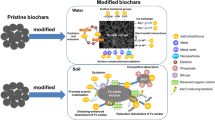
Abstract
Glyphosate is an herbicide used to control weeds and optimize agricultural production. However, since glyphosate is an emerging pollutant claimed to be potentially carcinogenic, glyphosate pollution of soils and water is a health issue. There is therefore a need for advanced techniques to remove glyphosate from the environment. Here, we review glyphosate properties and materials for glyphosate adsorption such as biochar and graphene, which display high glyphosate adsorption capacities.









Similar content being viewed by others
References
Aitbali Y, Ba-M’hamed S, Elhidar N, Nafis A, Soraa N, Bennis M (2018) Glyphosate based- herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice. Neurotoxicol Teratol 67(April):44–49. https://doi.org/10.1016/j.ntt.2018.04.002
Albers CN, Banta GT, Hansen PE, Jacobsen OS (2009) The influence of organic matter on sorption and fate of glyphosate in soil-comparing different soils and humic substances. Environ Pollut 157(10):2865–2870. https://doi.org/10.1016/j.envpol.2009.04.004
Aristilde L, Reed ML, Wilkes RA, Youngster T, Kukurugya MA, Katz V, Sasaki CRS (2017) Glyphosate-induced specific and widespread perturbations in the metabolome of soil Pseudomonas species. Front Environ Sci 5:1–13. https://doi.org/10.3389/fenvs.2017.00034
Arroyave JM, Waiman CC, Zanini GP, Avena MJ (2016) Effect of humic acid on the adsorption/desorption behavior of glyphosate on goethite. Isotherms kinetics. Chemosphere 145:34–41. https://doi.org/10.1016/j.chemosphere.2015.11.082
Assalin MR, de Moraes SG, Queiroz SCN, Ferracini VL, Duran N (2010) Studies on degradation of glyphosate by several oxidative chemical processes: ozonation, photolysis and heterogeneous photocatalysis. J Environ Sci Health B 45(1):89–94. https://doi.org/10.1080/03601230903404598
Autio S, Siimes K, Laitinen P, Rämö S, Oinonen S, Eronen L (2004) Adsorption of sugar beet herbicides to Finnish soils. Chemosphere 55(2):215–226. https://doi.org/10.1016/j.chemosphere.2003.10.015
Borba LL, Cuba RMF, Terán FJC, Castro MN, Mendes TA (2019) Use of adsorbent biochar from Pequi (Caryocar Brasiliense) husks for the removal of commercial formulation of glyphosate from aqueous media. Braz Arch Biol Technol. https://doi.org/10.1590/1678-4324-2019180450
Boruah PK, Sharma B, Hussain N, Das MR (2016) Chemosphere magnetically recoverable Fe3O4/graphene nanocomposite towards efficient removal of triazine pesticides from aqueous solution : investigation of the adsorption phenomenon and specific ion effect. Chemosphere. https://doi.org/10.1016/j.chemosphere.2016.10.103
Boudaoud N, Miloudi H, Bouazza D, Adjdir M (2020) Removal of zinc from aqueous solutions using with Cyanex 272. Characterization 2:1–17
Carneiro RTA, Taketa TB, Gomes RJ, Oliveira JL, Campos VR, De Moraes MA, Camila MG, Beppu MM, Fraceto LF (2015) Removal of glyphosate herbicide from water using biopolymer membranes. J Environ Manag 151:353–360. https://doi.org/10.1016/j.jenvman.2015.01.005
Cartigny B, Azaroual N, Imbenotte M, Mathieu D, Vermeersch G, Goullé JP, Lhermitte M (2004) Determination of glyphosate in biological fluids by 1H and 31P NMR spectroscopy. Forensic Sci Int 143(2–3):141–145. https://doi.org/10.1016/j.forsciint.2004.03.025
Cerdeira AL, Duke SO (2006) The Current status and environmental impacts of glyphosate-resistant crops. J Environ Qual 35(5):1633–1658. https://doi.org/10.2134/jeq2005.0378
Che H, Liu S (2014) Contaminant detection using multiple conventional water quality sensors in an early warning system. Procedia Eng 89:479–487. https://doi.org/10.1016/j.proeng.2014.11.239
