Abstract
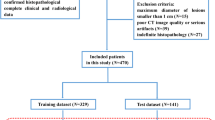
To explore the value of CT-based radiomics model in the differential diagnosis of benign ovarian tumors (BeOTs), borderline ovarian tumors (BOTs), and early malignant ovarian tumors (eMOTs). The retrospective research was conducted with pathologically confirmed 258 ovarian tumor patients from January 2014 to February 2021. The patients were randomly allocated to a training cohort (n = 198) and a test cohort (n = 60). By providing a three-dimensional (3D) characterization of the volume of interest (VOI) at the maximum level of images, 4238 radiomic features were extracted from the VOI per patient. The Wilcoxon–Mann–Whitney (WMW) test, least absolute shrinkage and selection operator (LASSO), and support vector machine (SVM) were employed to select the radiomic features. Five machine learning (ML) algorithms were applied to construct three-class diagnostic models. Leave-one-out cross-validation (LOOCV) was implemented to evaluate the performance of the radiomics models. The test cohort was used to verify the generalization ability of the radiomics models. The receiver-operating characteristic (ROC) was used to evaluate diagnostic performance of radiomics model. Global and discrimination performance of five models was evaluated by average area under the ROC curve (AUC). The average ROC indicated that random forest (RF) diagnostic model in training cohort demonstrated the best diagnostic performance (micro/macro average AUC, 0.98/0.99), which was then confirmed with by LOOCV (micro/macro average AUC, 0.89/0.88) and external validation (test cohort) (micro/macro average AUC, 0.81/0.79). Our proposed CT-based radiomics diagnostic models may effectively assist in preoperatively differentiating BeOTs, BOTs, and eMOTs.






Similar content being viewed by others
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CT:
-
Computed tomography
- BeOTs:
-
Benign ovarian tumors
- BOTs:
-
Borderline ovarian tumors
- eMOTs:
-
Early-stage malignant ovarian tumors
- LASSO:
-
Least absolute shrinkage and selection operator
- SVM:
-
Support vector machine
- LOOCV:
-
Leave-one-out cross-validation
- ML:
-
Five machine learning
- ROC:
-
Receiver-operating characteristics
- AUC:
-
Average area under the ROC curve
- AI:
-
Artificial intelligence
- ML:
-
Machine learning
- PACS:
-
Picture archiving and communication system
- PVP:
-
Portal-venous phase
- DICOM:
-
Digital Imaging and Communications in Medicine
- VOI:
-
Volume of interest
- ICC:
-
Intraclass correlation coefficient
- IBSI:
-
Image biomarker standardization initiative
- WMW:
-
Wilcoxon-Mann-Whitney
- LR:
-
Logistic regression
- SNN:
-
Standard neutral network
- RF:
-
Random forest
- KNN:
-
k-nearest neighbors
- DT:
-
Decision tree
- FIGO:
-
Federation International of Gynecology and Obstetrics
- CAD:
-
Computer-aided diagnosis
- 3D:
-
Three-dimentional
- 2D:
-
Two-dimentional
- AI:
-
Artificial intelligence
- IBSI:
-
Image biomarker standardization initiative
- ICC:
-
Interclass correlation coefficient
- Pre:
-
Precision
- SENS:
-
Sensitivity
- SPE:
-
Specificity
- PVP:
-
Portal-venous phase
References
Practice bulletin no. 174: evaluation and management of adnexal masses. Obstet Gynecol, 128(5): e210-e226,2016.
Jung K. W., Won Y. J., Hong S., Kong H. J., Im J. S. and Seo H. G. Prediction of cancer incidence and mortality in Korea, 2021. Cancer Res Treat, 53(2): 316-322,2021.
Matulonis U. A., Sood A. K., Fallowfield L., Howitt B. E., Sehouli J. and Karlan B. Y. Ovarian cancer. Nat Rev Dis Primers, 2(16061,2016.
