Abstract
Polycyclic aromatic hydrocarbons (PAHs) in atmospheric particulate matter have adverse effects on human health, yet total PAH concentrations should overestimate the toxicity compared to the bioavailable amount of PAHs. To explore this hypothesis, we measured PAHs oral bioavailability in vitro in particulate matter with aerodynamic diameter lower than 10 µm (PM10) using a test that mimics the human digestive system. This assay combines the use of simulated gastrointestinal fluids and a dialysis membrane to simulate intestinal absorption. Results show that oral PAH bioavailability was below 5%, with fluorene, anthracene, acenaphthene and phenanthrene as the most bioavailable PAHs. Data suggest no carcinogenic risk of oral bioavailable PM10-bound PAHs following a health risk assessment via inhalation-ingestion by using benzo(a)pyrene-equivalent carcinogenic concentration and hazard indexes. To our best knowledge, this is the first research study of in vitro oral bioavailability estimation of PM10-associated PAHs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inhalation of atmospheric particulate matter represents a significant exposure pathway for humans. Several studies have associated particulate matter exposure with an increase in morbidity and mortality due to its negative outcomes on human health, being particulate matter with aerodynamic diameter ≤ 10 µm (PM10) and ≤ 2.5 μm (PM2.5) the most studied particulate matter fractions due to their potential to penetrate and deposit in different regions of respiratory system after inhalation (Burnett et al. 2018). Also, particle-bound pollutants are thought to have a key role in adverse health effects of particulate matter, which can be get into the body by means of inhalation (Galvão et al. 2018). Among PM-bound pollutants, polycyclic aromatic hydrocarbons (PAHs) are a well-known group of compounds widely studied because of their high ubiquity in environmental samples and their adverse effects on human health (Abdel-Shafy and Mansour 2016). PAHs occurrence in particulate matter fractions has been attributed mainly as a result of organic materials combustion (Mesquita et al. 2014). Nevertheless, considering total particle-bound pollutant concentrations provides an overestimation of their toxicity and a non-realistic evaluation of the exposition and health risk assessment. In order to improve risk assessment, it is also important to consider how pollutants are assimilated by exposed people. In recent years, scientific community has been focused on the pollutant fraction that can be dissolved in biological fluids (i.e. bioaccessible fraction) and the fraction that diffuse across biological membranes to reach systemic circulation once dissolved (i.e. bioavailable fraction), to address a more realistic evaluation. For this purpose, different methodologies have been described in the literature to perform bioaccessibility/bioavailability estimation, distinguishing between in vivo methods and in vitro methods (using synthetic body fluids and physiological human conditions) to assess the maximum concentration of pollutant that can be dissolved (bioaccessible fraction), in combination with absorption sinks, semi-permeable dialysis membranes or cultured cells models to assess fraction of pollutant bioavailable (i.e. enters the blood stream) (Miller et al. 1981; Ruby et al. 1999; Kastury et al. 2017). In vivo methods may be unethical, expensive and impractical for large scale testing (Nemmar et al., 2013). On contrary, in vitro assays offer an attractive alternative to in vivo assays, due to the simplicity, rapidity, easy-control, low cost, high precision and reproducibility (Kastury et al. 2017). However, there is standardization shortage, which results in important differences in body fluid compositions and physiological human extraction conditions.
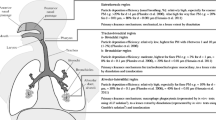
After air inhalation during breathing, particles come into the body and are deposited in different regions of respiratory depending on the particle properties (mainly the size) (Kastury et al. 2017). Concerning the deposition, particles with aerodynamic diameter < 2.5 µm are more likely to reach the alveolar region of lungs (where pollutants might be dissolved into lung fluids and absorbed to blood (inhalation bioavailability), while particles with aerodynamic diameter in the range of 2.5–10 µm are deposited in tracheobronchial region, which could be subjected to mucociliary clearance and expelled by coughing/sneezing or ingested. Those particles conducted to the gastrointestinal tract could be an important absorption pathway of particle-bound pollutants (oral bioaccessibility/bioavailability). Several oral in vitro bioaccessibility approaches have been applied mainly for metal(oid)s in particulate matter (Falta et al. 2008; Mukhtar and Limbeck 2011; Uzu et al. 2011; Hu et al. 2012; Puls et al. 2012; Sysalová et al. 2014; Mohr et al. 2017; Kastury et al. 2018; Nie et al. 2018; Gao et al. 2018) and indoor/outdoor dust (Turner and Simmonds 2006; Turner and Ip 2007; Turner and Hefzi 2010; Turner 2011; Boisa et al. 2014; Bradham et al. 2014; Huang et al. 2014a, b; Patinha et al. 2015; Goix et al. 2016; Padoan et al. 2017) samples. However, in vitro oral bioaccessibility studies of organic pollutants in particulate matter were not found in the literature, while there were some studies applied to indoor/outdoor dust and soils for organic compounds, i.e. PAHs (Tang et al. 2006; Kang et al. 2011; Collins et al. 2013; Li et al. 2015; Kademoglou et al. 2018a), organophosphate flame retardants (Quintana et al. 2017), semi-volatile organic compounds (Raffy et al. 2018), polychlorinated biphenyls (Wang et al. 2013) and polybrominated diphenyl ethers (Yu et al. 2011; Kademoglou et al. 2018b; Gao et al. 2019b). The stringent analytical requirements (target pollutants enrichment, due to the low levels; and pollutant isolation from the synthetic fluids matrix) for organic pollutant quantification in bioaccessible/bioavailable fractions might explain the scare information. Regarding in vitro oral bioavailable studies, in vitro physiologically based extraction test methodology developed by Miller et al. (Miller et al. 1981) was extensively used because of showing well correlations for metals with in vivo studies. According to the literature, some researches have based on this methodology (with some modifications) to assess metal(oid)s oral bioavailability in foods (Wolfgor et al. 2002; Haro-Vicente et al. 2006; Moreda-Piñeiro et al. 2013, 2015a), while it was recently applied to PM10 samples (Moreda–Piñeiro et al. 2019).