Chen S, Liu Y (2007) Study on the photocatalytic degradation of glyphosate by TiO2 photocatalyst. Chemosphere 67(5):1010–1017. https://doi.org/10.1016/j.chemosphere.2006.10.054
Chen F, Zhou C (2016) Thermodynamics and kinetics of glyphosate adsorption on resin D301. Arab J Chem 9:S1665–S1669. https://doi.org/10.1016/j.arabjc.2012.04.014
Chen J, Yao B, Li C, Shi G (2013) An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 64(1):225–229. https://doi.org/10.1016/j.carbon.2013.07.055
Chen W, Xing J, Lu Z, Wang J, Yu S, Yao W, Asiri AM, Alamry KA, Wang X, Wang S (2018) Citrate-modified Mg–Al layered double hydroxides for efficient removal of lead from water. Environ Chem Lett 16(2):561–567. https://doi.org/10.1007/s10311-017-0692-5
Cui H, Li Q, Qian Y, Zhang Q, Zhai J (2012) Adsorption of aqueous glyphosate n-phosphonomethylglycine by manganese oxides/mesoporous silica SBA-15 composite at high salinity condition. Asian J Chem 24(6):2685–2690
Damonte M, Torres RM, Afonso S (2007) Some aspects of the glyphosate adsorption on montmorillonite and its calcined form. Appl Clay Sci 36:86–94. https://doi.org/10.1016/j.clay.2006.04.015
De Jonge H, De Jonge LW (1999) pH influence on Gly Transport 39(5):753–763
Ding J, Guo H, Liu W, Zhang W, Wang J (2015) Current progress on the detection of glyphosate in environmental samples. J Sci Appl Biomed 03(06):88–95
Ding C, Wang X, Liu H, Li Y, Sun Y, Lin Y, Sun W, Zhu X, Dai Y, Luo C (2018) Glyphosate removal from water by functional three-dimensional graphene aerogels. Environ Chem 15(6):325–335. https://doi.org/10.1071/EN18087
Ezaka E, Akintokun AK, Akintokun PO, Taiwo LB, Uthman ACO, Oyedele OA, Aluko OI (2019) Glyphosate degradation by two plant growth promoting bacteria (PGPB) isolated from rhizosphere of maize. Microbiol Res J Int 26(6):1–11. https://doi.org/10.9734/mrji/2018/v26i630081
Fiorilli S, Rivoira L, Calì G, Appendini M, Concetta M, Coïsson M, Onida B (2017) applied surface science iron oxide inside SBA-15 modified with amino groups as reusable adsorbent for highly efficient removal of glyphosate from water. Appl Surf Sci 411:457–465. https://doi.org/10.1016/j.apsusc.2017.03.206
Fittschen UEA (2014) Strategies for ambient aerosols characterisation using synchrotron X-ray fluorescence: a review. Spectrosc Eur 26(3):10–14
Freitas VLS, da Silva MDMCR, Gomes JRB (2013) Efeitos energético-estruturais em compostos heteropolicíclicos com oxigénio ou enxofre. Quim Nova 36(6):840–847. https://doi.org/10.1590/S0100-40422013000600018
Garba J, Samsuri AW, Othman R, Ahmad Hamdani MS (2018) Adsorption-desorption and leaching potential of glyphosate and aminomethylphosphonic acid in acidic Malaysian soil amended with cow dung and rice husk ash. Environ Monit Assess. https://doi.org/10.1007/s10661-018-7034-3
Gasperi J, Laborie B, Rocher V (2012) Treatment of combined sewer overflows by ballasted flocculation: removal study of a large broad spectrum of pollutants. Chem Eng J 211–212(2012):293–301. https://doi.org/10.1016/j.cej.2012.09.025
Gimsing, AL, Afonso M (1871) Glyphosate, pp 263–277
Gimsing AL, Szilas C, Borggaard OK (2007) Sorption of glyphosate and phosphate by variable-charge tropical soils from Tanzania. Geoderma 138(1–2):127–132. https://doi.org/10.1016/j.geoderma.2006.11.001
Gomes MP, Gingras S, Manach L, Moingt M, Smedbol E, Paquet S, Labrecque M, Lucotte M, Juneau P, Manach L (2015) Title: impact of phosphate on glyphosate uptake and toxicity in willow. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2015.10.043
