Abstract
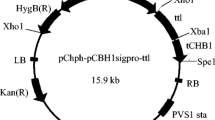
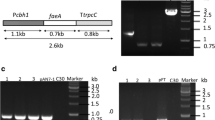
CBH1 (cellobiohydrolase) comprises the majority of secreted proteins by Trichoderma reesei. For expression of Talaromyces thermophilus lipase gene in T. reesei, a self-designed CBH1 promoter was applied to drive the lipase gene expression cassette which was bracketed by flanking sequences of cbh1 gene for homologous recombination. Protoplast and Agrobacterium-mediated plasmid transformations were performed and compared, resultantly, transformation mediated by Agrobacterium was overall proved to be more efficient. Stable integration of lipase gene into chromosomal DNA of T. reesei transformants was verified by PCR. After shaking flask fermentation, lipase activity of transformant reached 375 IU mL−1, whereas no cellobiohydrolase activity was detected. SDS-PAGE analysis further showed an obvious protein band about 39 kDa and no CBH1 band in fermentation broth, implying lipase gene was successfully extracellularly expressed in T. reesei via homologous recombination at cbh1 locus. This study herein would benefit genetic engineering of filamentous fungi and industrial application of thermo-alkaline lipase like in paper making and detergents addition.






Similar content being viewed by others
References
Abuodeh RO, Orbach MJ, Mandel MA, Das A, Galgiani JN (2000) Genetic transformation of Coccidioides immitis facilitated by Agrobacterium tumefaciens. J Infect Dis 181:2106–2110
Amore A, Faraco V (2012) Potential of fungi as category I Consolidated BioProcessing organisms for cellulosic ethanol production. Renew Sustain Energy Rev 16:3286–3301. doi:10.1016/j.rser.2012.02.050
Campoy S, Pérez F, Martín JF, Gutiérrez S, Liras P (2003) Stable transformants of the azaphilone pigment-producing Monascus purpureus obtained by protoplast transformation and Agrobacterium-mediated DNA transfer. Curr Genet 43:447–452. doi:10.1007/s00294-003-0417-0
Chen C-F, Chan K-G, Tan B-C, Khalid N (2015) Enhancement of Agrobacterium-mediated transformation efficiency of model plant using quorum sensing molecule, N-3-oxo-octanoyl-l-homoserine-lactone. Plant Cell Tissue Organ Cult (PCTOC) 121:481–487. doi:10.1007/s11240-015-0718-2
Ding WJ, Qian QF, Hou XL, Feng WY, Chen CY, Chai ZF, Zhang BR, Wang K (2002) A preliminary study of chromium distribution in chromium-rich brewer’s yeast cell by NAA. Biol Trace Elem Res 88:193–199. doi:10.1385/BTER:88:2:193
Fang H, Xia L (2013) High activity cellulase production by recombinant Trichoderma reesei ZU-02 with the enhanced cellobiohydrolase production. Bioresour Technol 144:693–697. doi:10.1016/j.biortech.2013.06.120
Giese H, Kruithof P, Meier K, Sieben M, Antonov E, Hommes RWJ, Büchs J (2014) Improvement and scale-down of a Trichoderma reesei shake flask protocol to microtiter plates enables high-throughput screening. J Biosci Bioeng 118:702–709. doi:10.1016/j.jbiosc.2014.05.016
Gu B, Xia L (2013) High expression of a neutral endo-β-glucanase gene from Humicola insolens in Trichoderma reesei. J Ind Microbiol Biotechnol 40:773–779. doi:10.1007/s10295-013-1267-5
Gusakov AV (2011) Alternatives to Trichoderma reesei in biofuel production. Trends Biotechnol 29:419–425. doi:10.1016/j.tibtech.2011.04.004
Hartl L, Seiboth B (2005) Sequential gene deletions in Hypocrea jecorina using a single blaster cassette. Curr Genet 48:204–211. doi:10.1007/s00294-005-0011-8
He R, Guo W, Wang L, Zhang D (2015) Construction of an efficient RNAi system in the cellulolytic fungus Trichoderma reesei. J Microbiol Methods 108:70–73. doi:10.1016/j.mimet.2014.11.010
He R, Ma L, Li C, Jia W, Li D, Zhang D, Chen S (2014) Trpac1, a pH response transcription regulator, is involved in cellulase gene expression in Trichoderma reesei. Enzyme Microbial Technol 67:17–26. doi:10.1016/j.enzmictec.2014.08.013
Iwashita K (2002) Recent studies of protein secretion by filamentous fungi. J Biosci Bioeng 94:530–535. doi:10.1016/S1389-1723(02)80191-8