Menon U., Gentry-Maharaj A., Burnell M., Singh N., Ryan A., Karpinskyj C., Carlino G., Taylor J., Massingham S. K., Raikou M., Kalsi J. K., Woolas R., Manchanda R., Arora R., Casey L., Dawnay A., Dobbs S., Leeson S., Mould T., Seif M. W., Sharma A., Williamson K., Liu Y., Fallowfield L., McGuire A. J., Campbell S., Skates S. J., Jacobs I. J. and Parmar M. Ovarian cancer population screening and mortality after long-Term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet, 397(10290): 2182-2193,2021.
Mathieu K. B., Bedi D. G., Thrower S. L., Qayyum A. and Bast R. C., Jr. Screening for ovarian cancer: imaging challenges and opportunities for improvement. Ultrasound Obstet Gynecol, 51(3): 293-303,2018.
Della Corte L., Mercorio A., Serafino P., Viciglione F., Palumbo M., De Angelis M. C., Borgo M., Buonfantino C., Tesorone M., Bifulco G. and Giampaolino P. The challenging management of borderline ovarian tumors (BOTs) in women of childbearing age. Front Surg, 9(973034,2022.
Shipeng G., Yongning C., Yadi Z., Chanyuan L. I. and Qifan J. [Comparison of serum cancer antigen 125, human epididymis protein 4, Roma, and Cph-I for diagnosis of ovarian cancer in Chinese patients with ovarian mass]. Nan Fang Yi Ke Da Xue Xue Bao, 39(12): 1393-1401,2019.
Cheng H. Y., Zeng L., Ye X., Ma R. Q., Tang Z. J., Chu H. L., Zhao Y. M., Zhu L. R., Gao Y. N., Chang X. H. and Cui H. Age and menopausal status are important factors influencing the serum human epididymis secretory protein 4 level: a prospective cross-sectional study in healthy Chinese people. Chin Med J (Engl), 133(11): 1285-1291,2020.
Fernandes M. C., Nikolovski I., Long Roche K. and Lakhman Y. Ct of Ovarian cancer for primary treatment planning: what the surgeon needs to know-radiology in training. Radiology, 304(3): 516–526,2022.
Wang S. Q., Li X., Cui J. L., Li H. X., Luk K. D. K. and Hu Y. Prediction of myelopathic level in cervical spondylotic myelopathy using diffusion tensor imaging. Journal of Magnetic Resonance Imaging, 41(6): 1682-1688,2015.
Wang S., Wang X., Shen Y., He B., Zhao X., Cheung W., Cheung J., Luk K. and Hu Y. An ensemble-based densely-connected deep learning system for assessment of skeletal maturity. IEEE Transactions on Systems, Man, and Cybernetics: Systems, PP(99): 1–12,
Lei B., Liang E., Yang M., Yang P., Zhou F., Tan E. L., Lei Y., Liu C. M., Wang T. and Xiao X. Predicting clinical scores for Alzheimer’s disease based on joint and deep learning. Expert Systems with Application, Jan.): 187,2022.
Li S., Liu J., Xiong Y., Pang P., Lei P., Zou H., Zhang M., Fan B. and Luo P. A Radiomics approach for automated diagnosis of ovarian neoplasm malignancy in computed tomography. Sci Rep, 11(1): 8730,2021.
Hu Y., Weng Q., Xia H., Chen T., Kong C., Chen W., Pang P., Xu M., Lu C. and Ji J. A radiomic nomogram based on arterial phase of Ct for differential diagnosis of ovarian cancer. Abdom Radiol (NY), 46(6): 2384-2392,2021.
Papanikolaou N., Matos C. and Koh D. M. How to develop a meaningful radiomic signature for clinical use in oncologic patients. Cancer Imaging, 20(1): 33,2020.
van Timmeren J. E., Cester D., Tanadini-Lang S., Alkadhi H. and Baessler B. Radiomics in medical imaging-“how-to” guide and critical reflection. Insights Imaging, 11(1): 91,2020.
Cuocolo R., Caruso M., Perillo T., Ugga L. and Petretta M. Machine learning in oncology: a clinical appraisal. Cancer Lett, 481(55–62,2020.
Li J., Zhang T., Ma J., Zhang N., Zhang Z. and Ye Z. Machine-learning-based contrast-enhanced computed tomography radiomic analysis for categorization of ovarian tumors. Front Oncol, 12(934735,2022.