Thus, the main objective of this research is the novel application and validation of an in vitro physiologically based extraction test, based on the use of a dialysis membrane to simulate the intestinal absorption (Miller et al. 1981; Moreda–Piñeiro et al. 2019), to assess the oral bioavailability of PAHs in PM10. Furthermore, the exposure and health risk assessment based on total and oral bioavailable contents of PAHs obtained were estimated and discussed.
Experimental
PM10 sample collection
An automatic high-volume sampler DIGITEL DHA-80 (Hegnau, Switzerland) was used to samples collection at an urban site of A Coruña city (northwest of Spain). Description of the sampling site was previously reported (Moreda-Piñeiro et al. 2015b). Atmospheric particulate matter was collected on Ahlstrom Munksjö MK360 (Falun, Sweden) quartz fibre filters, 150 mm of diameter, at 30 m3 h−1 for 24 h, 00:00 to 23:59 (Coordinated Universal Time), from 1st January to 31st December 2015 according to the European Norm 12,341 (EN 12,341:2015) (UNE 2015).
Due to the low PAHs levels expected in oral bioavailable fractions and limited PM10 filter amounts, monthly pooled samples (total filter area of 8.5 cm2) were made by combining one portion (0.28 cm2) of each daily PM10 sample collected during each month. Monthly PM10 mass ranged from 0.69 mg (September) to 1.0 mg (March).
In vitro oral bioavailability procedure
In vitro oral PAHs bioavailable fractions were obtained following the methodology as described in the Supplementary Material.
Total and oral bioavailable PAHs extraction and quantification procedures
Procedures for PAHs extraction and quantification in bioavailable fractions and in PM10 samples are summarized in Supplementary Material; details for validation procedures (Tables S1-3) are also shown in this section.
Exposure and health risk assessment and benzo(a)pyrene-equivalent carcinogenicity
Human health risk assessment of target PM10-associated PAHs via inhalation-ingestion was performed basing on United States Environmental Protection Agency’s (USEPA) Inhalation Dosimetry Methodology (as described in Supplementary Material) considering three scenarios (residents in the studied area (scenario I); residents working outside the studied area (scenario II); and workers not living in the area (scenario III), Table S4) (USEPA 2009, 2014; Hernández-Pellón et al. 2018).
Carcinogenicity of PM10-associated PAHs relatively to benzo(a)pyrene-equivalent toxic concentration was calculated as described in Supplementary Material (USEPA 1993).
Results and discussion
PM10 concentration levels and total PAH content
The daily PM10 mass concentrations ranged between 3 and 57 µg m−3. There is only two samples that exceed the daily limit value of 50 µg m−3 set by the European Commission, which cannot exceed more than 35 times per year (Directive 2008/50/EC) (EU 2008). Despite this, the annual mean PM10 concentration corresponds to 20 µg m−3, which is below the annual mean value of 40 µg m−3 set in the European Directive. The monthly average PM10 mass concentrations ranged between 17 µg m−3 (September) and 26 µg m−3 (March).
Monthly average PAHs concentrations obtained are shown in Figure S1, while detailed information is given in supporting material (Table S5). The contribution of each PAH to the Σ16PAHs (Figure S1) was dominated by benzo(e)pyrene (19–27%), chrysene (13–19%), benzo(b)fluoranthene + benzo(j)fluoranthene (8–20%) and indene(1,2,3-cd)pyrene (9–15%), while low contribution was achieved for volatile PAHs (mainly dominating the gas phase), such as naphthalene, anthracene, acenaphthene and fluorene (< 2%). The carcinogenic PAHs (benzo(b)fluoranthene + benzo(j)fluoranthene, chrysene, indene(1,2,3-cd)pyrene, benzo(a)pyrene, benzo(k)fluoranthene, benzo(a)anthracene and dibenzo(a,h)anthracene) concentrations (ΣcPAHs) and non-carcinogenic PAHs (acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(e)pyrene, benzo(ghi)perylene) concentrations (ΣncPAHs) represented 45–59% and 41–55% of PAHs, respectively. The average annual concentration of benzo(a)pyrene (628 pg m−3) is lower than the annual limit set in 1000 pg m−3 according to European Directive 2004/107/EC (EU 2004). Monthly average PAHs contents demonstrated high variation from month to month (Table S5 and Figure S1), due to the heterogeneousness of atmospheric particles (PM10 sources). In general, PAHs concentrations found in samples are according previous studies reported near the area. Regarding season variability, monthly average PAH profiles exhibited during cold season are higher than during the hot season due to the increasing emissions from domestic heating. Moreover, presence of heavier PAHs such as benzo(a)anthracene, benzofluoranthenes (benzo(k)fluoranthene, benzo(j)fluoranthene and benzo(b)fluoranthene), benzopyrenes (benzo(a)pyrene and benzo(e)pyrene) and indene(1,2,3-cd)pyrene might be associated with combustion sources such as vehicular emissions and biomass combustion (López-Mahía et al. 2003).