Jin X, Xia L (2011) Heterologous expression of an endo-β-1,4-glucanase gene from the anaerobic fungus Orpinomyces PC-2 in Trichoderma reesei. World J Microbiol Biotechnol 27:2913–2920. doi:10.1007/s11274-011-0774-7
Krishnamohan A, Balaji V, Veluthambi K (2001) Efficient vir gene induction in Agrobacterium tumefaciens requires virA, virG, and vir box from the same Ti plasmid. J Bacteriol 183:4079–4089
Leclerque A, Wan H, Abschütz A, Chen S, Mitina GV, Zimmermann G, Schairer HU (2003) Agrobacterium-mediated insertional mutagenesis (AIM) of the entomopathogenic fungus Beauveria bassiana. Curr Genet 45:111–119. doi:10.1007/s00294-003-0468-2
Li C, Yang Z, He Can Zhang R, Zhang D, Chen S, Ma L (2013) Effect of pH on cellulase production and morphology of Trichoderma reesei and the application in cellulosic material hydrolysis. J Biotechnol 168:470–477. doi:10.1016/j.jbiotec.2013.10.003
Li J, Wang J, Wang S, Xing M, Yu S, Liu G (2012) Achieving efficient protein expression in Trichoderma reesei by using strong constitutive promoters. Microb Cell Fact 11:1–10. doi:10.1186/1475-2859-11-84
Mach R, Zeilinger S (2003) Regulation of gene expression in industrial fungi: trichoderma. Appl Microbiol Biotechnol 60:515–522. doi:10.1007/s00253-002-1162-x
Martinez D, Berka RM, Henrissat B, Saloheimo M, Arvas M, Baker SE, Chapman J, Chertkov O, Coutinho PM, Cullen D, Danchin EGJ, Grigoriev IV, Harris P, Jackson M, Kubicek CP, Han CS, Ho I, Larrondo LF, de Leon AL, Magnuson JK, Merino S, Misra M, Nelson B, Putnam N, Robbertse B, Salamov AA, Schmoll M, Terry A, Thayer N, Westerholm-Parvinen A, Schoch CL, Yao J, Barabote R, Nelson MA, Detter C, Bruce D, Kuske CR, Xie G, Richardson P, Rokhsar DS, Lucas SM, Rubin EM, Dunn-Coleman N, Ward M, Brettin TS (2008) Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nat Biotech 26:553–560. http://www.nature.com/nbt/journal/v26/n5/suppinfo/nbt1403_S1.html
Meyer V (2008) Genetic engineering of filamentous fungi—progress, obstacles and future trends. Biotechnol Adv 26:177–185. doi:10.1016/j.biotechadv.2007.12.001
Meyer V, Arentshorst M, El-Ghezal A, Drews A-C, Kooistra R, van den Hondel CAMJJ, Ram AFJ (2007) Highly efficient gene targeting in the Aspergillus niger kusA mutant. J Biotechnol 128:770–775. doi:10.1016/j.jbiotec.2006.12.021
Meyer V, Mueller D, Strowig T, Stahl U (2003) Comparison of different transformation methods for Aspergillus giganteus. Curr Genet 43:371–377. doi:10.1007/s00294-003-0406-3
Michielse C, Hooykaas PJ, van den Hondel CMJJ, Ram AJ (2005) Agrobacterium-mediated transformation as a tool for functional genomics in fungi. Curr Genet 48:1–17. doi:10.1007/s00294-005-0578-0
Michielse CB, Arentshorst M, Ram AFJ, van den Hondel CAMJJ (2005) Agrobacterium-mediated transformation leads to improved gene replacement efficiency in Aspergillus awamori. Fungal Genet Biol 42:9–19. doi:10.1016/j.fgb.2004.06.009
Mullins ED, Chen X, Romaine P, Raina R, Geiser DM, Kang S (2001) Agrobacterium-mediated transformation of Fusarium oxysporum: an efficient tool for insertional mutagenesis and gene transfer. Phytopathology 91:173–180. doi:10.1094/PHYTO.2001.91.2.173
Nevalainen KMH, Te’o VSJ, Bergquist PL (2005) Heterologous protein expression in filamentous fungi. Trends Biotechnol 23:468–474. doi:10.1016/j.tibtech.2005.06.002
Ouedraogo JP, Arentshorst M, Nikolaev I, Barends S, Ram AFJ (2015) I-SceI-mediated double-strand DNA breaks stimulate efficient gene targeting in the industrial fungus Trichoderma reesei. Appl Microbiol Biotechnol 99:10083–10095. doi:10.1007/s00253-015-6829-1
Park S-M, Kim D-H (2004) Transformation of a filamentous fungus Cryphonectria parasitica using Agrobacterium tumefaciens. Biotechnol Bioprocess Eng 9:217–222. doi:10.1007/BF02942296
Penttila M, Nevalainen H, Ratto M, Salminen E, Knowles J (1987) A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene 61:155–164. doi:10.1016/0378-1119(87)90110-7
