Abstract
Long non-coding RNAs (lncRNAs) have emerged as crucial regulators in various cellular processes, including cancer progression and stress response. Recent studies have demonstrated that copper accumulation induces a unique form of cell death known as cuproptosis, with lncRNAs playing a key role in regulating cuproptosis-associated pathways. These lncRNAs may trigger cell-specific responses to copper stress, presenting new opportunities as prognostic markers and therapeutic targets. This paper delves into the role of lncRNAs in cuproptosis-mediated cancer, underscoring their potential as biomarkers and targets for innovative therapeutic strategies. A thorough review of scientific literature was conducted, utilizing databases such as PubMed, Google Scholar, and ScienceDirect, with search terms like 'lncRNAs,' 'cuproptosis,' and 'cancer.' Studies were selected based on their relevance to lncRNA regulation of cuproptosis pathways and their implications for cancer prognosis and treatment. The review highlights the significant contribution of lncRNAs in regulating cuproptosis-related genes and pathways, impacting copper metabolism, mitochondrial stress responses, and apoptotic signaling. Specific lncRNAs are potential prognostic markers in breast, lung, liver, ovarian, pancreatic, and gastric cancers. The objective of this article is to explore the role of lncRNAs as potential prognostic markers and therapeutic targets in cancers mediated by cuproptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
LncRNAs are emerging as pivotal regulators in cellular biology despite their lack of protein-coding potential [1]. These RNA molecules, typically longer than 200 nucleotides, have garnered significant attention due to their diverse roles in critical cellular processes such as gene expression regulation, chromatin remodeling, and cell differentiation [2]. By interacting with various molecules within the cell, lncRNAs can modulate gene expression at multiple levels, influencing mRNA stability, splicing, and even serving as endogenous RNAs (ceRNAs) to sequester microRNAs (miRNAs) [2]. Such functions have made lncRNAs central players in the development and progression of numerous diseases, including cancer, where specific lncRNAs like HOTAIR, MALAT1, and HOTAIR have been linked to metastasis, highlighting their potential as therapeutic targets and biomarkers [3].
In parallel with the growing understanding of lncRNAs, a novel form of programmed cell death, termed cuproptosis, has been discovered [4]. Unlike traditional cell death mechanisms such as apoptosis and necrosis, cuproptosis is triggered by the toxic accumulation of copper within cells [5]. This excess copper disrupts mitochondrial function, particularly impacting proteins involved in the Krebs cycle, leading to an overproduction of reactive oxygen species (ROS). The resulting oxidative stress induces cell death, marking cuproptosis as a unique and distinct pathway in cellular degradation [6].
The intersection of lncRNAs and cuproptosis presents a promising new avenue in cancer research. Tumor cells often exhibit dysregulated copper metabolism, leading to elevated copper levels that make them particularly susceptible to cuproptosis [7]. LncRNAs, with their ability to influence gene expression and cellular responses, are now being studied for their role in regulating cuproptosis-related pathways [8]. For example, lncRNAs like MALAT1 and HOTAIR have been found to interact with mitochondrial components and copper transporters, thereby modulating the sensitivity of cancer cells to cuproptosis [4]. This interaction suggests that lncRNAs could serve not only as biomarkers for cancer prognosis but also as potential therapeutic targets to induce selective cell death in tumors [9]. The potential of cuproptosis in cancer therapy lies in its ability to target cancer cells with minimal damage to normal tissues selectively. By leveraging the dysregulation of copper metabolism in tumor cells, researchers are exploring ways to harness cuproptosis as a novel treatment strategy [10].
Furthermore, the role of lncRNAs in this process adds another layer of complexity and opportunity, as these molecules could be manipulated to enhance the efficacy of cuproptosis-based therapies [3]. The purpose of this review is to explore and elucidate the emerging roles of lncRNAs in the regulation of cuproptosis, a novel form of programmed cell death driven by copper accumulation. By examining the intricate interactions between lncRNAs and cuproptosis-related pathways, this review aims to provide new insights into the molecular mechanisms underlying copper-induced cell death and its implications for cancer therapy.
Long non-coding RNAs: An overview
Biogenesis and classification
LncRNAs are transcribed from DNA by RNA polymerase II and processed through many post-transcriptional modifications that messenger RNAs go through, like 5' capping, poly-adenylation, or splicing [11]. Nonetheless, they also exhibit unique splicing and are less restricted than their protein-coding counterparts [12]. Biogenesis of lncRNAs involves transcription from different genomic locations and processing events that can affect their stability, localization, and function [13]. It is classified into lncRNAs depending on their location and functions within the genome [14]. Intergenic lncRNAs are transcribed in intergenic regions between coding genes and participate in further gene regulation chromatin dynamics [15]. The intronic lncRNAs are derived from their presence within introns in protein-coding genes, which regulate their host genes' splicing and gene expression levels [16]. LncRNAs transcribed from promoters that control gene activity are known as promoter-associated lncRNA [17]. LincRNAs (long intergenic non-coding RNAs) sometimes regulate gene expression over long genomic distances [18]. Antisense lncRNAs are divergently derived from the opposite strand of coding genes and work to regulate gene expression by interacting with RNA or chromatin [19]. The complexity of lncRNA function can be partially attributed to the specificity shown by each class to mediate cellular processes and gene regulation, thereby preserving normality within a cell or tissue while also contributing to the establishment of pathology.
Functions in cellular processes
LncRNAs play a central role in modulating many cellular processes by interacting with proteins, DNA, and other classes of RNA transcripts [1]. Through the recruitment of chromatin-modifying complexes, they exert widespread control over gene expression [20]. LncRNAs bring histone modifiers to promoters or enhancers and change chromatin structure, affecting the transcription of neighbor genes [21]. LncRNAs also participate in transcriptional control by being enhancers or repressors, with different mechanisms on transcriptions' initiation and elongation phases [22]. They regulate the expression of target genes at various levels post-transcriptionally by tweaking mRNA splicing, stability, and translation [23]. The lncRNAs can bind to the mRNA, altering alternative splicing events and replacing somatic hypermutation components like stem-loops or activating factors, modulating mRNA stability [24]. In addition to these functions, lncRNAs are also extremely important for cellular signaling, as they can scaffold signaling complexes or modulate the activity of different signaling proteins [25]. They are also required for proper cellular differentiation and development, controlling the expression of pivotal developmental genes and orchestrating transitions between cell fates [26]. In addition, lncRNAs mediate cellular stress responses by controlling key elements of the transcriptional and post-transcriptional machinery that govern expression levels of genes involved in apoptosis, survival or autophagy [27] (Fig. 1).
Cuproptosis: A novel cell death pathway
Mechanism of cuproptosis
The accumulation of copper ions through its direct interaction with mitochondrial components disturbs cellular homeostasis, leading to this particular mechanism of cell death [28]. The start of cuproptosis involves the accumulation of excess copper ions in a cell [29]. Copper is a fundamental micro-element that becomes toxic when it exceeds physiological limits [30]. The surplus copper is associated with lipoylated forms of tricarboxylic acid (TCA) pathway intermediates, a key mitochondrial energy production conduit [31]. For example, copper intercalates with lipoylated proteins in the TCA cycle, turning over and making these forms more unstable [32]. This defect leads to a major metabolic function of causative mitochondrial activity and excessive ROS production [33]. Oxidative stress produces mitochondria dysfunction, leading to cell death [34]. Moreover, the raised ROS per se damages cellular content and activates further cell stress/ damage response, accelerating apoptosis in a feed-forward manner [35]. Cuproptosis is not dependent on caspase activation nor shares the morphological features of apoptosis [36]. Instead, this results from copper's direct effects on mitochondrial proteins [37].
According to recent research, lncRNAs could be essential in controlling important cuproptosis pathway elements. The expression of copper transporters like CTR1 and ATP7A/B, which are essential for preserving intracellular copper levels, may be influenced by these lncRNAs [38]. When these transporters are dysregulated, copper may build up, which can cause mitochondrial malfunction and, eventually, cell death. Furthermore, lncRNAs may regulate genes related to mitochondrial respiration and the generation of ROS, which helps to fine-tune the mitochondrial responses to copper stress [39]. This control is essential because high levels of ROS from mitochondria may worsen cellular damage and trigger cuproptosis. Furthermore, under circumstances of copper toxicity, lncRNAs may interact with proteins directly involved in the cuproptosis machinery, affecting their stability or activity [40].