In vitro oral PAH bioavailable concentrations and oral bioavailability ratios in PM10
Monthly average PAHs concentrations (pg m−3) found were very low, within the ranges phenanthrene (< 0.21–3.4) > chrysene (0.25–2.6) > pyrene (< 0.05–2.9) > fluorene (< 0.5–2.2) > fluoranthene (< 0.1–3.6) > benzo(e)pyrene (< 0.02–1.3) > acenaphthene (< 0.10–0.97) > naphthalene (< 0.11–0.95) > benzo(a)anthracene (< 0.03–0.27) > benzo(k)fluoranthene (< 0.02–0.14). Oral bioavailable concentrations of anthracene, benzo(b)fluoranthene + benzo(j)fluoranthene, benzo(a)pyrene, benzo(ghi)perylene, dibenzo(a,h)anthracene and indene(1,2,3-cd)pyrene were found lower than limits of quantification. Furthermore, oral bioavailable carcinogenic PAHs plus non-carcinogenic PAHs concentrations represented 17–35% and 64–82% of the total oral bioavailable PAHs content, respectively. Results of monthly average PAH oral bioavailable concentrations as well as total carcinogenic PAHs and total non-carcinogenic PAHs are given in Table S6, while oral monthly average PAH bioavailability ratios (Bav, expressed as percentage), calculated using the following formula, are shown in Fig. 1:
where [PAH]oral bioavailable fraction is the monthly average PAH concentration obtained after in vitro oral bioavailability (pg m−3) and [PAH]total content is the total monthly average PAH concentration (pg m−3).
Monthly averaged oral bioavailability of polycyclic aromatic hydrocarbons (PAH), showing the inverse relationship between the PAHs bioavailability ratios and their hydrophobicity. Acenaphthene, Ace; fluorene, Fl; phenanthrene, Phe; anthracene, Ant; fluoranthene, Ft; pyrene, Pyr; benzo(a)anthracene, BaA; chrysene, Chry; benzo(e)pyrene, BeP; benzo(b)fluoranthene + benzo(j)fluoranthene, BbF + BjF; benzo(k)fluoranthene, BkF; benzo(a)pyrene, BaP; dibenz(a,h)anthracene, DBahA; benzo(g,h,i)perylene, BghiP; and indeno(1,2,3-cd)pyrene, IP)(acenaphthene, Ace; fluorene, Fl; phenanthrene, Phe; anthracene, Ant; fluoranthene, Ft; pyrene, Pyr; benzo(a)anthracene, BaA; chrysene, Chry; benzo(e)pyrene, BeP; benzo(b)fluoranthene + benzo(j)fluoranthene, BbF + BjF; benzo(k)fluoranthene, BkF; benzo(a)pyrene, BaP; dibenz(a,h)anthracene, DBahA; benzo(g,h,i)perylene, BghiP; and indeno(1,2,3-cd)pyrene, IP
Oral PAHs bioavailability percentages obtained for each PAH were below 5% (Fig. 1). As shown in the graph, fluorene offered the highest monthly average oral bioavailability ratio ± standard deviation (2.8 ± 2.1%), followed by anthracene (1.4 ± 1.1%), acenaphthene (1.2 ± 1.7%) and phenanthrene (0.71 ± 0.67%). Regarding fluoranthene, pyrene, benzo(a)anthracene, chrysene, benzo(e)pyrene, benzo(b)fluoranthene + benzo(j)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, dibenzo(a.h)anthracene, benzo(ghi)perylene and indene(1,2,3-cd)pyrene, bioavailability percentages obtained were below 0.5%. Naphthalene dialyzability percentages were not assessed because naphthalene concentrations in most of the dialysate fractions extracts were lower than limit of quantification. The low percentages obtained show that a low amount of PM10-bound inhaled PAHs could be available in the blood through intestinal absorption. Although 4–6 ring-condensed PAHs (benzo(e)pyrene, chrysene, indene(1,2,3-cd)pyrene and benzo(b)fluoranthene + benzo(j)fluoranthene) were the most predominant in monthly PM10 samples (considering total concentrations), the highest bioavailability ratios were obtained for 3 ring-condensed PAHs (fluorene, anthracene, acenaphthene and phenanthrene). This trend might be attributed to an increasing hydrophobicity (as increasing the condensed ring number) that obstructs PAHs mobilization from PM10 to biological simulated fluids (aqueous based), as published for other simulated fluids (Li et al. 2019; Sánchez-Piñero et al. 2021). Furthermore, the different chemical composition (different particulate matter sources) of PM10 samples could explain the high standard deviation of monthly average oral bioavailability ratios.