Jan Y. T., Tsai P. S., Huang W. H., Chou L. Y., Huang S. C., Wang J. Z., Lu P. H., Lin D. C., Yen C. S., Teng J. P., Mok G. S. P., Shih C. T. and Wu T. H. Machine learning combined with radiomics and deep learning features extracted from Ct images: a novel Ai model to distinguish benign from malignant ovarian tumors. Insights Imaging, 14(1): 68,2023.
Song X. L., Ren J. L., Zhao D., Wang L., Ren H. and Niu J. Radiomics derived from dynamic contrast-enhanced MRI pharmacokinetic protocol features: the value of precision diagnosis ovarian neoplasms. Eur Radiol, 31(1): 368-378,2021.
Wang Y., Zhang H., Wang T., Yao L., Zhang G., Liu X., Yang G. and Yuan L. Deep learning for the ovarian lesion localization and discrimination between borderline and malignant ovarian tumors based on routine MR imaging. Sci Rep, 13(1): 2770,2023.
Zwanenburg A., Vallières M., Abdalah M. A., Aerts H., Andrearczyk V., Apte A., Ashrafinia S., Bakas S., Beukinga R. J., Boellaard R., Bogowicz M., Boldrini L., Buvat I., Cook G. J. R., Davatzikos C., Depeursinge A., Desseroit M. C., Dinapoli N., Dinh C. V., Echegaray S., El Naqa I., Fedorov A. Y., Gatta R., Gillies R. J., Goh V., Götz M., Guckenberger M., Ha S. M., Hatt M., Isensee F., Lambin P., Leger S., Leijenaar R. T. H., Lenkowicz J., Lippert F., Losnegård A., Maier-Hein K. H., Morin O., Müller H., Napel S., Nioche C., Orlhac F., Pati S., Pfaehler E. A. G., Rahmim A., Rao A. U. K., Scherer J., Siddique M. M., Sijtsema N. M., Socarras Fernandez J., Spezi E., Steenbakkers R., Tanadini-Lang S., Thorwarth D., Troost E. G. C., Upadhaya T., Valentini V., van Dijk L. V., van Griethuysen J., van Velden F. H. P., Whybra P., Richter C. and Löck S. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology, 295(2): 328–338,2020.
Song J., Shi J., Dong D., Fang M., Zhong W., Wang K., Wu N., Huang Y., Liu Z., Cheng Y., Gan Y., Zhou Y., Zhou P., Chen B., Liang C., Liu Z., Li W. and Tian J. A new approach to predict progression-free survival in stage Iv Egfr-mutant Nsclc patients with Egfr-Tki therapy. Clin Cancer Res, 24(15): 3583-3592,2018.
Collewet G., Strzelecki M. and Mariette F. Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging, 22(1): 81-91,2004.
Nakamoto T., Takahashi W., Haga A., Takahashi S., Kiryu S., Nawa K., Ohta T., Ozaki S., Nozawa Y., Tanaka S., Mukasa A. and Nakagawa K. Prediction of malignant glioma grades using contrast-enhanced T1-weighted and T2-weighted magnetic resonance images based on a radiomic analysis. Sci Rep, 9(1): 19411,2019.
Rosati A., Gueli Alletti S., Capozzi V. A., Mirandola M., Vargiu V., Fedele C., Uccella S. and Vascone C. Role of ultrasound in the detection of recurrent ovarian cancer: a review of the literature. Gland Surg, 9(4): 1092–1101,2020.
Martínez-Más J., Bueno-Crespo A., Khazendar S., Remezal-Solano M., Martínez-Cendán J. P., Jassim S., Du H., Al Assam H., Bourne T. and Timmerman D. Evaluation of machine learning methods with Fourier transform features for classifying ovarian tumors based on ultrasound images. PLoS One, 14(7): e0219388,2019.
Forstner R., Thomassin-Naggara I., Cunha T. M., Kinkel K., Masselli G., Kubik-Huch R., Spencer J. A. and Rockall A. ESUR Recommendations for MR imaging of the sonographically indeterminate adnexal mass: an update. Eur Radiol, 27(6): 2248-2257,2017.
