relatively small, this finding seems to indicate that the observed variability between populations is not primarily caused by individual inter-annual variability in migratory behaviour. We could speculate that this result could be due to intrinsic differences between tagged individuals possibly related to their sex and age, which are known to influence the migration strategy of birds (Gauthreaux 1982; Terrill and Able 1988; Newton 2008; Chapman et al. 2011; Hegemann et al. 2019). We did not record the sex of Viterbo birds, but data collected on the remaining populations did not show a consistently different pattern in the migration strategy of males and females (data not shown). Furthermore, all birds considered in this study were adults (> 1 year old). Interestingly, the only exception was the single migrant in the Grosseto population, which migrated when it was one year old and then became resident for the next two years of monitoring. This suggests that age and/or competitive ability may indeed play a role in modulating the migratory behaviour of Stone-curlews, as observed in other partial migrants (see Newton 2008; Chapman et al. 2011; Hegemann et al. 2019 for references), but it is not very helpful in understanding the unexpected migratory propensity of Viterbo birds. It cannot be excluded that this result is due to genetic differences between the two populations, even though previous genetic analyses (not including the Viterbo population) suggested that the gene flow between Italian Stone-curlew populations is significant (Mori et al. 2017). Alternatively, it could be hypothesised that the resources available in the Viterbo area during the non-breeding season are more limited than in the Grosseto area, e.g., due to a more widespread use of intensive agricultural practices, thus leading a higher fraction of individuals to migrate (Cox 1985; Boyle 2008; Jahn et al. 2010). Beyond the possible explanations, this result highlights a high local variability in the migration strategy of this species that deserves further investigation.
Migration routes, speed and timing
Most birds followed a relatively straight and direct route both in autumn and in spring. Migratory movements were limited within the Mediterranean basin, but wintering sites showed a wide latitudinal range (from Sardinia to Libya) compared to the latitudinal range of breeding areas and to the scale of migratory movements. The large overlap of wintering sites between continental and Mediterranean populations indicates a telescopic migration, i.e., populations that are allopatric during the breeding period overwinter in sympatry (Newton 2008; Borras et al. 2011; Arizaga and Tamayo 2013). This pattern suggests a weak connection between breeding and non–breeding areas (migratory connectivity, Webster et al. 2002) which can have significant implications for the conservation of the species (Cresswell 2014; Finch et al. 2017). In this regard, it is interesting to observe that all tagged migrant Stone-curlews spent the winter season in areas that probably already hosted resident populations of the species (Delany et al. 2009; Brichetti and Fracasso 2004; Tinarelli et al. 2009; Hume and Kirwan 2020).
Our data were only partially in agreement with the expectation that total migration speed should be higher in spring than in autumn (Kokko 1999; Nilsson et al. 2013; Zhao et al. 2017; Schmaljohann 2018). Spring migration was indeed faster only for Mediterranean Stone-curlews, whereas no difference was observed for continental birds. Population variability in the difference in overall migration speed between seasons has been observed in other species (see e.g., Vansteelant et al. 2015; Schmaljohann 2018). The variability recorded in this study was relatively small, so we might assume that Stone-curlews did not significantly change their migration strategies in different seasons, given the short migration distance. Some data collected on shorebirds of different sizes also seemed to suggest that the seasonal difference in migration speed tended to be smaller for larger species (Zhao et al. 2017). It cannot be ruled out, however, that Stone-curlews do indeed adopt different strategies in spring and autumn, but no difference in migratory speed could be detected for continental birds due to the effect of unfavourable weather conditions encountered en route that may have delayed or interrupted the migratory journey (see e.g., Newton 2008; Shamoun-Baranes et al. 2010, 2017b). This effect might have been less relevant for Mediterranean individuals, because the greater proximity between the wintering and breeding areas might allow them to better adjust their timing of migration in function of weather conditions they expect to find en route and in the area of arrival (Lehikoinen et al. 2004; Rubolini et al. 2007; Møller et al. 2010; Knudsen et al. 2011). In this regard, it is interesting to note that birds from both areas tended to migrate during the day more frequently in spring than in autumn, as indicated by the significantly lower fraction of migratory fixes recorded during the night in spring. This extension of nocturnal migration into the daytime, which has been recorded for other nocturnal migrants especially when crossing ecological barriers (Newton 2008; Adamík et al. 2016; Malmiga et al. 2021), might suggest a tighter migratory schedule in spring and therefore seems to be in agreement with a time-minimization strategy.
Even though the latitudinal range of migratory movements was quite small, we observed a significant effect of breeding latitude on the timing of migration. Continental birds departed significantly earlier than Mediterranean ones in autumn while the opposite occurred for spring migration. The earlier autumn departure of animals breeding at higher latitudes is in contrast to what has been observed in long-distance migrants (Conklin et al. 2010; Briedis et al. 2016; Neufeld et al. 2021), but seems to confirm the expected effect of meteorological variables and the likely depletion of food resources in influencing the migratory timing of this species (see below). The pattern observed for arrival dates was the same as that observed for departure dates probably because the difference in the migration distance between the two areas was quite small. Interestingly, all autumn departures occurred after the end of primary moult estimated using data from the Taro population (17th October ± 14 days SD, Giunchi et al. 2008). This indicates that at southern latitudes animals have sufficient time to complete the moulting of the remiges before migrating and do not need to suspend the moult as appears to be the case at northern latitudes (see Giunchi et al. 2008 for details).
