Abstract
Sakis (genus Pithecia) are frugivorous primates with a preference for seeds that complete their diet with leaves and insects. Fruit pulp and seeds are known to have different nutritional characteristics that change during the process of ripening. The consumption of seeds can be an adaptation to changes in resource availability, as unripe seeds are a more steadily available resource than ripe pulp or young leaves. Here, we present the first study of the feeding ecology of monk sakis (Pithecia monachus). We investigated dietary composition and identified important feeding plants in a seasonally flooded forest within the Área de Conservación Regional Comunal Tamshiyacu–Tahuayo in Peruvian Amazonia. Throughout 20 months, we followed groups of monk sakis by foot and canoe and recorded 459 feeding events. Seeds were the most frequently consumed food item (49%), followed by pulp (mesocarp, pericarp or aril; 25%) and arthropods (22%). Leaves, bark, and flowers were ingested only sporadically. The importance of ripe seeds and arthropods in the diet of the monk sakis differed from other studies: we recorded the consumption of mostly ripe seeds and the share of arthropods was relatively high.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In tropical forests, fruits are an important food resource for many vertebrate species (Fleming et al. 1978). Fruits comprise several nutrient-rich components with different characteristics that change with the developmental stage and can differ between taxa (Janzen 1983). The pulp can be formed by a sugary pericarp or mesocarp, or a lipid-rich aril (Janzen 1983; Norconk 2021). Seeds additionally contain a high proportion of protein, but are usually physically and chemically protected to prevent seed predation (Janzen 1976; Norconk and Veres 2011). One vertebrate group that particularly depends on fruit as a resource are primates (Richard 1985). All South American primates (Platyrrhini) rely on fruit as part of their diet, although the proportion in the diet and which part of a fruit is used varies greatly (Rosenberger 2020).
The feeding ecology of Pithecia spp. is understudied, since sakis are difficult to habituate and very shy (Pinto et al. 2013). Of the 16 species considered by Marsh (2014), information on feeding ecology is available for only eight, and often restricted to short study periods or few observations. The majority of studies has been conducted on P. pithecia in the Guianan region. Only two studies have been conducted on sakis in seasonally flooded habitats, namely on P. rylandsiFootnote 1 (Palminteri et al. 2012) and P. isabelaFootnote 2 (Soini 1987).
Sakis are frugivores with a strong preference for seeds that made up 53–70% of their diet in previous studies (Norconk and Conklin-Brittain 2004: 63%, P. pithecia; Palminteri et al. 2012: 70%, P. rylandsi; Peres 1993: 53%, P. albicans). Seeds are usually masticated and sakis therefore act as “seed predators” (Ledogar et al. 2013; Norconk 2021). They have a highly specialized dental morphology to break open hard-shelled fruits and masticate seeds before swallowing (Kay et al. 2013; Kinzey and Norconk 1990; Norconk and Veres 2011). The consumption of seeds has been described as an adaptation to variation in fruit availability, as they are a more steadily available resource than ripe pulp (Norconk 1996; Palminteri et al. 2012). Like pulp, seeds change their chemical composition during the process of ripening of the fruit (Norconk and Conklin-Brittain 2004). For example, lipid levels increase and tannin levels decrease during seed ripening in certain plants consumed by sakis in Venezuela (Kinzey and Norconk 1993). The authors of previous studies found that > 95% of the seeds consumed by various saki species were unripe (Norconk 1996: 100%, P. pithecia; Oliveira et al. 1985: 100%, P. chrysocephala;Footnote 3 Palminteri et al. 2012: > 99%, P. rylandsi; Peres 1993: > 98%, P. albicans).