Differences from other cell death pathways
Cuproptosis is characterized by the retention of copper inside a cell that leads to apoptosis [41]. It significantly differs from other cell-dying methods, including apoptosis, necrosis, autophagy, pyroptosis, and ferroptosis [42, 43]. Apoptosis is initiated when a cell is damaged and results in a stream of intracellular response, which includes a series of events, such as activation of caspase and DNA fragmentation, causing cell death [44]. As for necrosis, it is elicited by acute injury and immediately causes cytoplasm retention and cell lysing [45]. However, cuproptosis is different since it involves more regulated and controlled methods of cuproptosis inside a cell [46]. Also, autophagy is a survival mechanism developed by a cell under stress, and it includes organelles digestion and recycling [47]. Thus, again, cuproptosis is different because it is related to the toxicity of copper inside a cell [48]. Finally, pyroptosis is initiated by inflammasome activation and gasdermins pore development [49]. Ferroptosis is related to iron lethality, but only in the case of cuproptosis, death is caused by the lethal amount [50].
Cuproptosis in cancer
Role of cuproptosis in cancer development and progression
Copper is a critical trace element that plays a crucial role in angiogenesis, oxidative stress response, enzyme function, and electron transfer [51]. Copper homeostasis is altered in cancer cells, significantly increasing copper levels [52]. It has also been shown that the role of cancer cell growth, metastasis, angiogenesis, and copper is directly related [53]. Therefore, the trace element may be considered essential in the survival and proliferation of cancer cells [54]. In this new model, cuproptosis, copper accumulates in the mitochondria, leading to protein aggregation and the formation of toxic protein levels [55]. This culminates in the loss of the mitochondrial potential function and cell death [56]. Cu is essential for cell metabolism and, simultaneously, deadly to the cells when present in either low or high amounts [57]. There are two ways to achieve cuproptosis: modulating the copper levels themselves or disrupting the copper-binding proteins [58]. Combining cancer therapies with these bronze-era chemicals is likely better than the use of singular [59]. Combining the two compounds increases the effectiveness of the therapeutic actions, consequently reducing the side effects [60].
Cuproptosis dysregulation in different cancer types
Cuproptosis, copper-induced cell death via mitochondrial dysfunction, results from specific copper metabolism dysregulations responsible for copper’s roles across different cancer types [61]. In breast cancer, abnormally elevated copper levels stimulate angiogenesis and metastasis and can be targeted effectively by cuproptosis [62]. The high intracellular copper concentration of lung cancer cells implies that cuproptosis-inducing agents could be used effectively against those cells [63]. Hepatocellular carcinoma exhibits some of the most substantial copper dysregulation, which might be related to the function of the liver in copper metabolism [64]. As a result, liver cancer might stand to benefit the most from this novel therapeutic avenue, especially in the advanced stable phase [65]. Colorectal cancer cells often have dysregulated copper metabolism, which means altering the copper levels could lead to cell death and possibly more effective inhibition of the tumor progression overall [8]. Pancreatic cancer is currently complicated to treat, but copper dysregulation offers another potential therapeutic use of cuproptosis [8]. Finally, increased copper amounts are also linked to prostate cancer’s progression, which, in the case of castration-resistant prostate cancer, could lead to potentially effective new treatments [8].
LncRNAs in cuproptosis regulation
LncRNAs influencing cuproptosis-related genes
LncRNAs control cuproptosis-related genes, which alters their impact on cells when exposed to copper-induced stress [66]. Mitochondria become dysfunctional and become the primary target for copper, resulting in cuproptosis [67]. LncRNA alters this stress's effects by controlling the expression of beneficial and necessary genes responsible for mitochondria’s correct function [68]. It can also change the concentration of intracellular transporters, such as CTR1 and ATP7A, which support the error of copper into and from the cell [69]. It means that it controls the amount of copper used and accumulated in mitochondria. Copper transport ATPases ATP7A and ATP7B are essential for the export and distribution of copper [70]. At the same time, lncRNAs also impact mitochondrial stress response and apoptotic pathways [71]. This occurs as the lncRNAs control the expression of genes, which mitochondria damage may lead to cell death [72]. In detail, mitochondria experience excessive stress and instigate the early stage of the apoptosis process [73]. Some lncRNAs can increase the genes that protect mitochondria from oxidation and free radicals or promote the creation of apoptosomes that cause cell death [74]. Scientists can create methods of targeting specific lncRNAs that control copper metabolism and mitochondria’s response to eliminate cuproptosis [75]. It would improve the effectiveness of treatment and decrease the chance of getting resistance [76].
The functional variety of lncRNAs makes it difficult to pinpoint their precise involvement in cuproptosis and to comprehend how they vary throughout cancer types. The lncRNA MALAT1 has been shown to be involved in a number of cellular functions, such as cell cycle control and metastasis [77]. More recently, research has indicated that it may affect cuproptosis by modifying mitochondrial activity and oxidative stress responses [78]. On the other hand, it seems that MALAT1's effects on cuproptosis varied depending on the cancer cell line. MALAT1 may have a protective effect by maintaining mitochondrial membranes in some malignancies. Still, it may also promote copper-induced cell death in other cancers by upregulating genes implicated in mitochondrial ROS generation [79].
In addition to its well-known functions in chromatin remodeling and gene silencing, HOTAIR is another lncRNA that may interact with pathways linked to cuproptosis, according to new research. Specifically, HOTAIR has been linked to the control of CTR1, a copper transporter that is essential for preserving intracellular copper concentrations. HOTAIR may alter cancer cells' sensitivity to cuproptosis by affecting CTR1 expression; however, the precise processes behind this effect are yet unknown and probably varied throughout cancer types [80].
Interaction between lncRNAs and cuproptosis pathways
The interaction of lncRNAs and cuproptosis pathways is an emerging study area with substantial implications for understanding and controlling cell responses to copper-induced stress [81]. Cuproptosis is a distinct form of cell death caused by copper accumulation, which disrupts mitochondria function and damages the cell [82, 83]. LncRNAs are central to adjusting the process and controlling the action of essential components of copper metabolism and mitochondrial function [84]. They target the copper transporter ATP7A, an integral component of the cellular machinery that manages copper levels [85]. Quantitative polymerase chain reaction studies have determined that modulation of CSNK1D by the lncRNA and other genes plays a vital role in cuproptosis [86]. The mitochondria are central to the process and are linked to the calculated signaling pathways of apoptosis, which is responsible for cuproptosis [87]. The novel interactions have substantial implications for cancer. In the context of loss of heterozygosity, cancer cells tend to turn into heterozygous ones and begin exhibiting sensitivity to copper-induced stress and death linked to cuproptosis [88]. The lncRNAs may serve as the primary target in the process associated with cancer and contribute to the anticipated outcomes, which offer insights into desirable treatment regimens that trigger cuproptosis [89].
Interplay of lncRNAs with key signaling pathways in cancer and cuproptosis
The ability of lncRNAs to interact with multiple signaling pathways makes them crucial players in modulating various aspects of tumor biology. The PI3K/AKT/mTOR signaling pathway is a key player in controlling metabolism, cell division, and survival. It has been shown to interact with a number of lncRNAs, affecting how they function in cuproptosis and the development of cancer [90]. It has been shown that the lncRNA H19 modulates the PI3K/AKT pathway by sponging miR-29a and serving as a ceRNA, thereby upregulating PI3K expression. In certain cancer types, this interaction may be able to offset the effects of cuproptosis by improving cell survival and proliferation [91]. Targeting this lncRNA may interfere with the PI3K/AKT/mTOR pathway in malignancies that overexpress H19, making cells more susceptible to copper-induced apoptosis.
Another important signaling cascade involved in cell migration, differentiation, and proliferation is the Wnt/β-catenin pathway [92]. It has been shown that the MALAT1 interacts with this pathway, facilitating the spread of cancer and increasing apoptotic resistance. MALAT1 can control β-catenin expression, which improves the transcription of Wnt target genes that encourage the development of tumors [93]. MALAT1 may shield cancer cells from copper-induced stress in the setting of cuproptosis by maintaining Wnt/β-catenin signaling, which promotes cell survival and proliferation. When Wnt signaling is active in malignancies, inhibiting MALAT1 may interfere with this protective function and increase the cells' susceptibility to cuproptosis [94].