The timing of migration was quite variable as both autumn and spring departures occurred over a period of two months. This variability is in line with the observed flexibility of the migratory schedule of short-distance migrants (Pulido and Widmer 2005; Newton 2008; Pulido and Berthold 2010; Monti et al. 2018a) which are often characterized by a sort of “relaxed migratory systems” (see Monti et al. 2018a), but has also been observed in long-distance migratory shorebirds (e.g., Black-tailed Godwit Limosa limosa, Senner et al. 2019). Contrary to the expectations (Shamoun‐Baranes et al. 2006; Nilsson et al. 2013) and to what has been observed in other shorebird species (e.g., Sociable Lapwing Vanellus gregarius, Donald et al. 2021), the variability of departure dates was comparable between autumn and spring. The wide interval of departures observed in both seasons could be at least partially due to a different timing of migration between age or sex classes (differential migration, Ketterson and Nolan 1993; Newton 2008). We cannot exclude an effect of sex, as the sex of some of the tracked birds was not available while the effect of age seems rather unlikely as all tracked birds were adults (> 1-year old).
Effects of meteorological variables on the start of autumn migration
The analysis of the meteorological proximate factors affecting the timing of migration suggests that departure dates are affected in particular by temperature and wind conditions. As expected, birds tended to depart in tailwind conditions (Åkesson and Hedenström 2000; Liechti 2006; Gill et al. 2014; but see Eikenaar and Schmaljohann 2015; Schwemmer et al. 2021), but the greatest effect on the probability to start autumn migration is due to the daily minimum temperature, probably for its negative effect on the availability of invertebrates (Eggleton et al. 2009; Abram et al. 2017) and on the cost of thermoregulation for a species with a low resting/basal metabolic rate (Duriez et al. 2010; Gutiérrez et al. 2012). The effect of temperature on migratory departure has been documented in several long- and short-distance migrants (e.g., Xu and Si 2019; Klinner and Schmaljohann 2020; Linek et al. 2021). In a medium-distance steppe migrant, the Asian Houbara Chlamydotis macqueenii, the effect of temperature was stronger in spring rather than in autumn, as departure decisions were less repeatable for temperature in autumn rather than in spring (Burnside et al. 2021). Unfortunately, this pattern cannot be verified in our species, as the small sample size available and the latitudinal spread of wintering areas did not allow robust modelling of the effect of temperature on the onset of spring migration. In addition, the lack of a significant number of repeated migration tracks did not allow an estimation of the repeatability of individual migration timing.
The relatively large increase in the probability of departure when temperatures were near or below 0 °C might suggest that Stone-curlews may tend to delay departure until winter conditions worsen abruptly (Haila et al. 1986; Newton 2008). This strategy could be effective at the considered latitudes, because the relatively mild environmental conditions combined with the short migration distance could allow for a relatively relaxed timing of the start of migration. This seems to be supported by the wide time interval of autumn departures, some of which occurred even in the second half of December. According to this hypothesis, it can be predicted that the frequency of migratory birds in the considered populations will decrease in the near future due to the effect of global warming in the Mediterranean (Gordo 2007; Morganti 2015). This could consequently modify the present distribution of the species throughout the year and should be taken into account when targeting conservation measures, as the ecological needs of the Stone-curlew could change between breeding and non-breeding seasons.