Sakis complement their diet with pulp, leaves, and insects (Happel 1982; Izawa 1975; Kinzey 1992; Ledogar et al. 2013; Norconk 1996; Norconk and Setz 2013; Oliveira et al. 1985; Peres 1993; Soini 1987). When consuming pulp, sakis prefer ripe mesocarp and arils (Charpentier et al. 2015; Norconk 1996; Peres 1993). Arthropods are not always listed as part of the diet or only represent a share of < 10% of the ingested food (Cunningham and Janson 2006: < 10%, P. pithecia; Kinzey and Norconk 1993: < 6%, P. pithecia; Peres 1993: < 1%, P. albicans). However, insect consumption can help to nutritionally complement the frugivorous diet (Rothman et al. 2014; Urbani et al. 2019).
Sakis are distributed throughout the Amazon basin and inhabit different types of forest, including high ground terra firme forest and seasonally flooded forest, white-water várzea, and black-water igapó (Marsh et al. 2018; Palminteri and Peres 2012). The former has usually an increased productivity due to the nutrient deposition during the annual flooding (Junk 1997; Melack and Forsberg 2001), although this is less distinguishable in western Amazonia (Prance 1979). Sakis use a variety of plant taxa, including many that are not as important in the diet of other platyrrhine species (Boyle et al. 2016; Norconk 2021). Plant families such as Moraceae, Fabaceae, Chrysobalanceae, Sapotaceae, Annonaceae, and Lecythidaceae have been repeatedly reported to be part of their diet (Charpentier et al. 2015; Happel 1982; Norconk 1996; Peres 1993; Setz 1993). Pitheciine biomass is positively correlated with the abundance of Eschweilera trees (Lecytidaceae) (Stevenson 2001). Inga (Fabaceae), Brosimum (Moraceae), and Pouteria (Sapotaceae) are particularly important in pitheciine diets across different habitats (Boyle et al. 2016).
Here, we present the first study on the feeding ecology of monk sakis (P. monachus). We determine the monk sakis’ dietary composition and identify important feeding plants. Based on previous studies on saki feeding ecology, we expected unripe seeds to be the most consumed food of monk sakis, followed by ripe fruit pulp. We anticipated that arthropods will be consumed occasionally, but will not make up a large proportion of the diet. Since Eschweilera, Pouteria, and Inga are present in the flooded forests and were reported to be important food items for other saki species, we expected these to also be included in the monk saki diet at our study site.
Material and methods
Study site
The Área de Conservación Regional Comunal Tamshiyacu-Tahuayo (ACRCTT) is located in northern Peruvian Amazonia in the department of Loreto. It was first established in 1991 as a Reserva Comunal, but was given added protections and expanded to its current size of 420,000 ha in 2009 (Gobierno Regional de Loreto 2009). The ACRCTT is known for its high biodiversity and is home to 13 primate species (Heymann and Aquino 1994; Valqui 2001). Before its designation as a protected area, primates, including saki monkeys, were hunted in the region (Bodmer 1995; Newing and Bodmer 2003). The area consists mostly of nonflooded terra firme habitat, but also contains seasonally flooded forests (Gobierno Regional de Loreto 2009). Mean monthly temperatures range from 25 to 27 °C and annual rainfall in the Tahuayo River Basin is ca. 3000 mm (Myster 2015).
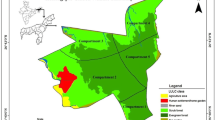
We collected the data around the Amazon Research Center (ARC) (4°22′23′′–4°24′16′′S 73°14′45′′–73°16′36′′W, Fig. 1). The ARC is located in the floodplain of the Tahuayo River, a tributary of the Amazon River, and run by the ecotourism company Amazonia Expeditions (www.perujungle.com). The Tahuayo is primarily an acidic, black-water river with a low nutrient content (Myster 2015). River water levels increase around mid-November, lower ecosystems begin to flood by the end of January, and widespread flooding is experienced from late March through to the end of May (“flood season”) (Gobierno Regional de Loreto 2009; Ronchail et al. 2018). From June to October, river levels are low (“dry season”) (Gobierno Regional de Loreto 2009). When referring to the “dry season” in our study area, we address the months with less rainfall and lower river levels, although precipitation levels are high throughout the year (Kelly et al. 2014).