In response to cellular stress, the tumor suppressor protein p53 is essential for controlling the cell cycle and programmed cell death [95]. Numerous lncRNAs have been linked to the regulation of the p53 pathway, such as PVT1 and NEAT1. PVT1 has the potential to influence the stability and transcriptional activity of p53, while NEAT1 is known to control p53-dependent apoptosis. LncRNAs such as PVT1 may affect the ratio of apoptosis to cuproptosis in tumors with p53 pathway mutations [96]. Overexpression of PVT1 may stabilize mutant p53 and allow cells to survive stress caused by copper. Autophagy is a cellular mechanism that facilitates the destruction and recycling of damaged proteins and organelles. It is also a route that interacts with the activity of lncRNA [27]. It has been shown that the lncRNA HOTAIR inhibits autophagy by downregulating the expression of genes linked to autophagy, including ATG5 and ATG7 [97]. In the case of copper stress, HOTAIR may worsen mitochondrial dysfunction and encourage cuproptosis by blocking autophagy, thus impeding the removal of damaged mitochondria [98].
LncRNAs in cuproptosis-mediated cancer
LncRNAs in cuproptosis-mediated breast cancer
Breast cancer is a condition concerning a large number of the population. It is one of the most commonly occurring cancers among women all over the world, with the chance of being diagnosed in men also [99]. Breast cancer is of various types, showing different manifestations, characteristics, and therapies, and has other implications for the prognosis [100]. Alongside cancers of other body organs, breast cancer has several subtypes, each having distinct characteristics [101]. The types of breast cancer discussed here are invasive ductal carcinoma and invasive lobular carcinoma, in which both types are invasive [102]. Breast cancer also has a noninvasive subtype known as ductal carcinoma in situ, which is confined in the ducts and, therefore, has a better prognosis with little chance of being life-threatening [103]. Triple-negative breast cancer is a subtype in which it is not positive for estrogen, progesterone, and HER, the three commonly occurring receptors in any breast cancer type [104]. NOMA is a subtype having the highest overexpression and growth rate [105]. Chemosensitivity is the susceptibility of cancer cells to chemotherapy agents [106]. Chemosensitivity is responsible for the treatment mechanism through personalized therapy [107]. A chemosensitivity test is an assay developed by the oncologist for their better understanding and indication or confirmation of the susceptibility or resistance or the testing conditions of chemotherapeutic agents developed [108]. Thus, chemosensitivity is the main or the first and foremost factor directing oncologists in identifying the drugs to which the cancer is susceptible [109]. The other factors determining the same factor are the genetic mutation or the tumor microenvironment of that particular person [110]. Wu et al. identified SLC31A1 as a cuproptosis-related gene in breast cancer. SLC31A1 in the breast cancer tissues was upregulated and indicated poor prognosis, immune cell infiltration, and chemosensitivity. The LINC01614/miR-204-5p/SLC31A1 axis may play a major role in cuproptosis-related breast cancer [111]. Therapy sensitivity means the patient's response to the treatment, whether drugs, psychotherapy, or other therapies [112]. It varies because of individuals and the disease’s nature [113]. The sensitivity can be assessed by symptom changes and biomarkers [114]. It is beneficial to understand the therapy of patients to individualize it, increasing the chances of recovery and success [115]. In this article, Wu et al. investigated the cuproptosis-related lncRNAs in breast cancer, showing how they are associated with prognosis and therapy sensitivity. They identified six lncRNAs, building a model that classified the patients in terms of risk groups. This model could be potentially used to predict the response to the therapy and the chances of survival [116].
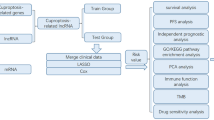
The immune microenvironment is a network of immune cells, stromal components, and signaling molecules around a tissue or tumor [117]. It controls immune response and affects disease development and response to therapy [118]. Understanding this microenvironment is vital for immunotherapy development and predicting the course of various diseases' responses to treatment [119]. Jiang et al. created a predictive model using 11 cuproptosis-related lncRNAs to determine breast cancer prognosis and immune microenvironment. In patients with high-risk, overall survival was lower and had different mutation profiles [120]. A similar study by Xu et al. developed a 2-lncRNA signature for breast cancer prognosis related to cuproptosis. The signature, BCCuS, was validated as a prognostic factor and could help predict therapy response and patient survival [121]. In another study, Pan et al. investigated cuproptosis-related lncRNAs for their prognostic role and effect on the immune microenvironment for breast cancer. The identified model based on ten lncRNAs could predict survival and drug sensitivity [122]. The immune checkpoint is an essential regulator of the immune system, which ensures that immune responses are proportionate, contain no autoimmunity, and that tissue damage during the reaction is minimized [123]. It is molecules on immune cells, especially T cells, that must be activated to start an immune response [124]. Although the immune checkpoint serves as a protection layer, it is activated by cancer cells to escape detection and elimination by the immune system [125]. Yu et al. established a predictive risk score system for breast cancer, which is based on 11 cuproptosis-related lncRNAs. This system can predict patients’ prognostic outcomes, immune status, and drug sensitivity to aid their treatment, as shown in Fig. 2 [126].
Jiang et al. described a target signature for cuproptosis-associated results and axon expression in breast cancer. lncRNAs correlated with the prognosis were detected in database mining analysis [127]. The study described the lncRNAs related to the prognosis. Another study by Li et al. analyzed the role of cuproptosis-related lncRNAs using prognosis prediction and immune infiltration in breast cancer. A predictive model was developed based on the lncRNA expression levels, and the signature was associated with specific outcomes and responses to therapeutics [116] (Fig. 2).
LncRNAs in cuproptosis-mediated lung cancer
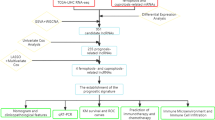
Lung cancer is a widespread type of cancer that remains highly lethal [128]. It occurs in the tissues of the lungs, commonly within the cells lining the air passages [129]. Lung cancer can be divided into two categories, NSCLC and SCLC, which differ in unique characteristics, influence factors, and therapy methods and outcomes [130]. About 85% of all cases are non-small cell lung cancer, which has several subtypes [131]. Squamous cell carcinoma is typically found within the central aspect of the lungs and is closely attached to smoking [132]. Large cell carcinoma does not apply to the other categories but can arise in any lung part; it generally grows rapidly and moves quickly to develop, which complicates the treatment process [133]. Small cell lung cancer accounts for nearly 15% of all cases [134]. It is strongly connected with smoking and demonstrates itself through its dominating cytologic profile, extreme acting, and speedy growth [135]. Generally, it begins in the central airways and quickly shifts to other body parts, such as the liver, bones, and brain [136]. Fast growth and high aggression suggest that it has already reached a practically developed level at a stage where it is detectable, which complicates the treatment and the following survival rates [137]. Wang et al. studied the cuproptosis-related lncRNAs in lung adenocarcinoma. They used the TCGA and GEO data to detect cuproptosis-related lncRNAs and detect 16 candidates before developing the prognostic signature. They found that the identified long non-coding RNAs are associated with poor overall survival and progression-free survival. They can predict prognosis and immune escape, indicating potential clinical applications and improvements [56].
Radiosensitization-related cuproptosis is a novel concept in the cancer treatment context, which combines the principles of radiosensitization and the recently discovered mechanism of cell death, cuproptosis [138]. Radiosensitization implies making a cancer cell more sensitive to the impacts of radiation therapy and, therefore, improving the effect of the treatment [139]. Cuproptosis is a type of programmed cell death, which is driven by copper ions serving to trigger the toxicity of a cancer cell [140]. Additionally, Xu et al. developed a cuproptosis-related lncRNA signature to estimate the prognosis of NSCLC patients after applying radiation therapy. The team constructed a prediction model of six lncRNAs and used it in training and testing groups. The results proved its accuracy in predicting patient prognosis more productively than traditional clinical factors [141]. According to another study by Yu et al., a cuproptosis-related lncRNA signature can predict prognosis and immunotherapy responses in LUAD patients. The authors identified six prognosis-associated key lncRNAs and employed numerous analytical approaches to validate their predictive values. Overall, the study demonstrated that patients at high risk often exhibit poorer survival rates; in addition, this research suggested some therapeutic options based on the analyzed lncRNA profile, as shown in Fig. 3 [142].
GSVA and GSEA are computational methods for interpreting gene expression profiles [143]. GSVA evaluates variation in pathway activity across samples, while GSEA identifies statistically significant gene sets enriched in different phenotypes [144]. Wang et al. constructed a novel ten-cuproptosis-related lncRNAs-based prognostic signature for LUAD. GSVA and GSEA, among other analyses, were used to evaluate the impact of the lncRNAs on prognosis and the immune landscape. Their findings showed that the signature could predict patient survival and guide therapeutics, including drug sensitivity [145]. Tumor cell proliferation is one of the most prominent characteristics of cancer, referring to the unrestricted and abnormally high reproduction of these cells [146]. This results from genetic aberrations as the unstable cell cycle continues due to mutations [147]. Importantly, this activity is vital for the growth, metastasis, and development of cancer, making it one of the most researched aspects of these conditions [148, 149]. Sun et al. presented a novel tested signature composed of six lncRNAs capable of reliably predicting the patient’s condition and guiding the optimum course of treatment. Additionally, their subsequent confirmation experiments uncovered that one of these cancers-suppressing lncRNAs, NIFK-AS1, could arrest the proliferation and migration of tumor cells [150].