References
Abram PK, Boivin G, Moiroux J, Brodeur J (2017) Behavioural effects of temperature on ectothermic animals: unifying thermal physiology and behavioural plasticity. Biol Rev 92:1859–1876. https://doi.org/10.1111/brv.12312
Adamík P, Emmenegger T, Briedis M, Gustafsson L, Henshaw I, Krist M, Laaksonen T, Liechti F, Procházka P, Salewski V, Hahn S (2016) Barrier crossing in small avian migrants: individual tracking reveals prolonged nocturnal flights into the day as a common migratory strategy. Sci Rep 6:21560. https://doi.org/10.1038/srep21560
Åkesson S, Hedenström A (2000) Wind selectivity of migratory flight departures in birds. Behav Ecol Sociobiol 47:140–144. https://doi.org/10.1007/s002650050004
Alerstam T, Lindström Å (1990) Optimal bird migration: the relative importance of time, energy, and safety. In: Gwinner E (ed) Bird migration. Springer, Berlin, pp 331–351
Alonso H, Correia RA, Marques AT, Palmeirim JM, Moreira F, Silva JP (2019) Male post-breeding movements and stopover habitat selection of an endangered short-distance migrant, the Little Bustard Tetrax tetrax. Ibis 162:279–292. https://doi.org/10.1111/ibi.12706
Amat JA (1986) Information on the diet of the Stone Curlew Burhinus oedicnemus in Doñana, southern Spain. Bird Study 33:71–73. https://doi.org/10.1080/00063658609476898
Ambrosini R, Cuervo JJ, du Feu C, Fiedler W, Musitelli F, Rubolini D, Sicurella B, Spina F, Saino N, Møller AP (2016) Migratory connectivity and effects of winter temperatures on migratory behaviour of the European robin Erithacus rubecula: a continent-wide analysis. J Anim Ecol 85:749–760. https://doi.org/10.1111/1365-2656.12497
Anderson CM, Gilchrist HG, Ronconi RA, Shlepr KR, Clark DE, Fifield DA, Robertson GJ, Mallory ML (2020) Both short and long-distance migrants use energy-minimizing migration strategies in North American herring gulls. Mov Ecol 8:26. https://doi.org/10.1186/s40462-020-00207-9
Arizaga J, Tamayo I (2013) Connectivity patterns and key non–breeding areas of white–throated bluethroat (Luscinia svecica) European populations. Anim Biodivers Conserv 36:69–78. https://doi.org/10.32800/abc.2013.36.0069
Barron DG, Brawn JD, Weatherhead PJ (2010) Meta-analysis of transmitter effects on avian behaviour and ecology. Methods Ecol Evol 1:180–187. https://doi.org/10.1111/j.2041-210X.2010.00013.x
Bartoń K (2020) MuMIn: Multi-model inference. R package version 1.43.17. https://CRAN.R-project.org/package=MuMIn
Bates D, Machler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Batschelet E (1981) Circular statistics in biology. Academic Press, London
Berthold P (1996) Control of bird migration. Chapman and Hall, London
BirdLife International (2021) European Red List of Birds. Luxembourg: Publications Office of the European Union
Biondi M, Pietrelli L (2015) Distribuzione dell’occhione (Burhinus oedicnemus) nel Lazio e dati preliminari sulla biologia riproduttiva. In: Occhione: ricerca, monitoraggi, conservazione di una specie a rischio, Belvedere. Massimo Biondi, Loris Pietrelli, Angelo Meschini, Dimitri Giunchi, Latina, pp 59–69
Birtsas P, Sokos C, Papaspyropoulos KG, Batselas T, Valiakos G, Billinis C (2013) Abiotic factors and autumn migration phenology of Woodcock (Scolopax rusticola Linnaeus, 1758, Charadriiformes: Scolopacidae) in a Mediterranean area. Ital J Zool 80:392–401. https://doi.org/10.1080/11250003.2013.805827
Borras A, Cabrera J, Colome X, Cabrera T, Senar JC (2011) Patterns of connectivity in Citril Finches Serinus citrinella: sympatric wintering of allopatric breeding birds? Bird Study 58:257–263. https://doi.org/10.1080/00063657.2011.587107
Both C, Van Turnhout CAM, Bijlsma RG, Siepel H, Van Strien AJ, Foppen RPB (2010) Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc R Soc B 277:1259–1266. https://doi.org/10.1098/rspb.2009.1525
Boyle WA (2008) Partial migration in birds: tests of three hypotheses in a tropical lekking frugivore. J Anim Ecol 77:1122–1128. https://doi.org/10.1111/j.1365-2656.2008.01451.x
Brichetti P, Fracasso G (2004) Ornitologia Italiana: Tetraonidae–Scolopacidae, vol 2. Perdisa, Bologna
Briedis M, Hahn S, Gustafsson L, Henshaw I, Träff J, Král M, Adamík P (2016) Breeding latitude leads to different temporal but not spatial organization of the annual cycle in a long-distance migrant. J Avian Biol 47:743–748. https://doi.org/10.1111/jav.01002
Bryant C, Wheeler NR, Rubel F, Roger H, French RH (2017) kgc: Koeppen-Geiger Climatic Zones. R package version 1.0.0.2. https://CRAN.R-project.org/package=kgc
