Abstract
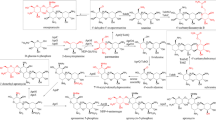
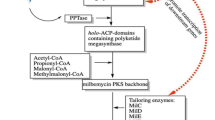
Branched chain amino acids (BCAA) are catabolized into various acyl-CoA compounds, which are key precursors used in polyketide productions. Because of that, BCAA catabolism needs fine tuning of flux balances for enhancing the production of polyketide antibiotics. To enhance BCAA catabolism for pikromycin production in Streptomyces venezuelae ATCC 15439, three key enzymes of BCAA catabolism, 3-ketoacyl acyl carrier protein synthase III, acyl-CoA dehydrogenase, and branched chain α-keto acid dehydrogenase (BCDH) were manipulated. BCDH overexpression in the wild type strain resulted in 1.3 fold increase in pikromycin production compared to that of WT, resulting in total 25 mg/L of pikromycin. To further increase pikromycin production, methylmalonyl-CoA mutase linked to succinyl-CoA production was overexpressed along with BCDH. Overexpression of the two enzymes resulted in the highest titer of total macrolide production of 43 mg/L, which was about 2.2 fold increase compared to that of the WT. However, it accumulated and produced dehydroxylated forms of pikromycin and methymycin, including their derivatives as well. It indicated that activities of pikC, P450 monooxygenase, newly became a bottleneck in pikromycin synthesis.






Similar content being viewed by others
References
Brakhage AA (2004) Molecular biotechnology of fungal beta-lactam antibiotics and related peptide synthetases. Springer Sciences and Business Media, Germany, pp 160–177
Chan YA, Podevels AM, Kevany BM, Thomas MG (2009) Biosynthesis of polyketide synthase extender units. Nat Prod Rep 26:90–114
Dekleva ML, Strohl WR (1988) Biosynthesis of epsilon-rhodomycinone from glucose by Streptomyces C5 and comparison with intermediary metabolism of other polyketide-producing streptomycetes. Can J Microbiol 34:1235–1240
Gajewski J, Buelens F, Serdjukow S, Janben M, Cortina N, Grumuller H, Grininger M (2017) Engineering fatty acid synthases for directed polyketide production. Nat Chem Biol 13:363–365
Gopalani M, Dhiman A, Rahi A, Bhatnagar R (2016) Overexpression of the pleiotropic regulator CodY decreases sporulation, attachment and pellicle formation in Bacillus anthracis. Biochem Biophys Res Commun 469:672–678
Julsing MK, Fichera MA, Malz F, Ebbelaar M, Bos R, Woerdenbag HJ, Quax WJ, Kayser O (2008) Bioconversion of mono- and sesquiterpenoids by recombinant human cytochrome P450 monooxygenases. Pharm Biol 46:710–718
Kaneda T (1991) Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev 55:288–302
Katz E, Brown D (1989) A possible rold of d-valine and related d-amino acids in repression of enzyme and actinomycin synthesis. Appl Microbiol Biotechnol 30:67–70
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical Streptomyces genetics. John Innes Foundation Norwich, England, pp 229–422
Kim M, Yi JS, Kim J, Kim JN, Kim MW, Kim BG (2014) Reconstruction of a high-quality metabolic model enables the identification of gene overexpression targets for enhanced antibiotic production in Streptomyces coelicolor A3(2). Biotechnol J 9:1185–1194
Komatsu M, Uchiyama T, Omura S, Cane DE, Ikeda H (2010) Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proc Natl Acad Sci USA 107:2646–2651
Laakel M, Lebrihi A, Khaoua S, Schneider F, Lefebvre G, Germain P (1994) Relationship between valine, fatty acids, and spiramycin biosynthesis in Streptomyces ambofaciens. Can J Microbiol 40:672–676