There are no oral PM10-bounded PAHs bioavailability data in the literature. Therefore, oral bioavailability ratios obtained in this study will be compared with oral bioaccessible ratios found in the literature. Although there are different bioaccessibility protocols reported in the literature to assess oral bioaccessible fractions of PAHs in soil (Tang et al. 2006; Collins et al. 2013; Li et al. 2015; Gao et al. 2019a) and indoor dust (Kang et al. 2011) samples, oral bioavailable ratios shown in Fig. 1 are lower than those previously published for bioaccessibility ratios. Oral PAHs bioaccessibility ratios in air conditioning filter dust, ranging from 2 to 17% (Kang et al. 2011), and soil, ranging from 3.9 to 54.9% (Tang et al. 2006), were assessed using a physiologically based extraction test. Within this context, the lower values obtained in this study might be attributed to the inclusion of a dialysis membrane filled with aqueous buffer solution, which hinders the capture of PM10-released PAHs (Fig. 1). Furthermore, several in vitro bioaccessibility approaches for organic compounds involved the use of sorption sinks (i.e. silicone sheet (Gao et al. 2019b), Tenax TA® absorption sink (Li et al. 2015; Kademoglou et al. 2018b) and activated carbon sink (Collins et al. 2013)) to capture pollutants as the same time they are released from matrix. The addition of a silicone sheet as sorption skin was observed to increase PAH bioaccessibility ratios up 44 − 67% (Gao et al. 2019b). In addition, activated carbon and Tenax TA® were used as sorption skins, increasing bioaccessibility ratios up to 16–31% in contaminated soils (Li et al. 2015) and up to 15–75% in spiked soil samples (Collins et al. 2013). According to the results obtained in the present study, the values are even much lower than those reported when including a sorption sink. Nevertheless, the inclusion of a sorption sink could create a sorption gradient higher than reality that might lead to overestimation.
Human health risk assessment of PM10-bound PAHs
Human health risk assessment was performed based on the inhalation dosimetry methodology for different scenarios and benzo(a)pyrene-equivalent carcinogenic concentrations approach, both models proposed by USEPA. Total and oral bioavailable PAH concentrations in monthly pooled PM10 samples were used for health risk assessment. Limit of quantification/2 criterion was considered for concentration values below limits of quantification. Exposure concentrations, carcinogenic risk and carcinogenic hazard index were evaluated for PM10-associated PAHs in the area studied. Maximum values of exposure concentrations estimated for each scenario are shown in Table 1, while carcinogenic risk and carcinogenic hazard index maximum values are shown in Table 2. Regarding exposure concentrations values, benzo(e)pyrene and fluoranthene are the most exposed PAHs for all scenarios considering total and oral bioavailable PAH concentrations, respectively. The highest exposure concentrations values were obtained for the most conservative scenario (scenario I for adults), with values of 2790 (total PAH concentrations) and 3.4 (oral bioavailable PAH concentrations) pg m−3 for benzo(e)pyrene and fluoranthene, respectively. Furthermore, estimated carcinogenic risks do not exceed the acceptable threshold lifetime cancer risk 1.0 × 10−6 set by USEPA, being benzo(a)pyrene the PAH that showed the highest values for all scenarios considering both total and oral bioavailable PAH concentrations. Maximum carcinogenic hazard index values estimated for each scenario do not exceed the cumulative cancer risk set in 1.0 × 10–4, showing values of 3.3 × 10–7 (total PAH concentrations) and 2.1 × 10–11 (oral bioavailable PAH concentrations) for scenario I (adults). Then, no carcinogenic risk was found for PM10-associated PAHs in the studied area.
Health risk assessment based on benzo(a)pyrene-equivalent carcinogenic concentrations was also performed. Benzo(a)pyrene-equivalent values obtained varied from 2100 to 5610 pg m−3 and 0.17 to 1.4 pg m−3 considering total and oral bioavailable PAH concentrations, respectively. Benzo(a)pyrene-equivalent concentrations exceeded the guideline value of 1.0 ng m−3 for benzo(a)pyrene (Directive 2004/107/EC) (EU 2004) when total PAH concentrations were used. However, no exceedances of benzo(a)pyrene limit value were observed when oral bioavailable PAH concentrations were used. In addition, lifetime cancer risk values estimated considering total and oral bioavailable PAH concentrations are shown in Fig. 2a–b, respectively. Attending to the graphs, all monthly average PM10 samples exceeded the acceptable carcinogenic risk limit of 1.0 × 10–6 for individual carcinogens when total PAH concentrations are considered (Fig. 2a). Nevertheless, none of them exceeded the cumulative cancer risk (1.0 × 10–4) for multiple carcinogens (USEPA 2001; Davie-Martin et al. 2017). Furthermore, lifetime risk calculated basing on oral bioavailable concentrations were much lower than the acceptable carcinogenic risk limit of 1.0 × 10–6 (Fig. 2b). Additionally, benzo(a)pyrene-equivalent concentrations approach offered slightly higher values than carcinogenic hazard index as a result of considering a high number of PAHs in risk calculation.