Burnside RJ, Salliss D, Collar NJ, Dolman PM (2021) Birds use individually consistent temperature cues to time their migration departure. Proc Natl Acad Sci USA 118:e2026378118. https://doi.org/10.1073/pnas.2026378118
Busse P, Meissner W (2015) Bird ringing station manual. De Gruyter Open Poland, Warsaw
Calenge C (2006) The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519. https://doi.org/10.1016/j.ecolmodel.2006.03.017
Cerritelli G, Vanni L, Baldaccini NE, Lenzoni A, Sorrenti M, Falchi V, Luschi P, Giunchi D (2020) Simpler methods can outperform more sophisticated ones when assessing bird migration starting date. J Ornithol 161:901–907. https://doi.org/10.1007/s10336-020-01770-z
Chapman BB, Brönmark C, Nilsson JÅ, Hansson LA (2011) The ecology and evolution of partial migration. Oikos 120:1764–1775. https://doi.org/10.1111/j.1600-0706.2011.20131.x
Chapman BB, Hulthén K, Wellenreuther M, Hansson LA, Nilsson JÅ, Brönmark C (2014) Patterns of animal migration. In: Hansson L-A, Åkesson S (eds) Animal movement across scales. Oxford University Press, pp 11–35
Chen D, Chen HW (2013) Using the Köppen classification to quantify climate variation and change: an example for 1901–2010. Environ Dev 6:69–79. https://doi.org/10.1016/j.envdev.2013.03.007
Conklin JR, Battley PF, Potter MA, Fox JW (2010) Breeding latitude drives individual schedules in a trans-hemispheric migrant bird. Nat Commun 1:67. https://doi.org/10.1038/ncomms1072
Cox GW (1985) The evolution of avian migration systems between temperate and tropical regions of the New World. Am Nat 126:451–474. https://doi.org/10.1086/284432
Cramp S, Simmons KEL (1983) The birds of the western palearctic, vol 3. Oxford University Press, Oxford
Cresswell W (2014) Migratory connectivity of Palaearctic-African migratory birds and their responses to environmental change: the serial residency hypothesis. Ibis 156:493–510. https://doi.org/10.1111/ibi.12168
Dänhardt J, Lindström Å (2001) Optimal departure decisions of songbirds from an experimental stopover site and the significance of weather. Anim Behav 62:235–243. https://doi.org/10.1006/anbe.2001.1749
Delany S, Scott D, Dodman T, Stroud D (eds) (2009) An atlas of wader populations in Africa and Western Eurasia. Wetlands International, Wageningen
Dingle H, Drake VA (2007) What is migration? Bioscience 57:113–121. https://doi.org/10.1641/B570206
Donald PF, Kamp J, Green RE, Urazaliyev R, Koshkin M, Sheldon RD (2021) Migration strategy, site fidelity and population size of the globally threatened Sociable Lapwing Vanellus gregarius. J Ornithol 162:349–367. https://doi.org/10.1007/s10336-020-01844-y
Doswald N, Willis SG, Collingham YC, Pain DJ, Green RE, Huntley B (2009) Potential impacts of climatic change on the breeding and non-breeding ranges and migration distance of European Sylvia warblers. J Biogeogr 36:1194–1208. https://doi.org/10.1111/j.1365-2699.2009.02086.x
Duijns S, Niles LJ, Dey A, Aubry Y, Friis C, Koch S, Anderson AM, Smith PA (2017) Body condition explains migratory performance of a long-distance migrant. ProR Soc Lond B 284:20171374. https://doi.org/10.1098/rspb.2017.1374
Duriez O, Eraud C, Bretagnolle V (2010) First measurements of metabolic rates in the Stone-Curlew, a nocturnal inland wader. Wader Study Group Bull 117:119–122
Eggleton P, Inward K, Smith J, Jones DT, Sherlock E (2009) A six year study of earthworm (Lumbricidae) populations in pasture woodland in southern England shows their responses to soil temperature and soil moisture. Soil Biol Biochem 41:1857–1865. https://doi.org/10.1016/j.soilbio.2009.06.007
Eikenaar C, Schmaljohann H (2015) Wind conditions experienced during the day predict nocturnal restlessness in a migratory songbird. Ibis 157:125–132. https://doi.org/10.1111/ibi.12210
Finch T, Butler SJ, Franco AMA, Cresswell W (2017) Low migratory connectivity is common in long-distance migrant birds. J Anim Ecol 86:662–673. https://doi.org/10.1111/1365-2656.12635
Gaget E, Fay R, Augiron S, Villers A, Bretagnolle V (2019) Long-term decline despite conservation efforts questions Eurasian Stone-curlew population viability in intensive farmlands. Ibis 161: 359–371. https://doi.org/10.1111/ibi.12646
García de la Morena ELG, Morales MB, Bota G, Silva JP, Ponjoan A, Suárez F, Mañosa S, Juana ED (2015) Migration patterns of Iberian little bustards Tetrax tetrax. Ardeola 62:95–112. https://doi.org/10.13157/arla.62.1.2015.95
Gauthreaux SA (1982) Age-dependent orientation in migratory birds. In: Papi F, Wallraff HG (eds) Avian navigation. Springer, Berlin, pp 68–74