Lee SK, Park JW, Kim JW, Jung WS, Park SR, Choi CY, Kim ES, Kim BS, Ahn JS, Sherman DH, Yoon YJ (2006) Neopikromycin and novapikromycin from the pikromycin biosynthetic pathway of Streptomyces venezuelae. J Nat Prod 69:847–849
Li S, Chaulagain MR, Knauff AR, Podust LM, Montgomery J, Sherman DH (2009) Selective oxidation of carbolide C–H bonds by an engineered macrolide P450 mono-oxygenase. Proc Natl Acad Sci USA 106:18463–18468
Li Y, Florova G, Reynolds KA (2005) Alteration of the fatty acid profile of Streptomyces coelicolor by replacement of the initiation enzyme 3-ketoacyl acyl carrier protein synthase III (FabH). J Bacteriol 187:3795–3799
Lounes A, Lebrihi A, Benslimane C, Lefebvre G, Germain P (1995) Regulation of valine catabolism by ammonium in Streptomyces ambofaciens, producer of spiramycin. Can J Microbiol 41:800–808
Maharjan S, Oh TJ, Lee HC, Sohng JK (2008) Heterologous expression of metK1-sp and afsR-sp in Streptomyces venezuelae for the production of pikromycin. Biotechnol Lett 30:1621–1626
McKenzie NL, Thaker M, Koteva K, Hughes DW, Wright GD, Nodwell JR (2010) Induction of antimicrobial activities in heterologous streptomycetes using alleles of the Streptomyces coelicolor gene absA1. J Antibiot (Tokyo) 63:177–182
Morvan C, Halpern D, Kenanian G, Hays C, Anba-Mondoloni J, Brinster S, Kennedy S, Trieu-Cuot P, Poyart C, Lamberet G, Gloux K, Gruss A (2016) Environmental fatty acids enable emergence of infectious Staphylococcus aureus resistant to FASII-targeted antimicrobials. Nat Commun 7:12944
Nah HJ, Pyeon HR, Kang SH, Choi SS, Kim ES (2017) Cloning and heterologous expression of a large-sized natural product biosynthetic gene cluster in Streptomyces species. Front Microbiol 8:394–404
Negretti S, Narayan AR, Chiou KC, Kells PM, Stachowski JL, Hansen DA, Podust LM, Montgomery J, Sherman DH (2014) Directing group-controlled regioselectivity in an enzymatic C–H bond oxygenation. J Am Chem Soc 136:4901–4904
Panagiotou G, Andersen MR, Grotkjaer T, Regueira TB, Nielsen J, Olsson L (2009) Studies of the production of fungal polyketides in Aspergillus nidulans by using systems biology tools. Appl Environ Microbiol 75:2212–2220
Phelan RM, Sachs D, Petkiewicz SJ, Barajas JF, Blake-Hedges JM, Thompson MG, Reider Apel A, Rasor BJ, Katz L, Keasling JD (2017) Development of next generation synthetic biology tools for use in Streptomyces Venezuelae. ACS Synth Biol 6:159–166
Podojil M, Steinerova N, Cudlin J (1989) Relationship between the fatty acid composition and the type of antibiotics produced by Streptomyces lasaliensis. J Basic Microbiol 29:605–609
Pulsawat N, Kitani S, Kinoshita H, Lee CK, Nihira T (2007) Identification of the BkdAB gene cluster, a plausible source of the starter-unit for virginiamycin M production in Streptomyces virginiae. Arch Microbiol 187:459–466
Reeves AR, Brikun IA, Cernota WH, Leach BI, Gonzalez MC, Weber JM (2007) Engineering of the methylmalonyl-CoA metabolite node of Saccharopolyspora erythraea for increased erythromycin production. Metab Eng 9:293–303
Ryu YG, Butler MJ, Chater KF, Lee KJ (2006) Engineering of primary carbohydrate metabolism for increased production of actinorhodin in Streptomyces coelicolor. Appl Environ Microbiol 72:7132–7139
She P, Olson KC, Kadota Y, Inukai A, Shimomura Y, Hoppel CL, Adams SH, Kawamata Y, Matsumoto H, Sakai R, Lang CH, Lynch CJ (2013) Leucine and protein metabolism in obese Zucker rats. PLoS ONE 8:e59443
Sonenshein AL (2007) Control of key metabolic intersections in Bacillus subtilis. Nat Rev Microbiol 5:917–927
Srinivasan A, Bach H, Sherman DH, Dordick JS (2004) Bacterial P450-catalyzed polyketide hydroxylation on a microfluidic platform. Biotechnol Bioeng 88:528–535