Lifetime cancer risks obtained for each month using benzo(a)pyrene-equivalent carcinogenic concentration approach, calculated using total PAHs contents in PM10 a and oral bioavailable concentrations b. The red line shows the acceptable limit of lifetime cancer risk, i.e. 1.0 × 10–6 as established by United States Environmental Protection Agency (USEPA). Note that the acceptable lifetime cancer risk limit was exceeded during all the months when total PAHs concentrations are used in contrast to oral bioavailable concentrations, which shows the overestimation in human health risk assessments when total concentrations are considered. PAH: polycyclic aromatic hydrocarbons
Estimated life cancer risk values assessed using total PAH concentrations are higher (by a factor of 104) than values achieved using oral bioavailable PAH concentrations. This great difference could be resulted in an overestimation of cancer risks when considering total concentrations instead of using bioaccessible/bioavailable concentrations. Within this context, many authors have already reported the necessity to consider bioaccessibilities and bioavailabilities on exposure and health risk models, providing a more realistic assessment (Huang et al. 2014b, 2018; Kastury et al. 2018; Raffy et al. 2018; Gao et al. 2018; Moreda-Piñeiro et al. 2019). In addition, it is important to point out that our health risk assessment could be overestimated even using oral bioavailable concentrations because of not considering other parameters such as PM10 deposition in different lung regions and clearance rates.
Conclusion
The present study describes a novel in vitro methodology for oral bioavailable PM10-associated PAHs estimation that encompasses a first in vitro physiologically based extraction test using gastrointestinal fluids in combination with a dialysis membrane to simulate intestinal absorption, which would provide a better understanding of how substances can interact with organisms. Also, procedure for the analysis of PAHs in oral PM10 bioavailable fractions was successfully validated, offering a simple, sensitive and accurate methodology to bioavailable PAHs quantification.
Applicability of the proposed method has been demonstrated by the analysis of a set of PM10 samples. Oral bioavailable ratios of investigated PAHs were found to be below 5%, showing that a low amount of PM10-bound PAHs could be available through intestinal absorption after inhalation. Among them, fluorene was found to be the most orally bioavailable, followed by anthracene, acenaphthene and phenanthrene (averaged bioavailable ratios of 2.8%, 1.4%, 1.2% and 0.71%, respectively), while carcinogenic PAHs (benzo(b)fluoranthene + benzo(j)fluoranthene, chrysene, indene(1,2,3-cd)pyrene, benzo(a)pyrene, benzo(k)fluoranthene, benzo(a)anthracene and dibenzo(a,h)anthracene) offered the lowest oral bioavailabilities (bioavailable ratios below 0.5%). Moreover, bioavailability percentages were observed to decrease when the number of condensed PAHs rings increases, which might be attributed to the increase in PAHs hydrophobicity as reported for other simulated biological fluids.
Furthermore, carcinogenic hazard index values and lifetime cancer risks based on benzo(a)pyrene-equivalent toxic concentrations by using oral bioavailable concentrations did not exceed cumulative cancer risk (1.0 × 10–4) set by USEPA, suggesting no carcinogenic risk was found to oral bioavailable PAHs in PM10-bound samples studied. However, a brief comparison among carcinogenic hazard index values using both total and bioavailable concentrations was performed, which resulted in an overestimation (by an average factor of almost 104) of PM10-associated risks PAHs. Attending to this, inclusion of biovailabilities in human health risk assessment models would be a step towards a more realistic assessment, as well as the development of pollutants’ bioavailability-based regulations that achieve the combination of pollutants limit levels safe for humans and ecosystems together with a realistic management of resources.
Finally, future efforts should be focused on the standardization of in vitro bioavailability methods so as to obtain more comparable data, as well as the suitability of including bioavailabilities and other biologically relevance parameters (such as deposition in lung regions and clearance rates) in current risk assessment models and toxicology studies.