Study species and groups
Our study species, the monk saki (P. monachus), is distributed in Peru and Brazil, in the interfluvium area between the Amazon/Solimões, lower-to-middle Ucayali, and lower Juruá rivers (Marsh et al. 2018). Their general shy behavior and inconspicuous coloration pose significant challenges to conducting field observations of P. monachus (Bartecki and Heymann 1987; Pinto et al. 2013). In addition to their position as a prey species for a variety of predators, these monkeys remain cryptic due to their history as a hunted population in this region (Marsh 2014).
We followed at least 12 groups of monk sakis inside the ACRCTT and in its buffer zone. Group size varied between two and seven individuals, most groups consisted of five individuals (median = mean = 5). Each group was composed of at least one adult male and one adult female (easily distinguished by their sexually dichromatic coloration), and some groups had more than two adult individuals. Juveniles were present in 11 groups. Infants were present in two groups during the study.
We searched for and followed groups of saki monkeys in the ACRCTT from July 2019 to July 2020 and from August 2021 to May 2022. The saki groups were not habituated and were typically very shy (Jackson 2016; Lehtonen 2017; Stenzel 2017). We left the ARC in the early morning, between 5 and 7 am. We alternately searched downstream (north of the ARC), upstream (southwest), and inland away from the river (southeast). We spent 931 h searching for sakis in the dry season and 1463 h in the flood season (Table 1). During the dry season, we used a canoe to move upstream or downstream and then followed by foot after locating a group of sakis. Searching inland was done by foot during the dry season. During the flood season, we used a small boat to move on the river and changed into a canoe after locating a group. Searching inland was done by canoe during the flood season. We located the sakis either visually, generally by witnessing movement, or via hearing their calls. Upon locating a group, we followed them for as long as possible. We defined contact hours as the amount of time that we were in visual contact with sakis or that we knew where the sakis were, e.g., hiding in a tree. In the latter case, we continuously watched the hiding place, to detect movement or observe feeding. If possible, we followed a group of sakis until nightfall and came back before sunrise the next morning. The total contact hours with each group varied between 4 and 90 h (total effort = 614 contact hours).
Feeding observations
We used behavioral sampling (Martin and Bateson 2007) to collect data on feeding with the help of binoculars. When we observed feeding, we noted the date, time, and number of sakis. We defined a feeding event as one individual feeding on a specific food item, independent of the time they spent feeding or the amount of food ingested. For example, if we saw a group of five sakis feeding on seeds in a tree, we recorded five feeding events, regardless of the number of seeds consumed by each individual. Accordingly, if we saw one saki feeding on several ants from the same branch, we recorded one feeding event. If a saki fed on plant parts, we recorded the Global Positioning System (GPS) location of the respective plant, marked it with tape for future recognition, and collected fruit, leaf, and bark samples for identification by botanists of the Herbarium AMAZ of the Universidad Nacional de la Amazonía Peruana in Iquitos.
We classified the type of food eaten as seed, mesocarp, pericarp, aril, leaf, bark, flower, or arthropod. For each feeding event on fruit parts, we assigned a category of ripeness: ripe, unripe, ripe + unripe (if both ripe and unripe fruits were consumed during the same feeding event), dry and NA (if we were unable to assign a category). We collected the fruits that the sakis dropped to inspect which part had been consumed and compared with intact fruits on the ground. We stored the fruits in 70% ethanol and labeled the vials with the number we assigned to the respective feeding tree. We determined ripeness preliminarily by looking at the size and color of the fruit and by opening an intact fruit of similar size to assess the stage of seed and pulp development. If possible, we took photos of the feeding plant and event (Fig. 2). Toward the end of our field study, we corrected some of our assignments of ripeness by comparing the stored fruit samples with ripe fruits collected throughout the year. If fruit pulp was consumed, we specified which part of the fruit becomes fleshy and constitutes the pulp (mesocarp, pericarp, or aril) following the genus-wise classification of pulp by Cornejo and Janovec (2010) and van Roosmalen (1985). For arthropods, we recorded the substrate from which the item was picked as stem, branch, leaf, epiphyte, or out of the air. If it was visible, we noted the class, order, or family of consumed arthropods; a higher taxonomic resolution was not possible.