Giannangeli L, De Sanctis A, Manginelli R, Medina FM (2004) Seasonal variation of the diet of the Stone Curlew Burhinus oedicnemus distinctus at the island of La Palma, Canary Islands. Ardea 92:175–184
Gill RE, Douglas DC, Handel CM, Tibbitts TL, Hufford G, Piersma T (2014) Hemispheric-scale wind selection facilitates bar-tailed godwit circum-migration of the Pacific. Anim Behav 90:117–130. https://doi.org/10.1016/j.anbehav.2014.01.020
Giovacchini P, Dragonetti M, Farsi F, Cianferoni F (2017) Winter diet of Eurasian Stone-curlew, Burhinus oedicnemus (L., 1758) (Aves: Charadriiformes) in a Mediterranean Area (Tuscany, Central Italy). Acta Zool Bulg 69:323–326
Giunchi D, Caccamo C, Pollonara E (2008) Pattern of wing moult and its relationship to breeding in the Eurasian Stone-curlew Burhinus oedicnemus. Ardea 96:251–260. https://doi.org/10.5253/078.096.0210
Giunchi D, Caccamo C, Mori A, Fox JW, Rodríguez-Godoy F, Baldaccini NE, Pollonara E (2015) Pattern of non-breeding movements by Stone-curlews Burhinus oedicnemus breeding in Northern Italy. J Ornithol 156:991–998. https://doi.org/10.1007/s10336-015-1219-0
Gordo O (2007) Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Clim Res 35:37–58. https://doi.org/10.3354/cr00713
Green RE, Hodson DP, Holness PR (1997) Survival and movements of Stone-curlews Burhinus oedicnemus ringed in England. Ringing Migr 18:102–112
Green RE, Tyler GA, Bowden CGR (2000) Habitat selection, ranging behaviour and diet of the Stone-curlew (Burhinus oedicnemus) in southern England. J Zool 250:161–183. https://doi.org/10.1111/j.1469-7998.2000.tb01067.x
Geen GR, Robinson RA, Baillie SR (2019) Effects of tracking devices on individual birds—a review of the evidence. J Avian Biol. https://doi.org/10.1111/jav.01823
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075. https://doi.org/10.1046/j.1365-294x.1998.00389.x
Grönroos J, Green M, Alerstam T (2012) To fly or not to fly depending on winds: shorebird migration in different seasonal wind regimes. Anim Behav 83:1449–1457. https://doi.org/10.1016/j.anbehav.2012.03.017
Gutiérrez JS, Abad-Gómez JM, Sánchez-Guzmán JM, Navedo JG, Masero JA (2012) Avian BMR in marine and non-marine habitats: a test using shorebirds. PLoS One 7:e42206. https://doi.org/10.1371/journal.pone.0042206
Gwinner E (1990) Circannual rhythms in bird migration: control of temporal patterns and interactions with photoperiod. In: Gwinner E (ed) Bird migration. Springer, Berlin, pp 257–268
Gwinner E, Helm B (2003) Circannual and Circadian contributions to the timing of avian migration. In: Berthold P, Gwinner E, Sonnenschein E (eds) Avian migration. Springer, Berlin, pp 81–95
Haest B, Hüppop O, Bairlein F (2018) The influence of weather on avian spring migration phenology: What, where and when? Global Change Biol 24:5769–5788. https://doi.org/10.1111/gcb.14450
Haest B, Hüppop O, van de Pol M, Bairlein F (2019) Autumn bird migration phenology: a potpourri of wind, precipitation and temperature effects. Global Change Biol 25:4064–4080. https://doi.org/10.1111/gcb.14746
Hartig F (2020) DHARMa: residual diagnostics for hierarchical (Multi-Level/Mixed) regression models, R package version 0.3.3.0. https://CRAN.R-project.org/package=DHARMa.
Hegemann A, Fudickar AM, Nilsson JÅ (2019) A physiological perspective on the ecology and evolution of partial migration. J Ornithol 160:893–905. https://doi.org/10.1007/s10336-019-01648-9
Heinze G, Ploner M, Jiricka L (2020) logistf: Firth's bias-reduced logistic regression. R package version 1.24. https://CRAN.R-project.org/package=logistf
Hijmans RJ (2019) geosphere: Spherical Trigonometry. R package version 1.5–14. https://CRAN.R-project.org/package=geosphere
Hulbert JH (1984) Pseudoreplication and the design of field experiments in ecology. Ecol Monogr 54:187–211
Hume R, Kirwan GM (2020) Eurasian thick-knee (Burhinus oedicnemus), version 1.0. In birds of the world. Cornell Lab of Ornithology, Ithaca
Jahn AE, Levey DJ, Hostetler JA, Mamani AM (2010) Determinants of partial bird migration in the Amazon Basin. J Anim Ecol 79:983–992. https://doi.org/10.1111/j.1365-2656.2010.01713.x
Jenni L, Kéry M (2003) Timing of autumn bird migration under climate change: advances in long–distance migrants, delays in short–distance migrants. Proc R Soc Lond B 270:1467–1471. https://doi.org/10.1098/rspb.2003.2394
Kassambara A, Kosinski M, Biecek P (2021) survminer: Drawing survival curves using 'ggplot2'. R package version 0.4.9. https://CRAN.Rproject.org/package=survminer