References
Abdel-Shafy HI, Mansour MSMM (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet 25:107–123. https://doi.org/10.1016/j.ejpe.2015.03.011
Boisa N, Elom N, Dean JR et al (2014) Development and application of an inhalation bioaccessibility method (IBM) for lead in the PM10 size fraction of soil. Environ Int 70:132–142. https://doi.org/10.1016/j.envint.2014.05.021
Bradham KD, Laird BD, Rasmussen PE et al (2014) Assessing the bioavailability and risk from metal-contaminated soils and dusts. Hum Ecol Risk Assess 20:272–286. https://doi.org/10.1080/10807039.2013.802633
Burnett R, Chen H, Szyszkowicz M et al (2018) Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1803222115
Collins CD, Mosquera-Vazquez M, Gomez-Eyles JL et al (2013) Is there sufficient “sink” in current bioaccessibility determinations of organic pollutants in soils? Environ Pollut 181:128–132. https://doi.org/10.1016/j.envpol.2013.05.053
Davie-Martin CL, Stratton KG, Teeguarden JG et al (2017) Implications of bioremediation of polycyclic aromatic hydrocarbon- contaminated soils for human health and cancer risk. Environ Sci Technol 51:9458–9468. https://doi.org/10.1021/acs.est.7b02956
EU (2008) Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe
EU (2004) Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air
Falta T, Limbeck A, Koellensperger G, Hann S (2008) Bioaccessibility of selected trace metals in urban PM2.5 and PM10 samples: a model study. Anal Bioanal Chem 390:1149–1157. https://doi.org/10.1007/s00216-007-1762-5
Galvão ES, Santos JM, Lima AT et al (2018) Trends in analytical techniques applied to particulate matter characterization: a critical review of fundaments and applications. Chemosphere 199:546–568. https://doi.org/10.1016/J.CHEMOSPHERE.2018.02.034
Gao P, da Silva EB, Townsend T et al (2019a) Emerging PAHs in urban soils: concentrations, bioaccessibility, and spatial distribution. Sci Total Environ 670:800–805. https://doi.org/10.1016/j.scitotenv.2019.03.247
Gao P, Guo H, Zhang Z et al (2018) Bioaccessibility and exposure assessment of trace metals from urban airborne particulate matter (PM10 and PM2.5) in simulated digestive fluid. Environ Pollut 242:1669–1677. https://doi.org/10.1016/J.ENVPOL.2018.07.109
Gao P, Liu D, Guo L et al (2019b) Ingestion bioaccessibility of indoor dust-bound PAHs: inclusion of a sorption sink to simulate passive transfer across the small intestine. Sci Total Environ 659:1546–1554. https://doi.org/10.1016/J.SCITOTENV.2018.12.459
Goix S, Uzu G, Oliva P et al (2016) Metal concentration and bioaccessibility in different particle sizes of dust and aerosols to refine metal exposure assessment. J Hazard Mater 317:552–562. https://doi.org/10.1016/j.jhazmat.2016.05.083
Haro-Vicente JF, Martínez-Graciá C, Ros G (2006) Optimisation of in vitro measurement of available iron from different fortificants in citric fruit juices. Food Chem 98:639–648. https://doi.org/10.1016/j.foodchem.2005.06.040
Hernández-Pellón A, Nischkauer W, Limbeck A, Fernández-Olmo I (2018) Metal(loid) bioaccessibility and inhalation risk assessment: a comparison between an urban and an industrial area. Environ Res 165:140–149. https://doi.org/10.1016/j.envres.2018.04.014
Hu X, Zhang Y, Ding Z et al (2012) Bioaccessibility and health risk of arsenic and heavy metals (Cd Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2.5 in Nanjing. China Atmos Environ 57:146–152. https://doi.org/10.1016/j.atmosenv.2012.04.056
Huang H, Jiang Y, Xu X, Cao X (2018) In vitro bioaccessibility and health risk assessment of heavy metals in atmospheric particulate matters from three different functional areas of Shanghai, China. Sci Total Environ 610–611:546–554. https://doi.org/10.1016/j.scitotenv.2017.08.074
Huang M, Chen X, Zhao Y et al (2014a) Arsenic speciation in total contents and bioaccessible fractions in atmospheric particles related to human intakes. Environ Pollut 188:37–44. https://doi.org/10.1016/j.envpol.2014.01.001
Huang M, Wang W, Chan CY et al (2014b) Contamination and risk assessment (based on bioaccessibility via ingestion and inhalation) of metal(loid)s in outdoor and indoor particles from urban centers of Guangzhou, China. Sci Total Environ 479–480:117–124. https://doi.org/10.1016/j.scitotenv.2014.01.115
Kademoglou K, Giovanoulis G, Palm-Cousins A et al (2018a) In vitro inhalation bioaccessibility of phthalate esters and alternative plasticizers present in indoor dust using artificial lung fluids. Environ Sci Technol Lett 5:329–334. https://doi.org/10.1021/acs.estlett.8b00113
Kademoglou K, Williams AC, Collins CD (2018b) Bioaccessibility of PBDEs present in indoor dust: a novel dialysis membrane method with a Tenax TA® absorption sink. Sci Total Environ 621:1–8. https://doi.org/10.1016/j.scitotenv.2017.11.097