Guerrero-Contreras J, Caballero-Briones F (2015) Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater Chem Phys 153:209–220. https://doi.org/10.1016/j.matchemphys.2015.01.005
Guo L, Cao Y, Jin K, Han L, Li G, Liu J, Ma S (2018) Adsorption characteristics of glyphosate on cross-linked amino-starch. J Chem Eng Data 63(2):422–428. https://doi.org/10.1021/acs.jced.7b00842
Guo D, Muhammad N, Lou C, Shou D, Zhu Y (2019) Synthesis of dendrimer functionalized adsorbents for rapid removal of glyphosate from aqueous solution. New J Chem 43(1):121–129. https://doi.org/10.1039/c8nj04433c
Hao C, Morse D, Morra F, Zhao X, Yang P, Nunn B (2011) Direct aqueous determination of glyphosate and related compounds by liquid chromatography/tandem mass spectrometry using reversed-phase and weak anion-exchange mixed-mode column. J Chromatogr A 1218(33):5638–5643. https://doi.org/10.1016/j.chroma.2011.06.070
Hébert MP, Fugère V, Gonzalez A (2019) The overlooked impact of rising glyphosate use on phosphorus loading in agricultural watersheds. Front Ecol Environ 17(1):48–56. https://doi.org/10.1002/fee.1985
Herath I, Kumarathilaka P, Al-wabel MI, Abduljabbar A (2015) Rice husk derived engineered biochar for glyphosate removal in aqueous media; engineered biochar for pesticides removal microporous and mesoporous materials mechanistic modeling of glyphosate interaction with rice husk derived engineered biochar. Micropor Mesopor Mater 225:280–288. https://doi.org/10.1016/j.micromeso.2016.01.017
Herath I, Kumarathilaka P, Al-Wabel MI, Abduljabbar A, Ahmad M, Usman ARA, Vithanage M (2016) Mechanistic modeling of glyphosate interaction with rice husk derived engineered biochar. Microporous Mesoporous Mater 225:280–288. https://doi.org/10.1016/j.micromeso.2016.01.017
Herath GAD, Poh LS, Ng WJ (2019) Statistical optimization of glyphosate adsorption by biochar and activated carbon with response surface methodology. Chemosphere 227:533–540. https://doi.org/10.1016/j.chemosphere.2019.04.078
Homola J (2003) Present and future of surface plasmon resonance biosensors. Anal Bioanal Chem 377(3):528–539. https://doi.org/10.1007/s00216-003-2101-0
Hosseini N, Toosi MR (2019) From water by polysulfone membranes mixed by graphene oxide/TiO2 nanocomposite: study of filtration and batch adsorption
Hu J-Y, Chen C-L, Li J-Z (2008) A simple method for the determination of glyphosate residues in soil by capillary gas chromatography with nitrogen phosphorus. J Anal Chem 63(4):371–375. https://doi.org/10.1134/s1061934808040102
Hu YS, Zhao YQ, Sorohan B (2011) Removal of glyphosate from aqueous environment by adsorption using water industrial residual. Desalination 271:150–156. https://doi.org/10.1016/j.desal.2010.12.014.10.1016/j.desal.2010.12.014
Huntscha S, Stravs MA, Bühlmann A, Ahrens CH, Frey JE, Pomati F, Hollender J, Buerge IJ, Balmer ME, Poiger T (2018) Seasonal dynamics of glyphosate and AMPA in Lake Greifensee: rapid microbial degradation in the epilimnion during summer. Environ Sci Technol 52(8):4641–4649. https://doi.org/10.1021/acs.est.8b00314
Ifebajo AO, Oladipo AA, Gazi M (2019) Efficient removal of tetracycline by CoO/CuFe2O4 derived from layered double hydroxides. Environ Chem Lett 17(1):487–494. https://doi.org/10.1007/s10311-018-0781-0
Jia DM, Li CH, Li AM (2017) Effective removal of glyphosate from water by resin-supported double valent nano-sized hydroxyl iron oxide. RSC Adv 7(39):24430–24437. https://doi.org/10.1039/c7ra03418k
Jiang X, Ouyang Z, Zhang Z, Yang C, Li X, Dang Z, Wu P (2018) Mechanism of glyphosate removal by biochar supported nano-zero-valent iron in aqueous solutions. Colloids Surf A 547(March):64–72. https://doi.org/10.1016/j.colsurfa.2018.03.041