Keller V, Herrando S, Voríšek P, Franch M, Kipson M, Milanesi P, Martí D, Anton M, Klvanová A, Kalyakin MV (eds) (2020) European breeding bird Atlas 2: distribution abundance and change. European Bird Census Council (EBCC) and Lynx Edicions, Barcelona
Knudsen E, Lindén A, Both C, Jonzén N, Pulido F, Saino N, Sutherland WJ, Bach LA, Coppack T, Ergon T, Gienapp P, Gill JA, Gordo O, Hedenström A, Lehikoinen E, Marra PP, Møller AP, Nilsson ALK, Péron G, Ranta E, Rubolini D, Sparks TH, Spina F, Studds CE, Sæther SA, Tryjanowski P, Stenseth NC (2011) Challenging claims in the study of migratory birds and climate change. Biol Rev 86:28–946. https://doi.org/10.1111/j.1469-185X.2011.00179.x
Kokko H (1999) Competition for early arrival in migratory birds. J Anim Ecol 68:940–950. https://doi.org/10.1046/j.1365-2656.1999.00343.x
Köppen W (2011) The thermal zones of the earth according to the duration of hot, moderate and cold periods and to the impact of heat on the organic world. Meteorol Z 20:351–360
Lehikoinen E, Sparks TH, Zalakevicius M (2004) Arrival and departure dates. Adv Ecol Res 35:1–31. https://doi.org/10.1016/S0065-2504(04)35001-4
Liechti F (2006) Birds: blowin’ by the wind? J Ornithol 147:202–211. https://doi.org/10.1007/s10336-006-0061-9
Linek N, Brzęk P, Gienapp P, O’Mara MT, Pokrovsky I, Schmidt A, Shipley JR, Taylor JRE, Tiainen J, Volkmer T, Wikelski M, Partecke J (2021) A partial migrant relies upon a range-wide cue set but uses population-specific weighting for migratory timing. Mov Ecol 9:63. https://doi.org/10.1186/s40462-021-00298-y
Lüdecke D, Ben-Shachar MS, Patil I, Waggoner P, Makowski D (2021) Performance: An R package for assessment, comparison and testing of statistical models. J Open Source Soft 6:60. https://doi.org/10.21105/joss.03139
Malmiga G, Tarka M, Alerstam T, Hansson B, Hasselquist D (2021) Individual and sex-related patterns of prolonged flights during both day and night by great reed warblers crossing the Mediterranean Sea and Sahara Desert. J Avian Biol. https://doi.org/10.1111/jav.02549
Mariotti A, Pan Y, Zeng N, Alessandri A (2015) Long-term climate change in the Mediterranean region in the midst of decadal variability. Clim Dyn 44:1437–1456. https://doi.org/10.1007/s00382-015-2487-3
McNamara JM, Welham RK, Houston AI (1998) The timing of migration within the context of an annual routine. J Avian Biol 29:416–423. https://doi.org/10.2307/3677160
Møller AP, Flensted-Jensen E, Klarborg K, Mardal W, Nielsen JT (2010) Climate change affects the duration of the reproductive season in birds. J Anim Ecol 79:777–784. https://doi.org/10.1111/j.1365-2656.2010.01677.x
Monti F, Grémillet D, Sforzi A, Sammuri G, Dominici JM, Bagur RT, Navarro AM, Fusani L, Duriez O (2018a) Migration and wintering strategies in vulnerable Mediterranean osprey populations. Ibis 160:554–567. https://doi.org/10.1111/ibi.12567
Monti F, Grémillet D, Sforzi A, Dominici JM, Bagur RT, Navarro AM, Fusani L, Klaassen RHG, Alerstam T, Duriez O (2018b) Migration distance affects stopover use but not travel speed: contrasting patterns between long- and short-distance migrating ospreys. J Avian Biol 49:e01839. https://doi.org/10.1111/jav.01839
Moore DF (2016) Applied survival analysis using R. Springer International Publishing, Cham
Morganti M (2015) Birds facing climate change: a qualitative model for the adaptive potential of migratory behaviour. Riv Ital Ornitol 85:3–13. https://doi.org/10.4081/rio.2015.197
Mori A, Giunchi D, Rodríguez-Godoy F, Grasso R, Baldaccini NE, Baratti M (2017) Multilocus approach reveals an incipient differentiation process in the Stone-curlew, Burhinus oedicnemus around the Mediterranean basin. Cons Gen 18:197–209. https://doi.org/10.1007/s10592-016-0894-6
Nakagawa S, Johnson PCD, Schielzeth H (2017) The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R S Interface 14:20170213. https://doi.org/10.1098/rsif.2017.0213
Neufeld LR, Muthukumarana S, Fischer JD, Ray JD, Siegrist J, Fraser KC (2021) Breeding latitude is associated with the timing of nesting and migration around the annual calendar among Purple Martin (Progne subis) populations. J Ornithol 162:1009–1024. https://doi.org/10.1007/s10336-021-01894-w
Newton I (2008) The migration ecology of birds. Academic Press, Amsterdam
Newton I (2012) Obligate and facultative migration in birds: ecological aspects. J Ornithol 153:171–180. https://doi.org/10.1007/s10336-011-0765-3
Newton I, Dale L (1996) Relationship between migration and latitude among West European birds. J Anim Ecol 65:137–146. https://doi.org/10.2307/5716