Results
Diet composition
Seeds were the most frequently consumed food item (49%, n = 226), followed by arthropods (22%, n = 99), mesocarp or pericarp (14%, n = 64), mesocarp or pericarp plus seed (7%, n = 33), aril plus seed (4%, n = 20), leaves (2%, n = 10), bark (1%, n = 5), and flowers (< 1%, n = 2). The majority of feeding events on fruit items (seed, mesocarp, pericarp, and aril) came from ripe fruits (Table 2).
Food items
We observed the monk sakis feeding on 212 plants, of which 108 could be identified to genus and 72 to species level. Food plants came from 29 plant families (Table 2). The plant items consumed by the sakis were from species that grow as tree (n = 49), vine (n = 14), shrub (n = 5), palm (n = 1), or epiphyte (n = 1). Most feeding events concerned plants from Eschweilera (n = 53, Lecythidaceae) and Pouteria (n = 30, Sapotaceae). We were not able to identify the species of consumed dry seeds, bark, or flowers (Table 3).
The sakis fed on arthropods picked up from branches (n = 27), leaves (n = 24), epiphytes (n = 11), the air (n = 5), or from the main stem of a tree (n = 3). For 29 feeding events on arthropods, we did not see the substrate from which the consumed arthropod was taken. We observed the consumption of termites (n = 5), ants (n = 4), katydids (n = 4), small spiders (n = 2), and butterfly (n = 1). We were not able to classify the majority of the remaining arthropods (n = 83)
Discussion
Observing sakis is generally difficult, and the groups encountered at our study site were shy. We spent many days in the field without seeing the sakis. Especially during the dry season, it was very difficult to observe feeding, since we made noise walking on the forest floor and the sakis hid for the rest of the day when perceiving us. The number of observations is therefore relatively low for some months. Nevertheless, we were able to contribute knowledge on saki feeding ecology that can help us discover species- and habitat-specific differences.
The high proportions of seed and pulp, respectively, in the diet support the classification of monk sakis as frugivores with an emphasis on seeds. However, we could only partially confirm our expectation that unripe seeds would be the most important food source for monk sakis, since most consumed seeds were already ripe. We could confirm the expectation that ripe fruit pulp would be the second most important resource for monk sakis.
Ripe seeds can be nutritionally different from unripe seeds. For example, ripe seeds had higher lipids and free simple sugar, and lower crude protein than unripe seeds consumed by sakis in Venezuela (Norconk and Conklin-Brittain 2004). Other studies that collected data throughout different seasons found changes in the dietary composition, such as changing proportion of ripe fruit items, increased use of certain plant species, or consumption of arthropods and leaves (Kinzey and Norconk 1993; Norconk 1996; Palminteri et al. 2012; Soini 1987). These changes do not appear to be generalizable across habitats and regions. We observed the majority of feeding events (86%) during the flood season. Although we did not conduct a phenological survey, it is likely that the availability of ripe and unripe seeds changes throughout the year, with implications for saki feeding ecology. Because of the difference in the number of observations during the wet and dry seasons, we refrained from examining possible seasonal patterns in dietary composition and use of feeding plants. However, it is possible that the high proportion of ripe seeds in the diet is due to seasonal variation in resource availability.
Contrary to our expectations, we found that arthropods were consumed regularly and accounted for the third-highest number of feeding events (22%). One possible explanation for this difference with other studies might be that we used a different way of quantification of feeding observations. While we used the number of feeding events to measure the importance of a resource in the saki diet, most other studies used feeding time as a measurement (e.g., Cunningham and Janson 2006; Kinzey and Norconk 1993; Peres 1993). Since the consumption of arthropods likely takes less time than, for example, opening a fruit to feed on the seed, the relative importance of arthropods in the sakis’ diet might be much higher when determined based on event instead of feeding time. Nevertheless, we clearly showed that arthropods are an important resource for monk sakis. Another possible explanation is the time period and habitat our study took place in. In seasonally flooded forests, arthropods might be more readily available for sakis in the flood season, since some taxa move into higher forest strata during flooding (Adis 1992; Irmler 1979; Souza et al. 2020). The authors of studies on arthropod diversity from saki feces suggest that the importance of arthropods in their diet might be underestimated due to the difficulties of observing sakis in the wild (Jesus et al. 2022; Pickett et al. 2012).