Kang Y, Cheung KC, Wong MH (2011) Mutagenicity, genotoxicity and carcinogenic risk assessment of indoor dust from three major cities around the Pearl River Delta. Environ Int 37:637–643. https://doi.org/10.1016/j.envint.2011.01.001
Kastury F, Smith E, Juhasz AL (2017) A critical review of approaches and limitations of inhalation bioavailability and bioaccessibility of metal(loid)s from ambient particulate matter or dust. Sci Total Environ 574:1054–1074. https://doi.org/10.1016/j.scitotenv.2016.09.056
Kastury F, Smith E, Karna RR et al (2018) An inhalation-ingestion bioaccessibility assay (IIBA) for the assessment of exposure to metal(loid)s in PM10. Sci Total Environ 631–632:92–104. https://doi.org/10.1016/j.scitotenv.2018.02.337
Li C, Cui XY, Fan YY et al (2015) Tenax as sorption sink for in vitro bioaccessibility measurement of polycyclic aromatic hydrocarbons in soils. Environ Pollut 196:47–52. https://doi.org/10.1016/j.envpol.2014.09.016
Li Y, Juhasz AL, Ma LQ, Cui X (2019) Inhalation bioaccessibility of PAHs in PM2.5: implications for risk assessment and toxicity prediction. Sci Total Environ 650:56–64. https://doi.org/10.1016/j.scitotenv.2018.08.246
López-Mahía P, Muniategui-Lorenzo S, López-Moure MP et al (2003) Determination of aliphatic and polycyclic aromatic hydrocarbons in atmospheric particulate samples of A Coruña city (Spain). Environ Sci Pollut Res 10:98–102. https://doi.org/10.1065/espr2001.12.105
Mesquita SR, van Drooge BL, Barata C et al (2014) Toxicity of atmospheric particle-bound PAHs: an environmental perspective. Environ Sci Pollut Res 21:11623–11633. https://doi.org/10.1007/s11356-014-2628-y
Miller D, Schricker R, Ph D, Rasmussen R (1981) An in vitro availability method for estimation iron availability from meals. Am J Clin Nutr 43:2248–2256
Mohr V, Miró M, Limbeck A (2017) On-line dynamic extraction system hyphenated to inductively coupled plasma optical emission spectrometry for automatic determination of oral bioaccessible trace metal fractions in airborne particulate matter. Anal Bioanal Chem 409:2747–2756. https://doi.org/10.1007/s00216-017-0219-8
Moreda-Piñeiro J, Moreda-Piñeiro A, Bermejo-Barrera P (2015a) In vivo and in vitro testing for selenium and selenium compounds bioavailability assessment in foodstuff. Crit Rev Food Sci Nutr 57:805–833. https://doi.org/10.1080/10408398.2014.934437
Moreda-Piñeiro J, Moreda-Piñeiro A, Romarís-Hortas V et al (2013) In vitro bioavailability of total selenium and selenium species from seafood. Food Chem 139:872–877. https://doi.org/10.1016/j.foodchem.2013.01.116
Moreda-Piñeiro J, Turnes-Carou I, Alonso-Rodríguez E et al (2015b) The influence of oceanic air masses on concentration of major ions and trace metals in PM2.5 fraction at a coastal European suburban site. Water, Air, Soil Pollut 226:2240. https://doi.org/10.1007/s11270-014-2240-2
Moreda-Piñeiro J, Dans-Sánchez L, Sánchez-Piñero J et al (2019) Oral bioavailability estimation of toxic and essential trace elements in PM10. Atmos Environ 213:104–115. https://doi.org/10.1016/j.atmosenv.2019.06.001
Mukhtar A, Limbeck A (2011) Development of an ETV-ICP-OES procedure for assessment of bio-accessible trace metal fractions in airborne particulate matter. J Anal at Spectrom 26:2081–2088. https://doi.org/10.1039/c1ja10125k
Nemmar A, Holme JA, Rosas I et al (2013) Recent advances in particulate matter and nanoparticle toxicology: a review of the in vivo and in vitro studies. Biomed Res Int 2013:1–22. https://doi.org/10.1155/2013/279371
Nie D, Wu Y, Chen M et al (2018) Bioaccessibility and health risk of trace elements in fine particulate matter in different simulated body fluids. Atmos Environ 186:1–8. https://doi.org/10.1016/j.atmosenv.2018.05.024
Padoan E, Romè C, Ajmone-Marsan F (2017) Bioaccessibility and size distribution of metals in road dust and roadside soils along a peri-urban transect. Sci Total Environ 601–602:89–98. https://doi.org/10.1016/j.scitotenv.2017.05.180
Patinha C, Durães N, Sousa P et al (2015) Assessment of the influence of traffic-related particles in urban dust using sequential selective extraction and oral bioaccessibility tests. Environ Geochem Health 37:707–724. https://doi.org/10.1007/s10653-015-9713-0
Puls C, Limbeck A, Hann S (2012) Bioaccessibility of palladium and platinum in urban aerosol particulates. Atmos Environ 55:213–219. https://doi.org/10.1016/j.atmosenv.2012.03.023
Quintana JB, Rosende M, Montes R et al (2017) In-vitro estimation of bioaccessibility of chlorinated organophosphate flame retardants in indoor dust by fasting and fed physiologically relevant extraction tests. Sci Total Environ 580:540–549. https://doi.org/10.1016/j.scitotenv.2016.11.210
Raffy G, Mercier F, Glorennec P et al (2018) Oral bioaccessibility of semi-volatile organic compounds (SVOCs) in settled dust: A review of measurement methods, data and influencing factors. J Hazard Mater 352:215–227. https://doi.org/10.1016/j.jhazmat.2018.03.035