Nilsson C, Klaassen RHG, Alerstam T (2013) Differences in speed and duration of bird migration between spring and autumn. Am Nat 181:837–845. https://doi.org/10.1086/670335
Patin R, Etienne M, Lebarbier E, Chamaillé-Jammes S, Benhamou S (2020) Identifying stationary phases in multivariate time series for highlighting behavioural modes and home range settlements. J Anim Ecol 89:44–56. https://doi.org/10.1111/1365-2656.13105
Pautasso M (2012) Observed impacts of climate change on terrestrial birds in Europe: an overview. Ital J Zool 79:296–314. https://doi.org/10.1080/11250003.2011.627381
Phipps WL, López-López P, Buechley ER, Oppel S, Álvarez E, Arkumarev V, Bekmansurov R, Berger-Tal O, Bermejo A, Bounas A, Alanís IC, de la Puente J, Dobrev V, Duriez O, Efrat R, Fréchet G, García J, Galán M, García-Ripollés C, Gil A, Iglesias-Lebrija JJ, Jambas J, Karyakin IV, Kobierzycki E, Kret E, Loercher F, Monteiro A, Morant Etxebarria J, Nikolov SC, Pereira J, Peške L, Ponchon C, Realinho E, Saravia V, Sekercioğlu CH, Skartsi T, Tavares J, Teodósio J, Urios V, Vallverdú N (2019) Spatial and temporal variability in migration of a soaring raptor across three continents. Front Ecol Evol. https://doi.org/10.3389/fevo.2019.00323
Podhrázský M, Musil P, Musilová Z, Zouhar J, Adam M, Závora J, Hudec K (2017) Central European greylag geese Anser anser show a shortening of migration distance and earlier spring arrival over 60 years. Ibis 159:52–365. https://doi.org/10.1111/ibi.12440
Pulido F, Berthold P (2010) Current selection for lower migratory activity will drive the evolution of residency in a migratory bird population. Proc Natl Acad Sci USA 107:7341–7346. https://doi.org/10.1073/pnas.0910361107
Pulido F, Widmer M (2005) Are long-distance migrants constrained in their evolutionary response to environmental change?: Causes of variation in the timing of autumn migration in a blackcap (S. atricapilla ) and two garden warbler (Sylvia borin) populations. Ann NY Acad Sci 1046:228–241. https://doi.org/10.1196/annals.1343.020
Pulido F, Berthold P, van Noordwijk AJ (1996) Frequency of migrants and migratory activity are genetically correlated in a bird population: evolutionary implications. Proc Natl Acad Sci USA 93:14642–14647. https://doi.org/10.1073/pnas.93.25.14642
QGIS Development Team (2020) Quantum GIS geographic information system. Open Source Geospatial Foundation Project. Version 3.4. https://qgis.org/en/site/
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/
Rappole JH, Tipton AR (1991) New harness design for attachment of radio transmitters to small passerines. J Field Ornithol 62:335–337
Rubolini D, Møller A, Rainio K, Lehikoinen E (2007) Intraspecific consistency and geographic variability in temporal trends of spring migration phenology among European bird species. Clim Res 35:135–146. https://doi.org/10.3354/cr00720
Schmaljohann H (2018) Proximate mechanisms affecting seasonal differences in migration speed of avian species. Sci Rep 8:4106. https://doi.org/10.1038/s41598-018-22421-7
Schwemmer P, Mercker M, Vanselow KH, Bocher P, Garthe S (2021) Migrating curlews on schedule: departure and arrival patterns of a long-distance migrant depend on time and breeding location rather than on wind conditions. Mov Ecol 9:9. https://doi.org/10.1186/s40462-021-00252-y
Senner NR, Verhoeven MA, Abad-Gómez JM, Alves JA, Hooijmeijer JC, Howison RA, Kentie R, Loonstra AJ, Masero JA, Rocha A, Stager M (2019) High migratory survival and highly variable migratory behavior in black-tailed godwits. Frontiers Ecol Evol 7:96. https://doi.org/10.3389/fevo.2019.00096
Shamoun-Baranes J, van Loon E, Alon D, Alpert P, Yom-Tov Y, Leshem Y (2006) Is there a connection between weather at departure sites, onset of migration and timing of soaring-bird autumn migration in Israel? Global Ecol Biogeogr 15:541–552. https://doi.org/10.1111/j.1466-8238.2006.00261.x
Shamoun-Baranes J, Leyrer J, van Loon E, Bocher P, Robin F, Meunier F, Piersma T (2010) Stochastic atmospheric assistance and the use of emergency staging sites by migrants. Proc R Soc Lond B 277:1505–1511. https://doi.org/10.1098/rspb.2009.2112
Shamoun-Baranes J, Burant JB, van Loon E, Bouten W, Camphuysen CJ (2017a) Short distance migrants travel as far as long distance migrants in lesser black-backed gulls Larus fuscus. J Avian Biol 48:49–57. https://doi.org/10.1111/jav.01229
Shamoun-Baranes J, Liechti F, Vansteelant WMG (2017b) Atmospheric conditions create freeways, detours and tailbacks for migrating birds. J Comp Physiol A 203:509–529. https://doi.org/10.1007/s00359-017-1181-9