We identified 70 species of feeding plants from 29 families. The most used plant family was Lecythidaceae, with seeds from Eschweilera being the most consumed. Eschweilera has been shown to be of special importance to sakis (Stevenson 2001) and its seeds were reported to be the most consumed by P. isabela in a seasonally flooded forest in western Amazonia (Soini 1987). The increased use of Mauritia flexuosa during some months was also described by Palminteri et al. (2012). Not all plant genera found to be of special importance for pitheciines in a meta study (Boyle et al. 2016) were recorded in our observations: seeds and pericarp of Pouteria were the second most consumed food items, but Inga was less important (n = 5), and Brosimum not recorded at all. Also, the plant families Moraceae and Annonaceae that were reported as important in previous studies, were not present in our observations. However, we were not able to identify all plant items the sakis fed on. The high diversity of plants at our study site and supra-annual patterns of fruiting make it likely that we only recorded a fraction of the plants actually used by monk sakis.
Altogether, we found monk sakis in a seasonally flooded forest in western Amazonia to have a similar feeding ecology to other saki species in distinct habitats. However, we also found some differences. Seeds were more often consumed in ripe state and arthropods were more important than we expected based on the literature. Monk sakis might have a slightly different feeding strategy than other saki species, although we suspect that these dietary differences result mainly from environmental conditions. It would be instrumental to study adjacent monk saki populations that are restricted to nonflooded forest. Research is needed to better understand saki feeding ecology, especially in western Amazonia.
Data availability
Not applicable.
References
Adis J (1992) How to survive six months in a flooded soil: strategies in chilopoda and symphyla from central Amazonian floodplains. Stud Neotrop Fauna Environ 27:117–129. https://doi.org/10.1080/01650529209360872
Bartecki U, Heymann EW (1987) Über Schweifaffen in Peru. Zeitschrift Des Kölner Zoo 30:79–92
Bodmer RE (1995) Managing Amazonian wildlife: Biological correlates of game choice by detribalized hunters. Ecol Appl 5:872–877. https://doi.org/10.2307/2269338
Boyle SA, Thompson CL, Deluycker A et al. (2016) Geographic comparison of plant genera used in frugivory among the pitheciids Cacajao, Callicebus, Chiropotes, and Pithecia. Am J Primat 78:493–506. https://doi.org/10.1002/ajp.22422
Charpentier E, Garcia G, Aquino R (2015) Uso y competición por plantas alimenticias entre Pithecia aequatorialis (Primates: Pitheciidae) y otros animales en la Amazonía peruana. Rev Peru Biol 22:225–232. https://doi.org/10.15381/rpb.v22i2.11356
Cornejo F, Janovec J (2010) Seeds of Amazonian plants. Princeton University Press. https://doi.org/10.1515/9781400834488
Cunningham EP, Janson CH (2006) Pithecia pithecia’s behavioral response to decreasing fruit abundance. Am J Primatol 68:491–497. https://doi.org/10.1002/ajp.20244
Fleming TH, Breitwisch R, Whitesides GH (1987) Patterns of tropical vertebrate frugivore diversity. Annu Rev Ecol Syst 18:91–109. https://doi.org/10.1146/annurev.es.18.110187.000515
Gobierno Regional de Loreto (2009) Plan maestro del Área de Conservación regional comunal tamshiyacu-tahuayo. Procrel, Loreto Peru
Happel RE (1982) Ecology of Pithecia hirsuta in Peru. J Hum Evol 11:581–590. https://doi.org/10.1016/S0047-2484(82)80005-5