Immune function is assessed to diagnose, monitor, and treat numerous medical conditions, including infections, autoimmune diseases, immunodeficiencies, and cancers [151]. Both clinical evaluation and laboratory tests are crucial components of comprehensive assessment [152]. Medical history and physical examination may reveal signs of recurrent infections, autoimmune symptoms, or a family history of immune disorders [153]. Physical examination may also show signs of acute or chronic infection, inflammation, or immunodeficiency[154]. According to the study by Zhao et al., their five-cuproptosis-related lncRNA signature for the prediction of prognosis, immune function, and drug sensitivity in LUSC clearly distinguished high- and low-risk patients and armed other specialists with considerable information also regarding immune function and drug response. The results showed differences in immune pathways and suggested potential therapies based on risk scores [150]. Copper homeostasis refers to the conjunction of processes to maintain optimal copper levels in the human body [155]. Copper is a vital trace element that performs a variety of functions in the human system, such as providing integrity while partaking in the DNA repair mechanism, vitalization of enzymes, forming strong ties between collagens and elastins in connective tissues, relaying chemical signals through the nervous system, and moisture distribution throughout the body [156]. At the same time, both copper deficiency and overload can induce devastating health effects, thus making it vital for the element’s amounts to be guarded cautiously [157]. Ma et al. considered the connection between copper homeostasis, cuproptosis, and lncRNA in LUAD. They used the public database TCGA and GEO and created a prognostic lncRNA signature, which can be vital for understanding the patients’ prognosis and available therapy methods. The authors studied the impact of this process in cancers focusing on individualized immunotherapy [158] (Fig. 3).
LncRNAs in cuproptosis-mediated ovarian cancer
Ovarian cancer is one of the most severe cases of gynecologic cancers due to a high degree of late diagnosis and aggressive growth of the tumor [159]. Depending on the type of cells from which cancerous formation emanates, it is generally accepted to distinguish three main types of the disease: epithelial tumors, germinative tumors, and tumors of the ovarian stroma [160]. Epithelial tumors form on the ovary's outer surface and are the most common, up to 90% of all cases [161]. The symptoms present are not characteristic of the disease, such as disturbing sensations of abdominal distension, periodic pains in the groin, problems with swallowing and eating, vomiting, and frequent urination [162]. It is possible to confuse this symptomatology with gastrointestinal diseases, bloating, and premenstrual syndrome, which can lead to additional difficulties in early diagnosis [163]. In their study, Wang et al. identified that battalions of immune cell abundance had a lower level in higher CTC.246B18.8 expression groups. In addition, a high CTC.246B18.8 expression was related to concerns for the lymphocyte level. LncRNAs identified as prognosis-associated, including RP5–1076A10.1, TM4SF1-AS1, RP11–409C1.2, AC020916.1, RP11–608G10.3, and CTC.246B18.8. The higher expression of the eighth RNA increased the possibility of bad results; there was also a change in the immune profile [164].
Paclitaxel, also known and marketed under the brand name Taxol, is a chemotherapy medication from the Pacific yew tree [165]. It is extensively used against different types of cancer, such as ovarian, breast, lung, and pancreatic cancers, and Kaposi’s sarcoma [166]. Gefitinib, also marketed as Iressa, is a targeted therapy mostly used against non-small cell lung cancer [167]. As a tyrosine kinase inhibitor, it is developed to affect the function of the epidermal growth factor receptor [168]. Li et al. analyzed cuproptosis-related lncRNAs in ovarian cancer to determine the molecular subtypes and develop a prognostic signature. Their results showed different links to prognosis, immunity, and drug reactions for the top 10 lncRNAs. The identified subtypes demonstrate the heterogeneity of ovarian cancer and the possible biomarkers of prognosis and drug response [169]. Characterizing immune profiles in the context of assessing immune system function and guiding the development of new immunotherapy strategies has relevance to several diseases, such as various types of cancer and autoimmune diseases [170]. Liu et al. developed a prognostic signature of four cuproptosis-related lncRNAs in ovarian cancer. They identified that the signature effectively separated patients with ovarian cancer into high- and low-risk subgroups, which was significantly associated with overall survival, immune profiles and drug sensitivity. Their approach introduces a new perspective with respect to individualized treatment strategies for ovarian cancer [171]. High tumor mutational burden (TMB) is often associated with an improved response to immunotherapy as more mutations could potentially generate novel antigens; as such, high TMB level might serve as a potential predictive marker of how cancer patients might respond to a certain type of treatment [172]. In a study by Guo et al., a prognostic model of ovarian serous cystadenocarcinoma was built by constructing cuproptosis-related lncRNAs. The results of the study showed that they identified 5 lncRNAs that were significant. The cross-validation has proven to have a strong predictive effect on patients’ prognosis and has a significant correlation with immune cell infiltration and TMB. Overall, the model provides information that can help patients better understand their outcomes and think about the appropriate treatment strategy [85].
LncRNAs in cuproptosis-mediated gastric cancer
Gastric cancer, or stomach cancer, is a malignant tumor originating from the gastric mucosa [173]. Gastric cancer is a matter of global health concern, with high incidence rates in East Asia, Eastern and Western Europe, and parts of South America [174]. Gastric cancer is generally asymptomatic in the early stages, as the symptoms may be vague and nonspecific [175]. Most patients are diagnosed in the advanced stages due to the absence of screening and the limited sensitivity of common endoscopic techniques [176]. The prognosis of gastric cancer varies depending on the stage at diagnosis [177]. The major factor affecting the prognosis of gastric cancer is the extent of the spread of the disease and whether the target organ has been invaded [178]. Early gastric cancer is curable with complete surgical resection and offers behind excellent 5-year survival rates [179]. However, advanced-stage cancer, which has a propensity for early metastasis, has dismal 5-year overall survival values for most current treatments [180]. The 5-year survival of advanced gastric cancer is still low, with a rate of no more than 5%-20%, emphasizing the need for early detection and prevention [181]. Zhao et al. constructed a prediction model based on cuproptosis-related gene expression levels to evaluate gastric cancer's clinical prognosis and immunotherapy efficacy. It concluded that high-risk patients had adverse overall survival, the risk score was an independent prognostic predictor, and a connection was established between the risk score and immune cell infiltration [182].
Cholesterol metabolism involves synthesis, absorption, transport, and excretion [183]. It is essential to cell membrane structure, hormone, and bile acid production [184]. Imbalance could cause cardiovascular disease [185]. The liver, through lipoprotein particles, such as low-density lipoprotein and high-density lipoprotein, controls the body's cholesterol level [186]. Feng et al. conducted a study on a novel nomogram based on cuproptosis-related lncRNA to improve prognosis prediction in gastric cancer. They discovered that the nomogram based on cuproptosis-related lncRNAs was effective, as patients in the high-risk group using this nomogram to predict outcome had a poorer prognosis than those in the low-risk group. This model was better than clinicopathological parameters in predicting outcomes, and it was found to be associated with cholesterol metabolism and immune function. The correlation between CD209 and HAVCR2 immune checkpoints and risk scores was validated in the experiment [187]. The oncogenes are an example of a gene mutation that may cause cancer development [188]. A somatic mutation occurs in non-germline cells after birth and is not inherited [189, 190]. Somatic mutations cause cancer or other diseases when they functionally affect cell division or function and are consigned due to environmental factors or other cellular errors [191]. Yin et al. developed and assessed a novel cuproptosis-related lncRNA signature to predict the prognosis of gastric cancer. The authors built a risk prediction model according to cuproptosis-related lncRNAs and proved that it could effectively classify patients into high- and low-risk groups. The model showed high prediction ability and was allied with the alterations in immune function, somatic mutation, and drug sensitivity [192].