Signer J, Fieberg J, Avgar T (2019) Animal movement tools (amt): R package for managing tracking data and conducting habitat selection analyses. Ecol Evol 9:880–890. https://doi.org/10.1002/ece3.4823
Taylor CM, Norris DR (2010) Population dynamics in migratory networks. Theor Ecol 3:65–73. https://doi.org/10.1007/s12080-009-0054-4
Tedeschi A, Sorrenti M, Bottazzo M, Spagnesi M, Telletxea I, Ibàñez R, Tormen N, De Pascalis F, Guidolin L, Rubolini D (2020) Interindividual variation and consistency of migratory behavior in the Eurasian woodcock. Curr Zool 66:155–163. https://doi.org/10.1093/cz/zoz038
Tellería JL, Fernández-López J, Fandos G (2016) Effect of climate change on mediterranean winter ranges of two migratory passerines. PLoS One 11:e0146958. https://doi.org/10.1371/journal.pone.0146958
Terrill SB, Able KP (1988) Bird migration terminology. Auk 105:205–206. https://doi.org/10.1093/auk/105.1.205
Therneau TM, Grambsch PM (2000) Modeling survival data: extending the cox model. Springer, New York
Therneau T (2020) A package for survival Analysis in R. R package version 3.2–7. https://CRAN.R-project.org/package=survival
Tinarelli R, Alessandria G, Giovacchini P, Gola L, Ientile R, Meschini A, Nissardi S, Parodi R, Perco F, Taiariol PL, Zucca C (2009) Consistenza e distribuzionedell’occhione in italia: aggiornamento al 2008. In: Giunchi D, Pollonara E, Baldaccini NE (eds) L’occhione (Burhinus oedicnemus): Biologia e conservazione di una specie di interesse comunitario - Indicazioni per la gestione del Territorio e Aree protette. Consorzio del Parco Fluviale Regionale del Taro, Collecchio, pp 45–50
Vansteelant WMG, Bouten W, Klaassen RHG, Koks BJ, Schlaich AE, van Diermen J, van Loon EE, Shamoun-Baranes J (2015) Regional and seasonal flight speeds of soaring migrants and the role of weather conditions at hourly and daily scales. J Avian Biol 46:25–39. https://doi.org/10.1111/jav.00457
Vaughan R, Jennings NV (2005) The stone curlew: Burhinus oedicnemus. Isabelline books, Falmouth
Webster MS, Marra PP, Haig SM, Bensch S, Holmes RT (2002) Links between worlds: unraveling migratory connectivity. Trends Ecol Evol 17:76–83. https://doi.org/10.1016/S0169-5347(01)02380-1
Xu F, Si Y (2019) The frost wave hypothesis: How the environment drives autumn departure of migratory waterfowl. Ecol Indic 101:1018–1025. https://doi.org/10.1016/j.ecolind.2019.02.024
Zhao M, Christie M, Coleman J, Hassell C, Gosbell K, Lisovski S, Minton C, Klaassen M (2017) Time versus energy minimization migration strategy varies with body size and season in long-distance migratory shorebirds. Mov Ecol 5:23. https://doi.org/10.1186/s40462-017-0114-0
Acknowledgements
This research was partially funded by The Taro River Regional Park, Gruppo Ornitologico Maremmano, Ekoclub Treviso, Azienda Agricola LaSelva, Department of Biology (University of Pisa). We would like to thank all the people who helped us in the field, and in particular: P. Berra, C. Caccamo, E. Pollonara, L. Passalacqua, F. Rodriguez-Godoy. The comments of two anonymous reviewers improved an earlier draft of the manuscript. All protocols performed in studies involving animals comply with the ethical standards and Italian laws on animal welfare. All procedures involving animals were approved by the Italian Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA). The data that support the findings of this study are stored on Movebank (Movebank ID: 12883793) and are available from the corresponding author upon reasonable request.
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
DG and VF are the principal authors of the work; DG suggested the initial idea for the paper and provided advice throughout the analysis and writing stages of the work along with VF; VF, GC and AC carried out data analyses and contributed to drafting the manuscript; DG, AB, CC, GD, MD, PG, AM, LP, AP contributed critically to data collection, drafting the manuscript and gave final approval.
Corresponding author
Additional information
Communicated by N. Chernetsov.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Falchi, V., Cerritelli, G., Barbon, A. et al. Inter and intra-population variability of the migratory behaviour of a short-distance partial migrant, the Eurasian Stone-curlew Burhinus oedicnemus (Charadriiformes, Burhinidae). J Ornithol 164, 85–100 (2023). https://doi.org/10.1007/s10336-022-02020-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-022-02020-0