Ruby MV, Schoof R, Brattin W et al (1999) Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environ Sci Technol 33:3697–3705. https://doi.org/10.1021/es990479z
Sánchez-Piñero J, Moreda-Piñeiro J, Concha-Graña E et al (2021) Inhalation bioaccessibility estimation of polycyclic aromatic hydrocarbons from atmospheric particulate matter (PM10): Influence of PM10 composition and health risk assessment. Chemosphere 263:127847. https://doi.org/10.1016/j.chemosphere.2020.127847
Sysalová J, Száková J, Tremlová J et al (2014) Methodological aspects of in vitro assessment of bio-accessible risk element pool in Urban particulate matter. Biol Trace Elem Res 161:216–222. https://doi.org/10.1007/s12011-014-0101-x
Tang XY, Tang L, Zhu YG et al (2006) Assessment of the bioaccessibility of polycyclic aromatic hydrocarbons in soils from Beijing using an in vitro test. Environ Pollut 140:279–285. https://doi.org/10.1016/j.envpol.2005.07.010
Turner A (2011) Oral bioaccessibility of trace metals in household dust: a review. Environ Geochem Health 33:331–341. https://doi.org/10.1007/s10653-011-9386-2
Turner A, Hefzi B (2010) Levels and bioaccessibilities of metals in dusts from an arid environment. Water Air Soil Pollut 210:483–491. https://doi.org/10.1007/s11270-009-0274-7
Turner A, Ip KH (2007) Bioaccessibility of metals in dust from the indoor environment: application of a physiologically based extraction test. Environ Sci Technol 41:7851–7856. https://doi.org/10.1021/es071194m
Turner A, Simmonds L (2006) Elemental concentrations and metal bioaccessibility in UK household dust. Sci Total Environ 371:74–81. https://doi.org/10.1016/j.scitotenv.2006.08.011
UNE (2015) UNE-EN 12341:2015. Air Quality - Determination of the PM10 fraction of suspended particulate matter - reference method and field test procedure to demonstrate reference equivalence of measurement methods. https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0054246. Accessed 23 Jul 2019
USEPA (1993) Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons
USEPA (2001) Supplemental guidance for developing soil screening levels for superfund sites. In: OSWER
USEPA (2009) Risk Assessment Guidance for Superfund, Volume I: Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment). Office of Superfund Remediation and Technology Innovation
USEPA (2014) Human Health Evaluation Manual, Supplemental Guidance: Update of Standard Default Exposure Factors. Office of Superfund Remediation and Technology Innovation, Assessment and Remediation Division
Uzu G, Sauvain JJ, Baeza-Squiban A et al (2011) In vitro assessment of the pulmonary toxicity and gastric availability of lead-rich particles from a lead recycling plant. Environ Sci Technol 45:7888–7895. https://doi.org/10.1021/es200374c
Wang W, Huang MJ, Zheng JS et al (2013) Exposure assessment and distribution of polychlorinated biphenyls (PCBs) contained in indoor and outdoor dusts and the impacts of particle size and bioaccessibility. Sci Total Environ 463–464:1201–1209. https://doi.org/10.1016/j.scitotenv.2013.04.059
Wolfgor R, Drago SR, Rodriguez V et al (2002) In vitro measurement of available iron in fortified foods. Food Res Int 35:85–90. https://doi.org/10.1016/S0963-9969(01)00122-3
Yu Y, Pang Y, Zhang X et al (2011) Optimization of an in vitro method to measure the bioaccessibility of polybrominated diphenyl ethers in dust using response surface methodology. J Environ Sci 23:1738–1746. https://doi.org/10.1016/S1001-0742(10)60571-2
Acknowledgements
This work was supported by Ministerio de Ciencia, Innovación y Universidades (MCIU), Agencia Estatal de Investigación (AEI) and Fondo Europeo de Desarrollo Regional (FEDER) (Programa Estatal de I+D+i Orientada a los Retos de la Sociedad, ref: RTI 2018-101116-B-I00) and Xunta de Galicia (Programa de Consolidación y Estructuración de Unidades de Investigación Competitivas ref: ED431C 2017/28-2017-2020). Joel Sánchez-Piñero acknowledges the Xunta de Galicia and the European Union (European Social Fund—ESF) for a predoctoral grant (ED481A-2018/164). The council of A Coruña is acknowledged for its assistance (collaboration agreement between the City of A Coruña and the University Institute of Environment (IUMA) of the University of A Coruña (UDC) for the measurement of PM10 particle levels in the area of Os Castros, A Coruña). The authors would like to thank EXPRELA group (UDC) for their support. Funding for open access charge: Universidade da Coruña/CISUG.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sánchez-Piñero, J., Gómez-Meijide, P., Concha-Graña, E. et al. Oral bioavailability reveals an overestimation of the toxicity of polycyclic aromatic hydrocarbons in atmospheric particulate matter. Environ Chem Lett 20, 49–57 (2022). https://doi.org/10.1007/s10311-021-01354-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-021-01354-0