Natural drug sensitivity refers to the inherent responsiveness of organisms or cells to drugs obtained from natural sources, such as plants, fungi, or microorganisms [193]. This sensitivity depends on various genetic, biochemical, and physiological factors and participates in the drug efficacy and development of approaches to personalized therapy [194]. Song et al. created a metal-dependent programmed cell death-related lncRNA prognostic signature for gastric cancer and assessed natural drug sensitivity. The 12-lncRNA signature model indicated the worse prognosis of high-risk patients and effectively predicted the natural antitumor drug sensitivity [195]. The tumor microenvironment can be defined as the cellular and extracellular composite surrounding a tumor [196]. It comprises immune cells, fibroblasts, blood vessels, and extracellular matrix [197]. The components in the tumor microenvironment may result in tumor growth and metastasis and affect a tumor's responsiveness to treatment [198]. Therefore, it is a critical area of interest for researchers who strive to understand and treat cancer [199, 200]. Huang et al. conducted a study investigating the diverse tumor microenvironment landscapes in gastric cancer using cuproptosis-related lncRNAs. The researchers developed a six-lncRNA signature that predicts patient survival and displays differences in immune activation and TME characteristics. Their study concludes that high-risk subgroups could have higher TME scores and receive better anti-PD-1 immune checkpoint blockade [201, 202].
Generally, antineoplastic drugs are defined as a broad group of medicines used to fight the development and spread of cancer [203]. Their main effect is achieved by targeting and killing abnormal and rapidly dividing cancer cells [204]. It is also important to note that there are several classes of these drugs, including alkylating agents, antimetabolites, and taxanes, among others, with all of them ultimately aimed at inhibiting tumor growth and spread [205]. It also follows from the definition that these drugs may negatively affect normal cells, causing a range of side effects [206, 207]. Tu et al. generated a cuproptosis-related lncRNA gene signature to predict the prognosis of gastric adenocarcinoma and assess the effect of antineoplastic drugs. In particular, the study has identified a six-lncRNA signature that correlated with patient survival, with high-risk patients demonstrating superior immunotherapy responses and different sensitivities to specific drugs [208]. Another study by Wang et al. developed a novel risk model by cuproptosis-related long non-coding RNAs for gastric cancer and analyzed the immune landscape. They identified 10 CRLs for predicting prognosis and their relevance with risk scores with immune cell infiltration, drug sensitivity, and tumor mutation burden. This model showed the potential to predict patient prognosis and guide immunotherapy [209, 210].
LncRNAs in cuproptosis-mediated pancreatic cancer
Pancreatic cancer is an aggressive form of cancer that starts in the tissues of the pancreas, an organ lying behind the lower part of the stomach [211]. The pancreas helps in digestion and the regulation of blood sugar through exocrine and endocrine functions [212, 213]. Most carcinomas of the pancreas start in the exocrine cells that produce digestive enzymes [214]. The most common type of pancreatic cancer is pancreatic ductal adenocarcinoma, constituting over 90% of all pancreatic carcinomas [215]. The prognosis of pancreatic cancer remains poor, having more than 10% five-year survival rate due to reasons including late diagnosis and the aggressiveness of the disease [216]. Despite this, pancreatic cancer is studied more and more extensively as continually accumulating science is unveiling more molecular mechanisms of the disease and new diagnostic markers and targets for therapy [217, 218].
Moreover, thanks to the advances in imaging technologies and genetic testing, personalized care can be adopted for better and more timely treatment [219]. CASC8, also known as CAN8 or Cancer Susceptibility 8, is a long non-coding RNA lncRNA associated with the susceptibility to, occurrence, and progress of this type of disease [220, 221]. It influences the process of gene expression and the development of a tumor [222]. As a lncRNA associated with cancer, its expression also correlates with other carcinomas, making the CASC8 a biomarker in blood and tumor tissue [223, 224]. Likewise, therapeutic agents can be developed to influence its expression [225]. A study by Yao et al. developed a signature of five lncRNAs associated with cuproptosis and affecting patients’ outcomes and the immune environment of the tumor in pancreatic cancer, retrieved from TCGA and ICGC cohorts. CASC8 was pointed out in the study as a five-gene signature influencing both patients’ outcomes and immune environment as having a higher expression and correlating to a worse outcome [226]. Similar research was conducted by Jiang et al. to construct a predictive model based on the cuproptosis-related lncRNAs in the case of pancreatic cancer. The researchers have found that 181 lncRNAs are relevant for the case in question and have identified the five specific lncRNA risk models. Overall, the model can be considered effective as it led to the difference in prognosis of the patients in the high- and low-risk groups [227, 228]. Sun et al. have constructed the predictive model by using the single-cell analysis of cuproptosis-related lncRNAs and their impact on the immune microenvironment in pancreatic cancer. The CIR-score, which integrates the factors of lncRNA signatures and clinical traits, could predict the prognosis, thus informing individual immunotherapy [229].
Glycolysis is a metabolic pathway that converts glucose to pyruvate while generating ATP and NADH [230]. Glycolysis is essential for energy production and metabolism, both anaerobically and aerobically, and it is also a precursor for many biosynthetic pathways [231]. Pancreatic adenocarcinoma’s cuproptosis-related lncRNA was studied through systemic analysis by Chen et al., focusing on the molecular mechanism of LINC00853. The study also developed a prognostic signature based on four lncRNAs and analyzed LINC00853’s role in glycolysis and cell proliferation [232, 233].
Tumor metabolism is a term used to describe the metabolic processes altered within cancer cells to promote their rapid growth, survival, and proliferation [234]. The defining feature of cancer cells is the presence of altered metabolic characteristics, distinct from those of normal cells, allowing them to out-compete normal cells in the harsh and often nutrient-poor conditions of tumors [235, 236]. For example, many cancer cells display increased glucose uptake and glycolysis rates. This metabolic characteristic has been used diagnostically in positron emission tomography by tagging a radiolabeled glucose analogue [237]. Upregulation of glucose transporters, such as GLUT1, and the key glycolytic enzymes, such as hexokinase 2, fuel the high glycolytic flux characteristic of many tumors [238]. The end product of glycolysis in cancer cells is lactate, which is perfused into the local microenvironment, contributing to the acidosis of the tumor microenvironment, which can promote cancer cell invasion and metastasis [239, 240]. Wang et al. studied the relationship between cuproptosis-related lncRNAs and the metabolism of tumors and the immune microenvironment and pancreatic cancer. They constructed a predictive model in their analysis, which determined that higher risk scores correlated with a worse cancer survival prognosis and a more immunosuppressive panorama in pancreatic cancer [241]. A study by Chen et al. developed a prognostic signature built on the cuproptosis-related lncRNAs and discovered its association with the prognosis of pancreatic cancer and tumor microenvironment. The study highlights the importance of the signature as a potential biomarker for prognosis and effect on drug targets [242].
LncRNAs in cuproptosis-mediated liver cancer
HCC accounts for roughly 75–85% of all liver cancer cases [243]. The prognosis for liver cancer is highly varied, dependent on how advanced the cancer is and the relative health of the patient [244]. Early liver cancer that is normally surgically removed or treated with transplantation has a relatively good prognosis [245]. The five-year survival rates are elevated, bridging 30–50% [246]. For advanced liver cancer patients, the prognosis is less good, with 5-year survival rates roughly under 20% [247]. Liu et al. developed and validated a predictive model for liver cancer based on cuproptosis-related mRNAs and lncRNAs. Five-year survival performances evaluate the prediction capacity of our model. With the model, other features, including the estimations of immune cell infiltration, TMB, and antitumor drug sensitivity, are also useful for clinical applications, as shown in Fig. 4 [248].
CYTOR is a novel, non-coding RNA regulating cell growth and proliferation [249]. It behaves as a molecular scaffold, which exerts a widespread effect on the signaling pathways and expression of the genes [250]. CYTOR’s dysregulation has been observed in cancer, creating an excellent peculiarity of a potential therapeutic target [251]. Chen et al. constructed a prognostic signature based on the cuproptosis-related lncRNAs in HCC, such as CYTOR, LINC00205, and LINC01184. The signature demonstrated an excellent perspective in forecasting the prognosis and the response to immunotherapy by the patient, which has been verified by both internal assessment and in vitro studies [252]. HCC is the most common type of liver cancer and develops in the context of chronic liver disease such as cirrhosis or hepatitis [253]. It is characterized by fast growth, poor prognosis, and resistance to therapy, so early detection and timely treatment are essential for the survival of patients [254, 255]. Wang et al. present a cuproptosis-related lncRNA risk model for HCC and identifies nine prognostic lncRNAs. When this model is applied, it is possible to precisely predict patients’ prognosis, their sensitivity to immunotherapy, and the condition of the immune microenvironment of their tumor. All this indicates that it is of high clinical value to predict which patients are likely to respond positively to immunotherapy [256]. Evaluating chemotherapy effectiveness means assessing whether the treatment has succeeded, which can be determined through a range of clinical outcomes, such as tumor shrinkage, progression-free survival, and overall survival [257]. Imaging studies, biomarker analysis, and patient-reported outcomes are the techniques for assessing these results [258]. Such evaluation can help decide improvement made in the regimen, while the outcomes are used to monitor patient care [259]. Liu et al. developed a predictive model for HCC with eight lncRNAs associated with cuproptosis.