Stirrett K, Denoya C, Westpheling J (2009) Branched-chain amino acid catabolism provides precursors for the type II polyketide antibiotic, actinorhodin, via pathways that are nutrient dependent. J Ind Microbiol Biotechnol 36:129–137
Swiatek MA, Gubbens J, Bucca G, Song E, Yang YH, Laing E, Kim BG, Smith CP, van Wezel GP (2013) The ROK family regulator Rok7B7 pleiotropically affects xylose utilization, carbon catabolite repression, and antibiotic production in Streptomyces coelicolor. J Bacteriol 195:1236–1248
Tanaka Y, Izawa M, Hiraga Y, Misaki Y, Watanabe T, Ochi K (2017) Metabolic perturbation to enhance polyketide and nonribosomal peptide antibiotic production using triclosan and ribosome-targeting drugs. Appl Microbiol Biotechnol 101:4417–4431
Tang ZK, Li XM, Pang AP, Lin CY, Zhang Y, Zhang J, Qiao J, Zhao GR (2017) Characterization of three pathway-specific regulators for high production of monensin in Streptomyces cinnamonensis. Appl Microbiol Biotechnol 101:6083–6097
Wallace KK, Zhao B, McArthur HA, Reynolds KA (1995) In vivo analysis of straight-chain and branched-chain fatty acid biosynthesis in three actinomycetes. FEMS Microbiol Lett 131:227–234
Wilson DJ, Xue Y, Reynolds KA, Sherman DH (2001) Characterization and analysis of the PikD regulatory factor in the pikromycin biosynthetic pathway of Streptomyces venezuelae. J Bacteriol 183:3468–3475
Woo MW, Nah HJ, Choi SS, Kim ES (2014) Pikromycin production stibulation through antibiotic down-regulatory gene disruption in Streptomyces venezuelae. Biotechnol Bioprocess Eng 19:973–977
Ye BC, Zhang Y, Yu H, Yu WB, Liu BH, Yin BC, Yin CY, Li YY, Chu J, Zhang SL (2009) Time-resolved transcriptome analysis of Bacillus subtilis responding to valine, glutamate, and glutamine. PLoS ONE 4:e7073
Yi JS, Kim MS, Kim SJ, Kim BG (2015) Effects of sucrose, phosphate, and calcium carbonate on the production of pikromycin from Streptomyces venezuelae. J Microbiol Biotechnol 24:496–502
Yi JS, Kim MW, Kim M, Jeong Y, Kim EJ, Cho BK, Kim BG (2017) A novel approach for gene expression optimization through native promoter and 5′ UTR combinations based on RNA-seq, Bibo-seq, and TSS-seq of Streptomyces coelicolor. ACS Synth Biol 6:555–565
Zabala D, Brana AF, Florez AB, Salas JA, Mendez C (2013) Engineering precursor metabolite pools for increasing production of antitumor mithramycins in Streptomyces argillaceus. Metab Eng 20:187–197
Zhang YX, Denoya CD, Skinner DD, Fedechko RW, McArthur HA, Morgenstern MR, Davies RA, Lobo S, Reynolds KA, Hutchinson CR (1999) Genes encoding acyl-CoA dehydrogenase (AcdH) homologues from Streptomyces coelicolor and Streptomyces avermitilis provide insights into the metabolism of small branched-chain fatty acids and macrolide antibiotic production. Microbiology 145:2323–2334
Zhang YX, Tang L, Hutchinson CR (1996) Cloning and characterization of a gene (msdA) encoding methylmalonic acid semialdehyde dehydrogenase from Streptomyces coelicolor. J Bacteriol 178:490–495
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016953757). The Institute of Engineering Research at Seoul National University provided research facilities for this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yi, J.S., Kim, M., Kim, EJ. et al. Production of pikromycin using branched chain amino acid catabolism in Streptomyces venezuelae ATCC 15439. J Ind Microbiol Biotechnol 45, 293–303 (2018). https://doi.org/10.1007/s10295-018-2024-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2024-6