Kaliannan P, Mohamed Naseer Ali M, Seethalakshmi T, Venuvanalingam P (2002) Electronic structure and conformation of glyphosate: an ab initio MO study. J Mol Struct (Thoechem) 618(1–2):117–125. https://doi.org/10.1016/S0166-1280(02)00467-0
Khenifi A, Derriche Z, Mousty C, Prévot V, Forano C (2010) Adsorption of glyphosate and glufosinate by Ni2AlNO3 layered double hydroxide. Appl Clay Sci 47(3–4):362–371. https://doi.org/10.1016/j.clay.2009.11.055
Kizil R, Irudayaraj J, Seetharaman K (2002) Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J Agric Food Chem 50(14):3912–3918. https://doi.org/10.1021/jf011652p
Kogan M, Metz A, Ortega R (2003) Adsorption of glyphosate in Chilean soils and its relationship with unoccupied phosphate binding sites. Pesquisa Agropecuaria Brasileira 38(4):513–519. https://doi.org/10.1590/S0100-204X2003000400010
la Cecilia D, Maggi F (2018) Analysis of glyphosate degradation in a soil microcosm. Environ Pollut 233(January):201–207. https://doi.org/10.1016/j.envpol.2017.10.017
Li F, Wang Y, Yang Q, Evans DG, Forano C, Duan X (2005) Study on adsorption of glyphosate ( - phosphonomethyl glycine) pesticide on MgAl-layered double hydroxides in aqueous solution. J Hazard Mater 125:89–95. https://doi.org/10.1016/j.jhazmat.2005.04.037
Li Y, Zhao C, Wen Y, Wang Y, Yang Y (2018) Adsorption performance and mechanism of magnetic reduced graphene oxide in glyphosate contaminated water. Environ Sci Pollut Res 25(21):21036–21048. https://doi.org/10.1007/s11356-018-2282-x
Liu ZL, Cui ZL, Zhang ZK (2005) The structural defects and UV–VIS spectral characterization of TiO2 particles doped in the lattice with Cr3 + cations. Mater Charact 54(2):123–129. https://doi.org/10.1016/j.matchar.2004.11.008
Liu C, Shi B, Zhou J, Tang C (2011) Quantification and characterization of microporosity by image processing, geometric measurement and statistical methods: application on SEM images of clay materials. Appl Clay Sci 54(1):97–106. https://doi.org/10.1016/j.clay.2011.07.022
Liu Z, Zhu M, Yu P, Xu Y, Zhao X (2013) Pretreatment of membrane separation of glyphosate mother liquor using a precipitation method. Desalination 313:140–144. https://doi.org/10.1016/j.desal.2012.12.011
Magonov SN, Reneker DH (1997) Characterization of polymer surfaces with atomic force microscopy. Annu Rev Mater Sci 27(1):175–222. https://doi.org/10.1146/annurev.matsci.27.1.175
Marin P, Bergamasco R, Módenes AN, Paraiso PR, Hamoudi S (2019) Synthesis and characterization of graphene oxide functionalized with MnFe2O4 and supported on activated carbon for glyphosate adsorption in fixed bed column. Process Saf Environ Prot 123:59–71. https://doi.org/10.1016/j.psep.2018.12.027
Martin FL, Martinez EZ, Stopper H, Garcia SB, Uyemura SA, Kannen V (2018) Increased exposure to pesticides and colon cancer: early evidence in Brazil. Chemosphere 209:623–631. https://doi.org/10.1016/j.chemosphere.2018.06.118
Mayakaduwa SS, Kumarathilaka P, Herath I, Ahmad M, Al-Wabel M, Ok YS, Usman A, Abduljabbar A, Vithanage M (2016) Equilibrium and kinetic mechanisms of woody biochar on aqueous glyphosate removal. Chemosphere 144:2516–2521. https://doi.org/10.1016/j.chemosphere.2015.07.080
Mesnage R, Antoniou MN (2017) Facts and fallacies in the debate on glyphosate toxicity. Front Public Health. https://doi.org/10.3389/fpubh.2017.00316
Mink PJ, Mandel JS, Sceurman BK, Lundin JI (2012) Epidemiologic studies of glyphosate and cancer: a review. Regul Toxicol Pharmacol 63(3):440–452. https://doi.org/10.1016/j.yrtph.2012.05.012