Along with predicting patient outcomes, the model can be used to evaluate chemotherapy effectiveness; the differences found in immune functions and drug sensitivity between high- and low-risk groups are significant [260]. Non-apoptotic therapeutics target processes not specifically involved in the induction of apoptosis [261]. These alternatives include actions related to necroptosis, autophagy, and pyroptosis, among other mechanisms [42]. Applying these therapies offers new approaches to modulating diseases and providing additional options for apoptotic pathways [262]. Non-apoptotic therapeutics are viable in various cases and bring greater advances in treatment methods [263]. Zhang et al. developed a cuproptosis-related lncRNA signature for hepatocellular carcinoma to predict patients’ overall survival and response to immunotherapy. Different analyses validated the examination and provided a new perspective on HCC treatment not based on apoptosis [264] (Fig. 4; Table 1).
Tumor heterogeneity and lncRNAs in cuproptosis
The expression and functionality of lncRNAs may be strongly impacted by this variability, which makes it more difficult to identify universal diagnostics or effective treatment approaches for cancer [267]. Due to genetic and epigenetic variations, many cancer types often have unique lncRNA expression patterns. For instance, lung cancer has high expression levels of MALAT1, which encourages metastasis and adds to treatment resistance [268]. On the other hand, MALAT1 in liver cancer has been linked to the promotion of tumor development via a variety of mechanisms, including angiogenesis and cell cycle modulation [269]. The difficulty of using MALAT1 as a universal biomarker for cuproptosis is highlighted by the variation in its function across various malignancies [270]. While MALAT1 may have a less direct role in this cell death pathway in liver cancer, it may affect lung cancer patients' responsiveness to medicines that cause cuproptosis by controlling genes associated with mitochondrial activity [271].
Different parts of a single tumor may express different amounts of lncRNA because of interactions with immune cells, hypoxia, or the availability of nutrients [272]. HOTAIR is elevated in some tumor areas in breast cancer, especially those with high hypoxia levels. HOTAIR can alter gene expression and chromatin remodeling, which varies the tumor reaction to treatment [273]. Because of improved survival signaling pathways, tumor areas with high HOTAIR expression may be more resistant to cuproptosis-inducing drugs. In contrast, tumor regions with low HOTAIR expression may be more vulnerable to such treatments. Because of this intra-tumoral heterogeneity, HOTAIR is not a reliable predictive diagnostic for the whole tumor [274].
The extracellular matrix, fibroblasts, and immune cells that make up the TME are important factors in the expression of lncRNA [275]. The TME affects the lncRNA PVT1 in pancreatic cancer, especially in regions with a high stromal composition. It has been shown that PVT1 interacts with TME elements to support cancer cell survival and apoptosis resistance [276]. PVT1 may influence the oxidative stress response and copper ion homeostasis in the setting of cuproptosis, increasing the resistance of certain tumor areas to cell death [277]. Rather than depending only on single lncRNAs for prognosis, biomarker panels must be used due to the variability in lncRNA expression [278]. LncRNAs, including MALAT1, H19, and NEAT1, have been suggested to predict patient outcomes in lung cancer [268] more precisely. The combined study of these lncRNAs may provide a more thorough knowledge of the behavior of the tumor, including its response to cuproptosis-inducing treatments. These lncRNAs are engaged in several aspects of tumor biology, including proliferation, metastasis, and cell death control [279].
Clinical relevance of lncRNAs in cuproptosis
LncRNAs have the potential to be predictive biomarkers in cuproptosis-mediated cancer treatment; nevertheless, in order to ensure practical application, extensive validation is necessary [280]. Because cancer is a very varied disease, biomarker validation is a multi-step procedure that involves determining the sensitivity, specificity, and repeatability of lncRNAs across a range of patient populations [281]. Determining the sensitivity and specificity of lncRNAs and their capacity to identify people with the illness and those without it accurately is essential to using them as trustworthy biomarkers [282]. The PVT1, which has been linked to several malignancies, has shown potential as a biomarker for prognosticating patients and therapeutic response. Its expression levels, however, may change dramatically across cancer types and even among people who have the same cancer type. Therefore, it's crucial to set a constant threshold for PVT1 expression that reliably forecasts cuproptosis-related events [283].
Another important component in the validation of biomarkers is reproducibility. In breast and colorectal malignancies, the lncRNA HOTAIR has been thoroughly investigated and has been linked to a poor prognosis as well as treatment resistance [284]. HOTAIR must, however, show consistent predictive efficacy across many cohorts, including individuals with various genetic backgrounds and clinical circumstances, in order to be validated as a biomarker for cuproptosis [281]. Clinical validation includes large-scale research to assess the biomarker's usefulness in real-world scenarios after its original discovery and validation in preclinical investigations. For example, the lncRNA MALAT1 has been investigated as a lung cancer prognostic marker [285].
Data integration
To better understand the involvement of lncRNAs in cuproptosis, a recently identified kind of programmed cell death connected to copper metabolism, it is imperative to integrate multiple datasets. Through the integration of genetic, transcriptome, proteome, and clinical data, scientists may reveal complex connections between lncRNAs and cuproptosis, which might provide novel treatment opportunities [81]. The GAE-LGA model uses graph autoencoders to integrate multi-omics data and find relationships between lncRNAs and protein-coding genes (PCGs). The model successfully predicts lncRNA-PCG connections by capturing functional similarities and cross-omics correlations. Using this method, scientists may investigate how lncRNAs might interfere with PCG activities and affect cuproptosis pathways. The incorporation of multi-omics characteristics considerably enhances the resilience of these forecasts, offering an effective instrument for comprehending the regulatory functions of lncRNAs in cuproptosis [286].
Cuproptosis-related lncRNAs in lung adenocarcinoma (LUAD) were identified by combining gene expression, clinical outcomes, and mutation data from The Cancer Genome Atlas (TCGA). Through the use of both univariate and multivariate Cox regression analysis, a predictive model including 15 lncRNAs linked to cuproptosis was created. This model demonstrates the potential of integrated data to aid clinical decision-making and treatment options since it not only predicted patient survival but also offered insights into immune escape pathways and medication sensitivity [287]. TCGA data were used in research on gastric cancer to create a nomogram based on lncRNAs associated with cuproptosis. Key lncRNAs that may function as prognostic indicators were found by the researchers by combining gene expression patterns with clinical and survival data. Kaplan–Meier survival analysis was used to evaluate the model's efficacy and show how integrated techniques might improve knowledge of lncRNA contributions to cancer development and patient outcomes [187].
Challenges
LncRNAs are exceedingly difficult to target therapeutically because of their complicated structures and the need for very specialized delivery methods to prevent off-target effects [288]. Since lncRNAs do not encode proteins, their modes of action often include complex interactions with proteins, RNA, and DNA that may vary greatly based on the biological setting [1]. Because of this intricacy, specific techniques for lncRNA regulation must be developed, especially when it comes to cuproptosis, where aberrant lncRNA activity may either promote or prevent cell death [289]. Secondary structures, including hairpins, loops, and pseudoknots, are often seen in lncRNAs and are essential to their function. It isn't easy to create compounds that can selectively target lncRNAs without interfering with other RNA species because of these structural characteristics [290]. Many strategies are being investigated to modify lncRNA activity, including CRISPR/Cas9-based technologies, siRNAs, and antisense oligonucleotides (ASOs) [291]. ASOs may be made to attach to certain sequences in lncRNAs, causing them to degrade or preventing them from interacting with target molecules. However, the stability of the lncRNA-ASO complexes in the cellular environment and the accessibility of the target sequences determine how successful these techniques are [292].
Another significant obstacle is the delivery of medicinal drugs that target lncRNAs. In order to minimize off-target effects, efficient delivery methods must guarantee that the therapeutic molecules reach the targeted cells and tissues in adequate amounts [293]. To improve the effectiveness and selectivity of lncRNA-targeted treatments, lipid-based carriers, viral vectors, and nanoparticle-based delivery methods are being developed [293]. Lipid nanoparticles have shown effective in delivering siRNAs that target the long non-coding RNA MALAT1 in preclinical models of lung cancer. Since lncRNAs may work in a variety of tissues and biological pathways, the possibility of off-target consequences is still a serious worry [293].