Heymann EW, Aquino R (1994) Exploraciones primatológicas en las quebradas Blanco, Blanquillo y Tangarana (Río Tahuayo, Amazonía peruana). Folia Amazon 6:135–149. https://doi.org/10.24841/fa.v6i1-2.252
Irmler U (1979) Abundance fluctuations and habitat changes of soil beetles in central Amazonian inundation forests (Coleoptera: Carabidae, Staphylinidae). Stud Neotrop Fauna Environ 14:1–16. https://doi.org/10.1080/01650527909360544
Izawa K (1975) Foods and feeding behavior of monkeys in the upper Amazon basin. Primates 16:295–316. https://doi.org/10.1007/BF02381557
Jackson R (2016) Habitat stratification of Pithecia species in the Área de Conservación Regional Comunal Tamshiyacu Tahuayo in the northeastern Peruvian Amazon. Master thesis, Winthrop University
Janzen DH (1976) The ecology and evolutionary biology of seed chemistry as relates to seed predation. In: Harborne JB (ed) Biochemical aspects of plant and animal coevolution. Academic Press, London, pp 163–206
Janzen DH (1983) Physiological ecology of fruits and their seeds. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology III: responses to the chemical and biological environment, 1st edn. Springer Science and Business Media, Berlin, pp 625–655
Jesus AS, Castilla Torres RI, Quadros JC, Cruz AN, Valsecchi J, El Bizri HR, Mayor P (2022) Are larger primates less faunivorous? Consumption of arthropods by Amazonian primates does not fulfil the Jarman-Bell and Kay models. Acta Amazon 52:208–217. https://doi.org/10.1590/1809-4392202200842
Junk WJ (1997) General aspects of floodplain ecology with special reference to Amazonian floodplains. In: Junk WJ (ed) The central Amazon floodplain: Ecology of a pulsing system, 1st editon. Springer, Berlin Heidelberg, pp 3–20
Kay RF, Meldrum DJ, Takai M (2013) Pitheciidae and other platyrrhine seed predators. In: Veiga LM, Barnett AA, Ferrari SF, Norconk MA (eds) Evolutionary biology and conservation of titis, sakis and uacaris, 1st edn. Cambridge University Press, Cambridge, pp 3–12
Kelly TJ, Baird AJ, Roucoux KH, Baker TR, Honorio Coronado EN, Ríos M, Lawson IT (2014) The high hydraulic conductivity of three wooded tropical peat swamps in northeast Peru: Measurements and implications for hydrological function. Hydrol Process 28:3373–3387. https://doi.org/10.1002/hyp.9884
Kinzey WG (1992) Dietary and dental adaptations in the Pitheciinae. Am J Phys Anthropol 88:499–514. https://doi.org/10.1002/ajpa.1330880406
Kinzey WG, Norconk MA (1990) Hardness as a basis of fruit choice in two sympatric primates. Am J Phys Anthropol 81:5–15. https://doi.org/10.1002/ajpa.1330810103
Kinzey WG, Norconk MA (1993) Physical and chemical properties of fruit and seeds eaten by Pithecia and Chiropotes in Surinam and Venezuela. Int J Primatol 14:207–227. https://doi.org/10.1007/BF02192632
Ledogar JA, Winchester JM, St. Clair EM, Boyer DM, (2013) Diet and dental topography in pitheciine seed predators. Am J Phys Anthropol 150:107–121. https://doi.org/10.1002/ajpa.22181
Lehtonen E (2017) The behavioural ecology of a potentially undescribed morph of saki monkey (genus Pithecia) in a highly diverse primate community. Master thesis, Uppsala Universitet
Marsh L (2014) A taxonomic revision of the saki monkeys, Pithecia Desmarest, 1804. Neotrop Primates 21:1–165. https://doi.org/10.1896/044.021.0101
Marsh L, Heymann E, Ravetta AL, Moura EF (2018) Pithecia monachus. In: IUCN Red List of Threatened Species. https://www.iucnredlist.org/species/70609726/17971958. Accessed 15 Sep 2022