Mohan A, Girdhar M (2018) Glyphosate toxicity for animals. Environ Chem Lett 16(2):401–426. https://doi.org/10.1007/s10311-017-0689-0
Naftaly M, Miles RE (2007) Terahertz time-domain spectroscopy for material characterization. Proc IEEE 95(8):1658–1665. https://doi.org/10.1109/JPROC.2007.898835
Nourouzi MM, Chuah TG, Choong TSY (2010) Adsorption of glyphosate onto activated carbon derived from waste newspaper. Desalin Water Treat 24(1–3):321–326. https://doi.org/10.5004/dwt.2010.1461
Nourouzi M, Putra U, Luqman A, Universiti C, Chuah AL (2014) Adsorption of glyphosate onto activated carbon derived from waste newspaper. Desalin Water Treat. https://doi.org/10.5004/dwt.2010.1461
Peng T, Xu L, Chen H (2010) Preparation and characterization of high specific surface area Mn3O4 from electrolytic manganese residue. Cent Eur J Chem 8(5):1059–1068. https://doi.org/10.2478/s11532-010-0081-4
Pereira EAO, Melo VF, Abate G, Masini JC (2019a) Adsorption of glyphosate on Brazilian subtropical soils rich in iron and aluminum oxides. J Environ Sci Health B 54(11):906–914. https://doi.org/10.1080/03601234.2019.1644947
Pereira RC, Anizelli PR, Di Mauro E, Valezi DF, Da Costa ACS, Zaia CTBV, Zaia DAM (2019b) The effect of pH and ionic strength on the adsorption of glyphosate onto ferrihydrite. Geochem Trans 20(1):1–14. https://doi.org/10.1186/s12932-019-0063-1
Piccolo A, Celano G, Arienzo M (2008) Adsorption and desorption of glyphosate in some European soils. J Environ Sci Health Part B 39:1105–1115
Portier CJ, Armstrong BK, Baguley BC, Baur X, Belyaev I, Bellé R, Belpoggi F, Biggeri A, Bosland MC, Bruzzi P, Budnik LT, Bugge MD, Burns K, Calaf GM, Carpenter DO, Carpenter HM, López-Carrillo L, Clapp R, Cocco P, Zhou SF (2016) Differences in the carcinogenic evaluation of glyphosate between the international agency for research on cancer (IARC) and the european food safety authority (EFSA). J Epidemiol Commun Health 70(8):741–745. https://doi.org/10.1136/jech-2015-207005
Ramrakhiani L, Ghosh S, Mandal AK, Majumdar S (2019) Utilization of multi-metal laden spent biosorbent for removal of glyphosate herbicide from aqueous solution and its mechanism elucidation. Chem Eng J 361:1063–1077. https://doi.org/10.1016/j.cej.2018.12.163
Romero-Natale A, Palchetti I, Avelar M, González-Vergara E, Garate-Morales JL, Torres E (2019) Spectrophotometric detection of glyphosate in water by complex formation between bis 5-phenyldipyrrinate of nickel (II) and glyphosate. Water. https://doi.org/10.3390/w11040719
Roustan A, Aye M, De Meo M, Di Giorgio C (2014) Genotoxicity of mixtures of glyphosate and atrazine and their environmental transformation products before and after photoactivation. Chemosphere 108:93–100. https://doi.org/10.1016/j.chemosphere.2014.02.079
Rubí-Juárez H, Cotillas S, Sáez C, Cañizares P, Barrera-Díaz C, Rodrigo MA (2016) Use of conductive diamond photo-electrochemical oxidation for the removal of pesticide glyphosate. Sep Purif Technol 167:127–135. https://doi.org/10.1016/j.seppur.2016.04.048
Salman JM, Al-Saad K (2012) Batch study for herbicide bentazon adsorption onto palm oil fronds activated carbon. Int J Chem Sci 10(2):731–740
Samuel L, Wang R, Dubois G, Allen R, Wojtecki R, La YH (2017) Amine-functionalized, multi-arm star polymers: a novel platform for removing glyphosate from aqueous media. Chemosphere 169:437–442. https://doi.org/10.1016/j.chemosphere.2016.11.049
Sansom M, Saborido AA, Dubois M (2013) Control of Conyza spp. with glyphosate A review of the situation in Europe. Plant Prot Sci 49(1):44–53. https://doi.org/10.17221/67/2011-pps