Prasetyo J, Sumita S, Okuda N, Park E (2010) Response of cellulase activity in pH-controlled cultures of the Filamentous fungus Acremonium cellulolyticus. Appl Biochem Biotechnol 162:52–61. doi:10.1007/s12010-009-8826-2
Punt PJ, van Biezen N, Conesa A, Albers A, Mangnus J, van den Hondel C (2002) Filamentous fungi as cell factories for heterologous protein production. Trends Biotechnol 20:200–206. doi:10.1016/S0167-7799(02)01933-9
Qin L-N, Cai F-R, Dong X-R, Huang Z-B, Tao Y, Huang J-Z, Dong Z-Y (2012) Improved production of heterologous lipase in Trichoderma reesei by RNAi mediated gene silencing of an endogenic highly expressed gene. Bioresour Technol 109:116–122. doi:10.1016/j.biortech.2012.01.013
Rahman Z, Shida Y, Furukawa T, Suzuki Y, Okada H, Ogasawara W, Morikawa Y (2009) Application of Trichoderma reesei cellulase and xylanase promoters through homologous recombination for enhanced production of extracellular β-glucosidase I. Biosci Biotechnol Biochem 73:1083–1089. doi:10.1271/bbb.80852
Rajesh EM, Arthe R, Rajendran R, Balakumar C, Pradeepa N, Anitha S (2010) Investigation of lipase production by Trichoderma reesei and optimization of production parameters. Electron J Environ Agric Food Chem 9:1177–1189
Richardson T, Thistleton J, Higgins TJ, Howitt C, Ayliffe M (2014) Efficient Agrobacterium transformation of elite wheat germplasm without selection. Plant Cell. Tissue Organ Cult (PCTOC) 119:647–659. doi:10.1007/s11240-014-0564-7
Romdhane IB-B, Fendri A, Gargouri Y, Gargouri A, Belghith H (2010) A novel thermoactive and alkaline lipase from Talaromyces thermophilus fungus for use in laundry detergents. Biochem Eng J 53:112–120. doi:10.1016/j.bej.2010.10.002
Romdhane IB-B, Romdhane ZB, Gargouri A, Belghith H (2011) Esterification activity and stability of Talaromyces thermophilus lipase immobilized onto chitosan. J Mol Catal B Enzym 68:230–239. doi:10.1016/j.molcatb.2010.11.010
Ruiz-Díez B (2002) Strategies for the transformation of filamentous fungi. J Appl Microbiol 92:189–195
Singh A, Taylor Ii LE, Vander Wall TA, Linger J, Himmel ME, Podkaminer K, Adney WS, Decker SR (2015) Heterologous protein expression in Hypocrea jecorina: a historical perspective and new developments. Biotechnol Adv 33:142–154. doi:10.1016/j.biotechadv.2014.11.009
Su X, Schmitz G, Zhang M, Mackie RI, Cann IKO (2012) Heterologous gene expression in filamentous fungi. Adv Appl Microbiol 81:1–61. doi:10.1016/b978-0-12-394382-8.00001-0
Sugui JA, Chang YC, Kwon-Chung KJ (2005) Agrobacterium tumefaciens-mediated transformation of Aspergillus fumigatus: an efficient tool for insertional mutagenesis and targeted gene disruption. Appl Environ Microbiol 71:1798–1802
Sun A, Peterson R, Te’o J, Nevalainen H (2016) Expression of the mammalian peptide hormone obestatin in Trichoderma reesei. New Biotechnol 33:99–106. doi:10.1016/j.nbt.2015.08.004
Wang B, Xia L (2011) High efficient expression of cellobiase gene from Aspergillus niger in the cells of Trichoderma reesei. Bioresour Technol 102:4568–4572. doi:10.1016/j.biortech.2010.12.099
Weld RJ, Plummer KM, Carpenter MA, Ridgway HJ (2006) Approaches to functional genomics in filamentous fungi. Cell Res 16:31–44
Zhang X, Li X, Xia L (2015) Heterologous expression of an alkali and thermotolerant lipase from Talaromyces thermophilus in Trichoderma reesei. Appl Biochem Biotechnol 176:1722–1735. doi:10.1007/s12010-015-1673-4
Acknowledgements
This work was supported by the National High-tech R&D Program (2007AA05Z401) and the Program for Zhejiang Leading Team of S&T Innovation (2011R50002) of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Xia, L. Expression of Talaromyces thermophilus lipase gene in Trichoderma reesei by homologous recombination at the cbh1 locus. J Ind Microbiol Biotechnol 44, 377–385 (2017). https://doi.org/10.1007/s10295-016-1897-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-016-1897-5