A thorough grasp of lncRNA function in cuproptosis and other cancer-related pathways is necessary in order to integrate them into precision medicine approaches. Personalized techniques that modify therapy according to the unique long non-coding RNA expression patterns of a patient's tumor [294]. Tailored medicines may be created to block the action of the lncRNA HOTAIR, which is increased in malignancies and linked to a bad prognosis. This would make the tumor more susceptible to cuproptosis. Nevertheless, more testing is necessary to guarantee that focusing on HOTAIR won't negatively impact regular cellular processes [295].
Conclusion
The exploration of lncRNAs can be used as prognostic markers and therapeutic targets in cuproptosis-mediated cancer; it presents a promising way to develop cancer diagnostics and treatment. Cuproptosis is a newly identified form of programmed cell death regulated by copper ions and distinguished from apoptosis, necroptosis, and ferroptosis, which are induced by similar biochemical pathways but regulated by glucose restriction. The findings from various research studies emphasize the significant involvement of lncRNAs in modulating key pathways associated with cuproptosis across different cancer types, including breast, lung, ovarian, gastric, and pancreatic cancers. Our analysis reveals that specific lncRNAs, such as MALAT1, HOTAIR, and PVT1, interact with crucial proteins and pathways that govern mitochondrial function and oxidative stress responses, thereby influencing the susceptibility of cancer cells to cuproptosis. The identification of these lncRNAs as potential biomarkers opens new avenues for predicting cancer prognosis and therapy response. For instance, the SLC31A1-related axis in breast cancer and the role of specific lncRNAs in LAUD have shown promising results in predicting patient outcomes and guiding therapeutic strategies.
Moreover, several studies have developed predictive models based on cuproptosis-related lncRNAs, showing their potential to predict patient outcomes, therapy sensitivity, and immune response. These models, such as those applied in lung adenocarcinoma and hepatocellular carcinoma, underscore the clinical relevance of lncRNAs in personalized cancer treatment. Furthermore, the study underscores the potential of leveraging these lncRNAs as therapeutic targets to induce cuproptosis selectively in cancer cells, offering a novel approach to overcome resistance to conventional therapies. The development of predictive models based on cuproptosis-related lncRNAs, as seen in ovarian and pancreatic cancers, further supports the clinical relevance of these molecules in personalizing cancer treatment.
Future perspectives
Emerging research directions
Emerging research direction in cuproptosis and lncRNAs emerges and can revolutionize our understanding and treatment of diseases, mostly cancer [296]. Mechanistic insights in cuproptosis have been a focus of investigations. However, on the one hand, the interests of scientists are directed at understanding how mitochondrial failure and cell death are triggered by the accumulation of copper [297]. Several investigations have been conducted to understand molecular interactions and signaling pathways of copper, mitochondrial proteins, and events in cuproptosis [298]. On the other hand, intensive attention is paid to understanding how the processes of copper metabolism and cuproptosis are regulated by lncRNAs [299]. There are lncRNAs capable of controlling the expression of copper transfer systems, including CTR1 ATP7A and ATP7B, responsible for the uptake and efflux of copper [300]. The assumption is that these carriers and modulators contribute to increased intracellular copper levels and cuproptosis susceptibility [301]. Moreover, the production of COUP-TFII lncRNA is considered to govern mitochondrial-driven stress response and, thus, copper-induced damage [302].
Therapeutic research has not been left behind, and an emerging research direction is the cultivation of novel treatments modulating cuproptosis. The efforts are directed to damage cancer cells [299] specifically. As a result, several copper-modulating agents catching scientists' attention are created [303]. Copper chelators are produced for intracellular copper level reduction [304]. Some drugs induce cuproptosis only; these novel drugs kill cancer cells and contribute to resistance overcoming [305]. An emerging research direction of biomarker discovery is to identify cuproptosis-associated expression profiles of lncRNA [306]. This may lead to novel methods of cancer diagnosis, early cancer identification, and monitoring of the disease process to administer the best allergy treatment. Individualized treatment strategies may also be formed as a result [307].
Potential clinical applications
The investigation into cuproptosis and its regulation by lncRNAs presents valuable clinical applications, particularly in cancer treatment [308]. One of the primary target treatment options revolves around investigating and utilizing the mechanism through which cuproptosis kills cancerous cells rather than just healthy ones [309]. Copper-induced cell death is a potentially promising treatment avenue due to the improved susceptibility of cancer cells to this particular symptom [310]. Scientists are developing both cuproptosis-inducing drugs and medications that alter the metabolism of copper as a potential approach to treatment [311]. For instance, copper chelators could affect the intracellular levels of copper [312]. While present in healthy cells, the compounds that only kill tumor cells could also revolutionize the treatment industry, rescuing many patients who are resistant to existing therapeutic processes [313].
Similarly, cuproptosis-related and lncRNA monitoring biomarkers can significantly increase the accuracy of cancer diagnostics and overall prognosis [314]. This mechanism can allow scientists to find cancer solutions within a timeframe starting earlier than the first symptoms and until the imminent danger of metastasis [315]. The monitoring processes can assess the individual treatment plans for effectiveness against a particular type of cancer and plan new product development and testing accordingly [316]. Combining different therapy types based on the conference material, such as chemotherapy, immunotherapy, or targeted therapy in conjunction with cuproptosis-inducing agents, can also improve the overall practical effectiveness of these treatments and counter-resistance [317].
Data availability
No datasets were generated or analyzed during the current study.
References
Statello L, et al. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22(2):96–118.
Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220(2):e202009045.
Ahadi A. Functional roles of lncRNAs in the pathogenesis and progression of cancer. Genes Dis. 2021;8(4):424–37.
Wang X, Jing H, Li H. A novel cuproptosis-related lncRNA signature to predict prognosis and immune landscape of lung adenocarcinoma. Transl Lung Cancer Res. 2023;12(2):230–46.
Wu L, et al. A novel cuproptosis-related lncRNAs signature predicts prognosis in bladder cancer. Aging (Albany NY). 2023;15(13):6445–66.
Guo C, et al. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8(21):2003–14.
Abdullah KM, et al. Copper metabolism and cuproptosis in human malignancies: Unraveling the complex interplay for therapeutic insights. Heliyon. 2024;10(5):e27496.
Wang Z, et al. Regulatory roles of copper metabolism and cuproptosis in human cancers. Front Oncol. 2023;13:1123420.
Liang XR, et al. Cell cycle-related lncRNAs as innovative targets to advance cancer management. Cancer Manag Res. 2023;15:547–61.
Luan M, et al. Mechanism of metal ion-induced cell death in gastrointestinal cancer. Biomed Pharmacother. 2024;174:116574.
Yoon JH, Abdelmohsen K, Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol. 2013;425(19):3723–30.
Nitsche A, et al. Comparison of splice sites reveals that long noncoding RNAs are evolutionarily well conserved. RNA. 2015;21(5):801–12.
Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193(3):651–69.
Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10(6):925–33.
Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–66.
Zhang X, et al. Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int J Mol Sci. 2019;20(22):5573.
Vance KW, Ponting CP. Transcriptional regulatory functions of nuclear long noncoding RNAs. Trends Genet. 2014;30(8):348–55.
Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19(3):143–57.
Villegas VE, Zaphiropoulos PG. Neighboring gene regulation by antisense long non-coding RNAs. Int J Mol Sci. 2015;16(2):3251–66.
Schvartzman JM, Thompson CB, Finley LWS. Metabolic regulation of chromatin modifications and gene expression. J Cell Biol. 2018;217(7):2247–59.
Saxena A, Carninci P. Long non-coding RNA modifies chromatin: epigenetic silencing by long non-coding RNAs. BioEssays. 2011;33(11):830–9.
Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–14.
McKay BC. Post-transcriptional regulation of DNA damage-responsive gene expression. Antioxid Redox Signal. 2014;20(4):640–54.
Malakar P, et al. The nexus of long noncoding RNAs, splicing factors, alternative splicing and their modulations. RNA Biol. 2024;21(1):1–20.
Nadhan R, et al. Signaling by LncRNAs: Structure, cellular homeostasis, and disease pathology. Cells. 2022;11(16):2517.
Perrimon N, Pitsouli C, Shilo BZ. Signaling mechanisms controlling cell fate and embryonic patterning. Cold Spring Harb Perspect Biol. 2012;4(8):a005975.