Santos TRT, Andrade MB, Silva MF, Bergamasco R, Hamoudi S (2019) Development of α- and γ-Fe2O3 decorated graphene oxides for glyphosate removal from water. Environ Technol 40(9):1118–1137. https://doi.org/10.1080/09593330.2017.1411397
See HH, Hauser PC, Ibrahim WAW, Sanagi MM (2010) Rapid and direct determination of glyphosate, glufosinate, and aminophosphonic acid by online preconcentration CE with contactless conductivity detection. Electrophoresis 31(3):575–582. https://doi.org/10.1002/elps.200900380
Sen K, Kumar N, Soumya M, Jayanta C, Datta K (2017) Statistical optimization study of adsorption parameters for the removal of glyphosate on forest soil using the response surface methodology. Environ Earth Sci. https://doi.org/10.1007/s12665-016-6333-7
Sen K, Datta JK, Mondal NK (2019) Glyphosate adsorption by Eucalyptus camaldulensis bark-mediated char and optimization through response surface modeling. Appl Water Sci. https://doi.org/10.1007/s13201-019-1036-3
Serra-Clusellas A, De Angelis L, Beltramo M, Bava M, De Frankenberg J, Vigliarolo J, Di Giovanni N, Stripeikis JD, Rengifo-Herrera JA, Fidalgo De Cortalezzi MM (2019) Glyphosate and AMPA removal from water by solar induced processes using low Fe(III) or Fe(II) concentrations. Environ Sci Water Res Technol 5(11):1932–1942. https://doi.org/10.1039/c9ew00442d
Silva V, Montanarella L, Jones A, Fernández-Ugalde O, Mol HGJ, Ritsema CJ, Geissen V (2018) Distribution of glyphosate and aminomethylphosphonic acid (AMPA) in agricultural topsoils of the European Union. Sci Total Environ 621:1352–1359. https://doi.org/10.1016/j.scitotenv.2017.10.093
Singh S, Kumar V, Datta S, Wani AB, Dhanjal DS, Romero R, Singh J (2020) Glyphosate uptake, translocation, resistance emergence in crops, analytical monitoring, toxicity and degradation: a review. Springer International Publishing, In Environmental Chemistry Letters. https://doi.org/10.1007/s10311-020-00969-z
Society WS (2019) Adsorption, mobility, and microbial degradation of glyphosate in the soil author (s): Paul Sprankle WF. Meggitt and Donald Penner Published by : Cambridge University Press on behalf of the Weed Science Society of America Stable 23(3):229–234
Sok V, Fragoso A (2018) Kinetic, spectroscopic and computational docking study of the inhibitory effect of the pesticides 2,4,5-T, 2,4-D and glyphosate on the diphenolase activity of mushroom tyrosinase. Int J Biol Macromol 118:427–434. https://doi.org/10.1016/j.ijbiomac.2018.06.098
Tarone RE (2018) On the international agency for research on cancer classification of glyphosate as a probable human carcinogen. Eur J Cancer Prev 27(1):82–87. https://doi.org/10.1097/CEJ.0000000000000289
Thamaphat K, Limsuwan P, Ngotawornchai B (2008) Phase characterization of TiO2 powder by XRD and TEM. Kasetsart J Nat Sci 42:357–361
Torretta V, Katsoyiannis IA, Viotti P, Rada EC (2018) Critical review of the effects of glyphosate exposure to the environment and humans through the food supply chain. Sustainability 10(4):1–20. https://doi.org/10.3390/su10040950
Tsai WT (2019) Trends in the use of glyphosate herbicide and its relevant regulations in Taiwan: a water contaminant of increasing concern. Toxics. https://doi.org/10.3390/toxics7010004
Ueda N, Bergamasco R, Hamoudi S (2016) Magnetic MnFe2O4—graphene hybrid composite for efficient removal of glyphosate from water. Chem Eng 295:391–402. https://doi.org/10.1016/j.cej.2016.03.051
Valle AL, Mello FCC, Alves-Balvedi RP, Rodrigues LP, Goulart LR (2019) Glyphosate detection: methods, needs and challenges. Environ Chem Lett 17(1):291–317. https://doi.org/10.1007/s10311-018-0789-5