Martin P, Bateson M (2007) Measuring behaviour: an introductory guide, 3rd editon. Cambridge University Press
Melack JM, Forsberg BR (2001) Biogeochemistry of Amazon floodplain. In: McClain ME, Victoria R, Richey JE (eds) The biogeochemistry of the Amazon basin, 1st editon. Oxford University Press, Oxford, pp 235–274
Myster RW (2015) Flooding × tree fall gap interactive effects on blackwater forest floristics and physical structure in the Peruvian Amazon. J Plant Interact 10:126–131. https://doi.org/10.1080/17429145.2015.1029018
Newing H, Bodmer R (2003) Collaborative wildlife management and adaptation to change: the tamshiyacu-tahuayo communal reserve, Peru. Nomad People 7:110–122. https://doi.org/10.3167/082279403782088859
Norconk MA (1996). In: Norconk MA, Rosenberger AL, Garber PA (eds) Adaptive radiations of neotropical primates, 1st editon. Springer, New York, pp 403–423
Norconk MA (2021) Historical antecedents and recent innovations in pitheciid (titi, saki and uakari) feeding ecology. Am J Primatol 83:e23177. https://doi.org/10.1002/ajp.23177
Norconk MA, Conklin-Brittain NL (2004) Variation on frugivory: the diet of venezuelan white-faced sakis. Int J Primatol 25:1–26. https://doi.org/10.1023/B:IJOP.0000014642.68751.ed
Norconk MA, Setz EZ (2013) Ecology and behavior of saki monkeys (genus Pithecia). In: Veiga LM, Barnett AA, Ferrari SF, Norconk MA (eds) Evolutionary biology and conservation of titis, sakis and uacaris, 1st editon. Cambridge University Press, Cambridge, pp 262–271
Norconk MA, Veres M (2011) Physical properties of fruit and seeds ingested by primate seed predators with emphasis on sakis and bearded sakis. Anat Rec 294:2092–2111. https://doi.org/10.1002/ar.21506
Oliveira JMS, Lima MGD, Bonvincino C, Ayres JM, Fleagle JG (1985) Preliminary notes on the ecology and behavior of the Guianan Saki (Pithecia pithecia, Linnaeus 1766; Cebidae, Primate). Acta Amazon 15:249–264. https://doi.org/10.1590/1809-43921985152263
Palminteri S, Peres CA (2012) Habitat selection and use of space by bald-faced sakis (Pithecia irrorata) in southwestern Amazonia: lessons from a multiyear, multigroup study. Int J Primatol 33:401–417. https://doi.org/10.1007/s10764-011-9573-0
Palminteri S, Powell GV, Peres CA (2012) Advantages of granivory in seasonal environments: feeding ecology of an arboreal seed predator in Amazonian forests. Oikos 121:1896–1904. https://doi.org/10.1111/j.1600-0706.2012.20456.x
Peres CA (1993) Notes on the ecology of buffy saki monkeys (Pithecia albicans, Gray 1860): a canopy seed-predator. Am J Primatol 31:129–140. https://doi.org/10.1002/ajp.1350310205
Pickett SB, Bergey CM, Di Fiore A (2012) A metagenomic study of primate insect diet diversity. Am J Primatol 74:622–631. https://doi.org/10.1002/ajp.22014
Pinto L, Barnett A, Bezerra B, Boubli JP, Bowler M, Cardoso N, Caselli C, Ospina Rodríguez MJ, Rodrigues Santos R, Setz E, Veiga LM (2013) Why we know so little: The challenges of field work on pitheciines. In: Veiga LM, Barnett AA, Ferrari SF, Norconk MA (eds) Evolutionary biology and conservation of titis, sakis and uacaris, 1st edn. Cambridge University Press, Cambridge, pp 145–150
Prance GT (1979) Notes on the vegetation of Amazonia III. The terminology of Amazonian forest types subject to inundation. Brittonia 31:26–38. https://doi.org/10.2307/2806669
Richard AF (1985) Primates in nature. W.H.Freeman and Company, New York
Ronchail J, Espinoza JC, Drapeau G, Sabot M, Cochonneau G, Schor T (2018) The flood recession period in western Amazonia and its variability during the 1985–2015 period. J Hydrol Reg Stud 15:16–30. https://doi.org/10.1016/j.ejrh.2017.11.008
