Abstract
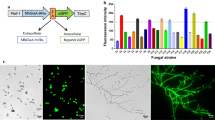
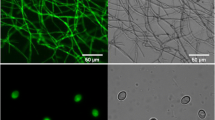
Trehalase catalyzes the hydrolysis of the non-reducing disaccharide trehalose. The highly active trehalase MthT from Myceliophthora thermophila was screened from the trehalase genes of six species of filamentous fungi. An ingenious multi-copy knock-in expression strategy mediated by the CRISPR/Cas9 tool and medium optimization were used to improve MthT production in Aspergillus niger, up to 1698.83 U/mL. The protein background was dramatically abated due to insertion. The recombinant MthT showed optimal activity at pH 5.5 and 60 °C, and exhibited prominent thermal stability between 50 and 60 °C under acid conditions (pH 4.5–6.5). The ethanol conversion rate (ethanol yield/total glucose) was significantly improved by addition of MthT (51.88%) compared with MthT absence (34.38%), using 30% starch saccharification liquid. The results of this study provided an effective strategy, established a convenient platform for heterologous expression in A. niger and showed a potential strategy to decrease production costs in industrial ethanol production.





Similar content being viewed by others
References
Inagaki K, Ueno N, Tamura T, Tanaka H (2001) Purification and characterization of an acid trehalase from Acidobacterium capsulatum. J Biosci Bioeng 91:141–146
Shukla E, Thorat L, Bhavnani V, Bendre AD, Pal JK, Nath BB, Gaikwad SM (2016) Molecular cloning and in silico studies of physiologically significant trehalase from Drosophila melanogaster. Int J Biol Macromol 92:282–292. https://doi.org/10.1016/j.ijbiomac.2016.06.097
Ai D, Cheng S, Chang H, Yang T, Wang G, Yu C (2018) Gene cloning, prokaryotic expression, and biochemical characterization of a soluble trehalase in Helicoverpa armigera Hubner (Lepidoptera: Noctuidae). J Insect Sci. https://doi.org/10.1093/jisesa/iey056
Guo Q, Hao YJ, Li Y, Zhang YJ, Ren S, Si FL, Chen B (2015) Gene cloning, characterization and expression and enzymatic activities related to trehalose metabolism during diapause of the onion maggot Delia antiqua (Diptera: Anthomyiidae). Gene 565:106–115. https://doi.org/10.1016/j.gene.2015.03.070
de Almeida FM, Bonini BM, Beton D, Jorge JA, Terenzi HF, da Silva AM (2009) Heterologous expression in Escherichia coli of Neurospora crassa neutral trehalase as an active enzyme. Protein Express Purif 65:185–189. https://doi.org/10.1016/j.pep.2008.11.010
Shi Z, Liu X, Xu Q, Qin Z, Wang S, Zhang F, Wang S, Tang B (2016) Two novel soluble trehalase genes cloned from Harmonia axyridis and regulation of the enzyme in a rapid changing temperature. Comp Biochem Physiol B Biochem Mol Biol 198:10–18. https://doi.org/10.1016/j.cbpb.2016.03.002
Jin K, Peng G, Liu Y, Xia Y (2015) The acid trehalase, ATM1, contributes to the in vivo growth and virulence of the entomopathogenic fungus, Metarhizium acridum. Fungal Genet Biol 77:61–67. https://doi.org/10.1016/j.fgb.2015.03.013
Peng G, Jin K, Liu Y, Xia Y (2015) Enhancing the utilization of host trehalose by fungal trehalase improves the virulence of fungal insecticide. Appl Microbiol Biotechnol 99:8611–8618. https://doi.org/10.1007/s00253-015-6767-y
Tang B, Yang M, Shen Q, Xu Y, Wang H, Wang S (2017) Suppressing the activity of trehalase with validamycin disrupts the trehalose and chitin biosynthesis pathways in the rice brown planthopper, Nilaparvata lugens. Pestic Biochem Physiol 137:81–90. https://doi.org/10.1016/j.pestbp.2016.10.003
Cheng Q, Gao H, Hu N (2016) A trehalase from Zunongwangia sp.: characterization and improving catalytic efficiency by directed evolution. BMC Biotechnol 16:9. https://doi.org/10.1186/s12896-016-0239-z
Kocot B, Vroemen C, Torres Pazmino DE, Cannon DM, Ward DE, Foerster H, Shetty JK, Clarkson KA, Albers K, Van Brussel M, Chow M, Scheffers M, Schelle MW, Ward M, Kruithof P, Sala RF, Pratt RJ, Rabinovich R, Barends S, Torres PDE, Vroeman C, Joe M, Brussel MV, Cannon D, Forsten HH, Ii RJP, Pazmino DT, Schelle M, Vroemen CW (2015) Increasing production of ethanol from liquefact in fermentation reaction, involves fermenting liquefact with glucoamylase, fermenting organism and trehalase, and recovering ethanol and other fermentation products at end of fermentation. Danisco Us Inc (Dupo-C) Danisco Us Inc (Dupo-C)