Villarreal-Chiu JF, Acosta-Cortés AG, Kumar S, Kaushik G (2017) Biological limitations on glyphosate biodegradation. In: Green technologies and environmental sustainability. Springer, Berlin, pp 179–201. https://doi.org/10.1007/978-3-319-50654-8_8
Xu ML, Gao Y, Li Y, Li X, Zhang H, Han XX, Zhao B, Su L (2018) Indirect glyphosate detection based on ninhydrin reaction and surface-enhanced Raman scattering spectroscopy. Spectrochim Acta Part A Mol Biomol Spectrosc 197:78–82. https://doi.org/10.1016/j.saa.2018.01.014
Xu S, Zhao J, Yu Q, Qiu X, Sasaki K (2019) Effect of natural organic matter model compounds on the structure memory effect of different layered double hydroxides. ACS Earth Space Chem 3(10):2175–2189. https://doi.org/10.1021/acsearthspacechem.9b00175
Yang Q, Wang J, Zhang W, Liu F, Yue X (2016) Interface engineering of metal organic framework on graphene oxide with enhanced adsorption capacity for organophosphorus pesticide. Chem Eng J. https://doi.org/10.1016/j.cej.2016.12.041
Yang Y, Deng Q, Yan W, Jing C, Zhang Y (2018) Comparative study of glyphosate removal on goethite and magnetite: adsorption and photo-degradation. Chem Eng J 352(July):581–589. https://doi.org/10.1016/j.cej.2018.07.058
Yu Y, Zhou QX (2005) Adsorption characteristics of pesticides methamidophos and glyphosate by two soils. Chemosphere 58(6):811–816. https://doi.org/10.1016/j.chemosphere.2004.08.064
Yuan J, Duan J, Saint CP, Mulcahy D (2018) Removal of glyphosate and aminomethylphosphonic acid from synthetic water by nanofiltration. Environ Technol (United Kingdom) 39(11):1384–1392. https://doi.org/10.1080/09593330.2017.1329356
Yue Y, Zhang Y, Zhou L, Qin J, Chen X (2008) In vitro study on the binding of herbicide glyphosate to human serum albumin by optical spectroscopy and molecular modeling. J Photochem Photobiol B 90(1):26–32. https://doi.org/10.1016/j.jphotobiol.2007.10.003
Yusof N, Ismail AF (2012) Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: a review. J Anal Appl Pyrol 93:1–13. https://doi.org/10.1016/j.jaap.2011.10.001
Zavareh S, Farrokhzad Z, Darvishi F (2018) Modification of zeolite 4A for use as an adsorbent for glyphosate and as an antibacterial agent for water. Ecotoxicol Environ Saf 155(February):1–8. https://doi.org/10.1016/j.ecoenv.2018.02.043
Zhan H, Feng Y, Fan X, Chen S (2018) Recent advances in glyphosate biodegradation. Appl Microbiol Biotechnol 102(12):5033–5043. https://doi.org/10.1007/s00253-018-9035-0
Zhang Z, Ouyang Z, Yang J, Liu Y, Yang C, Dang Z (2019) High mineral adsorption of glyphosate versus diethyl phthalate and tetracycline, during visible light photodegradation with goethite and oxalate. Environ Chem Lett 17(3):1421–1428. https://doi.org/10.1007/s10311-019-00877-x
Zheng T, Sun Y, Lin Y, Wang N, Wang P (2016) Study on preparation of microwave absorbing MnOx/Al2O3 adsorbent and degradation of adsorbed glyphosate in MW-UV system. Chem Eng J 298:68–74. https://doi.org/10.1016/j.cej.2016.03.143
Zhou C, Jia D, Liu M, Liu X, Li C (2017) Removal of glyphosate from aqueous solution using nanosized copper hydroxide modi feiled resin : equilibrium isotherms and kinetics. https://doi.org/10.1021/acs.jced.7b00569
Zhu Y, Zhang F, Tong C, Liu W (1999) Determination of glyphosate by ion chromatography. J Chromatogr A 850(1–2):297–301. https://doi.org/10.1016/S0021-9673(99)00558-0
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pereira, H.A., Hernandes, P.R.T., Netto, M.S. et al. Adsorbents for glyphosate removal in contaminated waters: a review. Environ Chem Lett 19, 1525–1543 (2021). https://doi.org/10.1007/s10311-020-01108-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-020-01108-4