Zhang H., Mao Y., Chen X., Wu G., Liu X., Zhang P., Bai Y., Lu P., Yao W., Wang Y., Yu J. and Zhang G. Magnetic resonance imaging radiomics in categorizing ovarian masses and predicting clinical outcome: a preliminary study. Eur Radiol, 29(7): 3358-3371,2019.
Wang G., Sun Y., Chen Y., Gao Q., Peng D., Lin H., Zhan Z., Liu Z. and Zhuo S. Rapid identification of human ovarian cancer in second harmonic generation images using radiomics feature analyses and tree-based pipeline optimization tool. J Biophotonics, 13(9): e202000050,2020.
Wang X. and Lu Z. Radiomics analysis of Pet and Ct components of (18)F-Fdg Pet/Ct imaging for prediction of progression-free survival in advanced high-grade serous ovarian cancer. Front Oncol, 11(638124,2021.
Rizzo S., Botta F., Raimondi S., Origgi D., Buscarino V., Colarieti A., Tomao F., Aletti G., Zanagnolo V., Del Grande M., Colombo N. and Bellomi M. Radiomics of high-grade serous ovarian cancer: association between quantitative Ct features, residual tumour and disease progression within 12 months. Eur Radiol, 28(11): 4849-4859,2018.
Vargas H. A., Veeraraghavan H., Micco M., Nougaret S., Lakhman Y., Meier A. A., Sosa R., Soslow R. A., Levine D. A., Weigelt B., Aghajanian C., Hricak H., Deasy J., Snyder A. and Sala E. A novel representation of inter-site tumour heterogeneity from pre-treatment computed tomography textures classifies ovarian cancers by clinical outcome. Eur Radiol, 27(9): 3991-4001,2017.
Lu H., Arshad M., Thornton A., Avesani G., Cunnea P., Curry E., Kanavati F., Liang J., Nixon K., Williams S. T., Hassan M. A., Bowtell D. D. L., Gabra H., Fotopoulou C., Rockall A. and Aboagye E. O. A mathematical-descriptor of tumor-mesoscopic-structure from computed-tomography images annotates prognostic- and molecular-phenotypes of epithelial ovarian cancer. Nat Commun, 10(1): 764,2019.
Zargari A., Du Y., Heidari M., Thai T. C., Gunderson C. C., Moore K., Mannel R. S., Liu H., Zheng B. and Qiu Y. Prediction of chemotherapy response in ovarian cancer patients using a new clustered quantitative image marker. Phys Med Biol, 63(15): 155020,2018.
Wei W., Rong Y., Liu Z., Zhou B., Tang Z., Wang S., Dong D., Zang Y., Guo Y. and Tian J. Radiomics: a novel Ct-based method of predicting postoperative recurrence in ovarian cancer. Annu Int Conf IEEE Eng Med Biol Soc, 2018(4130–4133,2018.
Vamvakas A., Williams S. C., Theodorou K., Kapsalaki E., Fountas K., Kappas C., Vassiou K. and Tsougos I. Imaging biomarker analysis of advanced multiparametric MRI for glioma grading. Phys Med, 60(188–198,2019.
Acknowledgements
We appreciate Dong Xie and Zheng Wang for providing medical guidance to the manuscript.
Funding
This study was funded in part by grants from the National Natural Science Foundation of China (Grant No. 81560425), Guangxi Clinical Research Center for Medical Imaging Construction (Grant No. Guike AD 20238096), and Beijing Medical Award Foundation (Grant No. YXJL-2022–0665-0210).
Author information
Authors and Affiliations
Contributions
Conception and design: DS and CL Development of methodology: JC, LL, DS, and CL Acquisition of data: LL and ZH Analysis and interpretation of data: JC, LL, and ZH Writing, review, and/or revision of the manuscript: JC Administrative, technical, or material support: DS and CL Study supervision: DS and CL.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of Guangxi Medical University Cancer Hospital (LW2022153). Written informed consent was obtained from individual or guardian participants.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, J., Liu, L., He, Z. et al. CT-Based Radiomics and Machine Learning for Differentiating Benign, Borderline, and Early-Stage Malignant Ovarian Tumors. J Digit Imaging. Inform. med. 37, 180–195 (2024). https://doi.org/10.1007/s10278-023-00903-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10278-023-00903-z