Rosenberger AL (2020) New world monkeys. Princeton University Press, Princeton, The evolutionary odyssey. https://doi.org/10.1515/9780691189512
Rothman JM, Raubenheimer D, Bryer MAH, Takahashi M, Gilbert CC (2014) Nutritional contributions of insects to primate diets: Implications for primate evolution. J Hum Evol 71:59–69. https://doi.org/10.1016/j.jhevol.2014.02.016
Setz EZF (1993) Ecologia alimentar de um grupo de parauacus (Pithecia pithecia chrysocephala) em um fragmento florestal na Amazônia Central. PhD Thesis, Universidade Estadual de Campinas
Soini P (1987) La dieta del mono huapo (Pithecia monachus). Informe De Pacaya: Investigaciones En La Estación Biológica Cahuana 25:1–12
Souza CS, Baronio GJ, Weirich CE, Oliveira AF, dos Santos Ferreira BH, Arruda R, Aoki C (2020) Ants climb plants because they cannot swim: ant presence on flowers during the flood season reduces the frequency of floral visitors. Ecol Entomol 45:1337–1345. https://doi.org/10.1111/een.12917
Stenzel C (2017) Anti-predator behavior and discriminative abilities: Playback experiments with free-ranging equatorial saki monkeys (Pithecia aequatorialis) in the Peruvian Amazon. Master thesis, Winthrop University
Stevenson PR (2001) The relationship between fruit production and primate abundance in neotropical communities. Biol J Linn Soc 72:161–178. https://doi.org/10.1111/j.1095-8312.2001.tb01307.x
Urbani B, Norconk MA, Urbani F (2019) Geofagia e ingestión de ortópteros y avisperos por monos viudo (Pithecia pithecia) en Guri, sureste de Venezuela. In: Urbani B, Ceballos-Mago N (eds) La primatología en Venezuela, 1st edn. Editorial Equinoccio, Caracas, pp 241–270
Valqui MH (2001) Mammal diversity and ecology of terrestrial small rodents in western Amazonia. PhD thesis, University of Florida, Gainesville
van Roosmalen MGM (1985) Fruits of the guianan flora. Institute of Systematic Botany, Utrecht
Acknowledgements
We would like to thank Paul Beaver for providing the research opportunity and valuable advice throughout all stages of this project. MG and ALM are grateful for the support by the staff of Amazonia Expeditions, especially to Aladino Hidalgo and Aladino Hidalgo, Jr. We thank José Eduardo Serrano-Villavicencio and Edgardo M. Rengifo for their help in obtaining the research permit; and Cesar Grández Ríos and the team from Herbarium AMAZ of the Universidad Nacional de la Amazonía Peruana in Iquitos for identifying the plant species. Júlio César Bicca-Marques, Marilyn Norconk, and an anonymous reviewer provided comments that significantly improved the manuscript. This study received funding from Amazonia Expeditions. MG was employed by Amazon Research Center & Company S.A.C. during study conception and data collection. MG and KH were supported by the Eva Mayr-Stihl Foundation. The study was authorized by the Servicio Nacional Forestal y de Fauna Silvestre Peru under the permit N° AUT-IFS-2020-031.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MG, KH, and EWH contributed to the study conception and design. Data collection and analysis were performed by MG and ALM. The first draft of the manuscript was written by MG and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Gottstein, M., Morris, A.L., Heer, K. et al. Feeding ecology of monk sakis (Pithecia monachus) in a seasonally flooded forest in western Amazonia. Primates 64, 527–537 (2023). https://doi.org/10.1007/s10329-023-01074-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-023-01074-9