Liu Y, Wang Z, Yin Y, Cao Y, Zhao H, Xia Y (2007) Expression, purification, and characterization of recombinant Metarhizium anisopliae acid trehalase in Pichia pastoris. Protein Expr Purif 54:66–72. https://doi.org/10.1016/j.pep.2007.02.016
Carroll JD, Pastuszak I, Edavana VK, Pan YT, Elbein AD (2007) A novel trehalase from Mycobacterium smegmatis—purification, properties, requirements. FEBS J 274:1701–1714. https://doi.org/10.1111/j.1742-4658.2007.05715.x
Lucio-Eterovic AK, Jorge JA, Polizeli Mde L, Terenzi HF (2005) Biochemical characterisation of the trehalase of thermophilic fungi: an enzyme with mixed properties of neutral and acid trehalase. Biochem Biophys Acta 1723:201–207. https://doi.org/10.1016/j.bbagen.2005.02.011
de Aquino AC, Peixoto-Nogueira SC, Jorge JA, Terenzi HF, Polizeli Mde L (2005) Characterisation of an acid trehalase produced by the thermotolerant fungus Rhizopus microsporus var. rhizopodiformis: biochemical properties and immunochemical localisation. FEMS Microbiol Lett 251:169–175. https://doi.org/10.1016/j.femsle.2005.07.045
Kadowaki MK, Polizeli ML, Terenzi HF, Jorge JA (1996) Characterization of trehalase activities from the thermophilic fungus Scytalidium thermophilum. Biochem Biophys Acta 1291:199–205. https://doi.org/10.1016/s0304-4165(96)00065-7
He Y, Pan L, Wang B (2019) Efficient over-expression and application of high-performance pectin lyase by screening Aspergillus niger pectin lyase gene family. Biotechnol Bioprocess Eng 23:662–669. https://doi.org/10.1007/s12257-018-0387-1
Fleissner A, Dersch P (2010) Expression and export: recombinant protein production systems for Aspergillus. Appl Microbiol Biotechnol 87:1255–1270. https://doi.org/10.1007/s00253-010-2672-6
Mekmouche Y, Zhou S, Cusano AM, Record E, Lomascolo A, Robert V, Simaan AJ, Rousselot-Pailley P, Ullah S, Chaspoul F, Tron T (2014) Gram-scale production of a basidiomycetous laccase in Aspergillus niger. J Biosci Bioeng 117:25–27. https://doi.org/10.1016/j.jbiosc.2013.06.013
Chen X, Wang B, Pan L (2019) Heterologous expression and characterization of Penicillium citrinum nuclease P1 in Aspergillus niger and its application in the production of nucleotides. Protein Express Purif 156:36–43. https://doi.org/10.1016/j.pep.2018.12.004
Liu F, Wang B, Ye Y, Pan L (2018) High level expression and characterization of tannase tan7 using Aspergillus niger SH-2 with low-background endogenous secretory proteins as the host. Protein Expr Purif 144:71–75. https://doi.org/10.1016/j.pep.2017.11.003
Benghazi L, Record E, Suarez A, Gomez-Vidal JA, Martinez J, de la Rubia T (2014) Production of the Phanerochaete flavido-alba laccase in Aspergillus niger for synthetic dyes decolorization and biotransformation. World J Microbiol Biotechnol 30:201–211. https://doi.org/10.1007/s11274-013-1440-z
Nodvig CS, Nielsen JB, Kogle ME, Mortensen UH (2015) A CRISPR-Cas9 system for genetic engineering of filamentous fungi. PLoS One 10:e0133085. https://doi.org/10.1371/journal.pone.0133085
Shi TQ, Liu GN, Ji RY, Shi K, Song P, Ren LJ, Huang H, Ji XJ (2017) CRISPR/Cas9-based genome editing of the filamentous fungi: the state of the art. Appl Microbiol Biotechnol 101:7435–7443. https://doi.org/10.1007/s00253-017-8497-9
Zheng X, Zheng P, Zhang K, Cairns TC, Meyer V, Sun J, Ma Y (2018) 5S rRNA promoter for guide RNA expression enabled highly efficient CRISPR/Cas9 genome editing in Aspergillus niger. ACS Synth Biol. https://doi.org/10.1021/acssynbio.7b00456
Yin C, Wang B, He P, Lin Y, Pan L (2014) Genomic analysis of the aconidial and high-performance protein producer, industrially relevant Aspergillus niger SH2 strain. Gene 541:107–114. https://doi.org/10.1016/j.gene.2014.03.011
Liu Q, Gao R, Li J, Lin L, Zhao J, Sun W, Tian C (2017) Development of a genome-editing CRISPR/Cas9 system in thermophilic fungal Myceliophthora species and its application to hyper-cellulase production strain engineering. Biotechnol Biofuels 10:1. https://doi.org/10.1186/s13068-016-0693-9
Xu Y, Wang YH, Liu TQ, Zhang H, Zhang H, Li J (2018) The GlaA signal peptide substantially increases the expression and secretion of alpha-galactosidase in Aspergillus niger. Biotechnol Lett 40:949–955. https://doi.org/10.1007/s10529-018-2540-5
van Hartingsveldt W, Mattern IE, van Zeijl CM, Pouwels PH, van den Hondel CA (1987) Development of a homologous transformation system for Aspergillus niger based on the pyrG gene. Mol Gen Genet 206:71–75
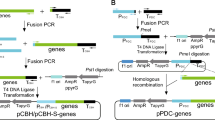
Dong H, Zheng J, Yu D, Wang B, Pan L (2019) Efficient genome editing in Aspergillus niger with an improved recyclable CRISPR-HDR toolbox and its application in introducing multiple copies of heterologous genes. J Microbiol Methods. https://doi.org/10.1016/j.mimet.2019.105655
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Pel HJ, de Winde JH, Archer DB, Dyer PS, Hofmann G, Schaap PJ, Turner G, de Vries RP, Albang R, Albermann K, Andersen MR, Bendtsen JD, Benen JA, van den Berg M, Breestraat S, Caddick MX, Contreras R, Cornell M, Coutinho PM, Danchin EG, Debets AJ, Dekker P, van Dijck PW, van Dijk A, Dijkhuizen L, Driessen AJ, d’Enfert C, Geysens S, Goosen C, Groot GS, de Groot PW, Guillemette T, Henrissat B, Herweijer M, van den Hombergh JP, van den Hondel CA, van der Heijden RT, van der Kaaij RM, Klis FM, Kools HJ, Kubicek CP, van Kuyk PA, Lauber J, Lu X, van der Maarel MJ, Meulenberg R, Menke H, Mortimer MA, Nielsen J, Oliver SG, Olsthoorn M, Pal K, van Peij NN, Ram AF, Rinas U, Roubos JA, Sagt CM, Schmoll M, Sun J, Ussery D, Varga J, Vervecken W, van de Vondervoort PJ, Wedler H, Wosten HA, Zeng AP, van Ooyen AJ, Visser J, Stam H (2007) Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol 25:221–231. https://doi.org/10.1038/nbt1282
Mary Ann Stringer KD, Kirk Schnorr HD, Jesper Vind VD (2005) Expression cloning methods in filamentous fungi
Lee JH, Saito S, Mori H, Nishimoto M, Okuyama M, Kim D, Wongchawalit J, Kimura A, Chiba S (2007) Molecular cloning of cDNA for trehalase from the European honeybee, Apis mellifera L., and its heterologous expression in Pichia pastoris. Biosci Biotechnol Biochem 71:2256–2265. https://doi.org/10.1271/bbb.70239
Xia Y, Gao M, Clarkson JM, Charnley AK (2002) Molecular cloning, characterisation, and expression of a neutral trehalase from the insect pathogenic fungus Metarhizium anisopliae. J Invertebr Pathol 80:127–137
Jorge CD, Sampaio MM, Hreggvidsson GO, Kristjanson JK, Santos H (2007) A highly thermostable trehalase from the thermophilic bacterium Rhodothermus marinus. Extremophiles Life Under Extreme Conditions 11:115–122. https://doi.org/10.1007/s00792-006-0021-6
Thatipamala R, Rohani S, Hill GA (1992) Effects of high product and substrate inhibitions on the kinetics and biomass and product yields during ethanol batch fermentation. Biotechnol Bioeng 40:289–297. https://doi.org/10.1002/bit.260400213
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69:627–642. https://doi.org/10.1007/s00253-005-0229-x
Funding
This work was supported by the National Natural Science Foundation of China (Grant number 31871736), the Research and Development Plan in Key Areas of Guangdong Province (Grant number 2018B020205002) and the Guangdong Provincial Key Laboratory of Advanced Biofermentation Technology Enterprise in Flavoring & Food (Grant number 2017B030302002).
Author information
Authors and Affiliations
Contributions
LD, XL and DY performed the experiments. LD, LH and LP designed the study, analyzed the data and wrote the paper. BW interpreted and polished the writing. All authors discussed the results and implications and commented on the manuscript at all stages. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, L., Lin, X., Yu, D. et al. High-level expression of highly active and thermostable trehalase from Myceliophthora thermophila in Aspergillus niger by using the CRISPR/Cas9 tool and its application in ethanol fermentation. J Ind Microbiol Biotechnol 47, 133–144 (2020). https://doi.org/10.1007/s10295-019-02252-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-019-02252-9