Kumar A, et al. Targeting autophagy using long non-coding RNAs (LncRNAs): New landscapes in the arena of cancer therapeutics. Cells. 2023;12(5):810.
Wu J, et al. Cuproptosis: Mechanism, role, and advances in urological malignancies. Med Res Rev. 2024;44(4):1662–82.
Huang M, Zhang Y, Liu X. The mechanism of cuproptosis in Parkinson’s disease. Ageing Res Rev. 2024;95:102214.
Xiong C, et al. Cuproptosis: p53-regulated metabolic cell death? Cell Death Differ. 2023;30(4):876–84.
Yang Y, et al. Exploring cuproptosis as a mechanism and potential intervention target in cardiovascular diseases. Front Pharmacol. 2023;14:1229297.
Yuan HJ, Xue YT, Liu Y. Cuproptosis, the novel therapeutic mechanism for heart failure: a narrative review. Cardiovasc Diagn Ther. 2022;12(5):681–92.
Cobine PA, Brady DC. Cuproptosis: Cellular and molecular mechanisms underlying copper-induced cell death. Mol Cell. 2022;82(10):1786–7.
Guo J, Sun Y, Liu G. The mechanism of copper transporters in ovarian cancer cells and the prospect of cuproptosis. J Inorg Biochem. 2023;247:112324.
Li D, et al. Cuproptosis-a potential target for the treatment of osteoporosis. Front Endocrinol (Lausanne). 2023;14:1135181.
Liu N, Chen M. Crosstalk between ferroptosis and cuproptosis: From mechanism to potential clinical application. Biomed Pharmacother. 2024;171:116115.
Tang D, Kroemer G, Kang R. Targeting cuproplasia and cuproptosis in cancer. Nat Rev Clin Oncol. 2024;21(5):370–88.
Zhu Z, et al. Comprehensive analysis of cuproptosis-related lncRNAs to predict prognosis and immune infiltration characteristics in colorectal cancer. Front Genet. 2022;13:984743.
Gao L, Zhang A. Copper-instigated modulatory cell mortality mechanisms and progress in oncological treatment investigations. Front Immunol. 2023;14:1236063.
Vo TTT, et al. The crosstalk between copper-induced oxidative stress and cuproptosis: a novel potential anticancer paradigm. Cell Commun Signal. 2024;22(1):353.
Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol. 2020;21(11):678–95.
Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18(5):1106–21.
Thapa R, et al. From LncRNA to metastasis: The MALAT1-EMT axis in cancer progression. Pathol-Res Practice, 2023: 154959.
Chakraborty A, et al. Programmed cell death in aortic aneurysm and dissection: A potential therapeutic target. J Mol Cell Cardiol. 2022;163:67–80.
Chi H, Chang HY, Sang TK. Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci. 2018;19(10):3082.
D’Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43(6):582–92.
Denton D, Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019;26(4):605–16.
Kist M, Vucic D. Cell death pathways: intricate connections and disease implications. Embo j. 2021;40(5):e106700.
Li J, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88.
Peng F, et al. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther. 2022;7(1):286.
Cheng B, et al. Cuproptosis illustrates tumor micro-environment features and predicts prostate cancer therapeutic sensitivity and prognosis. Life Sci. 2023;325:121659.
Huang Y, Yin D, Wu L. Identification of cuproptosis-related subtypes and development of a prognostic signature in colorectal cancer. Sci Rep. 2022;12(1):17348.
Sha S, et al. Prognostic analysis of cuproptosis-related gene in triple-negative breast cancer. Front Immunol. 2022;13:922780.
Shi B, et al. The therapeutic and prognostic role of cuproptosis-related genes in triple negative breast cancer. BMC Bioinformatics. 2023;24(1):223.
Song Q, et al. Cuproptosis scoring system to predict the clinical outcome and immune response in bladder cancer. Front Immunol. 2022;13:958368.
Wang F, et al. Cuproptosis-related lncRNA predict prognosis and immune response of lung adenocarcinoma. World J Surg Oncol. 2022;20(1):275.
Wang Y, et al. Cuproptosis: A novel therapeutic target for overcoming cancer drug resistance. Drug Resist Updat. 2024;72:101018.
Zhang W, et al. Identification of cuproptosis and immune-related gene prognostic signature in lung adenocarcinoma. Front Immunol. 2023;14:1179742.
Zhao Q, Qi T. The implications and prospect of cuproptosis-related genes and copper transporters in cancer progression. Front Oncol. 2023;13:1117164.
Lehár J, et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat Biotechnol. 2009;27(7):659–66.
Springer C, Humayun D, Skouta R. Cuproptosis: Unraveling the mechanisms of copper-induced cell death and its implication in cancer therapy. Cancers (Basel). 2024;16(3):647.
Bian C, et al. Copper homeostasis and cuproptosis in tumor pathogenesis and therapeutic strategies. Front Pharmacol. 2023;14:1271613.
Xie J, et al. Cuproptosis: mechanisms and links with cancers. Mol Cancer. 2023;22(1):46.
Zhou C, et al. Copper metabolism and hepatocellular carcinoma: current insights. Front Oncol. 2023;13:1186659.
Singh AK, et al. Current therapeutic modalities and chemopreventive role of natural products in liver cancer: Progress and promise. World J Hepatol. 2023;15(1):1–18.
Wang L, et al. A novel copper-induced cell death-related lncRNA prognostic signature associated with immune infiltration and clinical value in gastric cancer. J Cancer Res Clin Oncol. 2023;149(12):10543–59.
Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022;7(1):378.
Zhang C, et al. The biological function and potential mechanism of long non-coding RNAs in cardiovascular disease. J Cell Mol Med. 2020;24(22):12900–9.
Gupta A, Lutsenko S. Human copper transporters: mechanism, role in human diseases and therapeutic potential. Future Med Chem. 2009;1(6):1125–42.
Petruzzelli R, Polishchuk RS. Activity and trafficking of copper-transporting atpases in tumor development and defense against platinum-based drugs. Cells. 2019;8(9):1080.
Jiang N, et al. Progress in understanding the role of lncRNA in programmed cell death. Cell Death Discovery. 2021;7(1):30.
Cavalcante GC, et al. Mitochondrial epigenetics: Non-coding RNAs as a novel layer of complexity. Int J Mol Sci. 2020;21(5):1838.
Picard M, McEwen BS. Psychological stress and mitochondria: A systematic review. Psychosom Med. 2018;80(2):141–53.
Kang L, et al. Long noncoding RNA ANPODRT overexpression protects nucleus pulposus cells from oxidative stress and apoptosis by activating keap1-Nrf2 signaling. Oxid Med Cell Longev. 2021;2021:6645005.
Zhang L, et al. Cuproptosis combined with lncRNAs predicts the prognosis and immune microenvironment of breast cancer. Comput Math Methods Med. 2022;2022:5422698.
Lee CR, et al. Strategies to minimize antibiotic resistance. Int J Environ Res Public Health. 2013;10(9):4274–305.
Mahmoudi Z, et al. Efficacy of DMARDs and methylprednisolone treatment on the gene expression levels of HSPA5, MMD, and non-coding RNAs MALAT1, H19, miR-199a-5p, and miR-1-3p, in patients with rheumatoid arthritis. Int Immunopharmacol. 2022;108:108878.
Wang J, et al. Progress in the research of cuproptosis and possible targets for cancer therapy. World J Clin Oncol. 2023;14(9):324–34.
Feng Q, et al. Research progress on cuproptosis in cancer. Front Pharmacol. 2024;15:1290592.
Bhan A, Mandal SS. LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim Biophys Acta. 2015;1856(1):151–64.
Bai X, et al. Cuproptosis-related lncRNA signature as a prognostic tool and therapeutic target in diffuse large B cell lymphoma. Sci Rep. 2024;14(1):12926.
Chen Y, et al. Novel cuproptosis-related lncRNAs can predict the prognosis of patients with multiple myeloma. Transl Cancer Res. 2023;12(11):3074–87.
Thapa R, et al. New horizons in lung cancer management through ATR/CHK1 pathway modulation. Future Med Chem. 2023;15(19):1807–18.
Chen YT, et al. Identification of three cuproptosis-specific expressed genes as diagnostic biomarkers and therapeutic targets for atherosclerosis. Int J Med Sci. 2023;20(7):836–48.
Guo J, et al. The prognosis and immunotherapy prediction model of ovarian serous cystadenocarcinoma patient was constructed based on cuproptosis-related LncRNA. Tohoku J Exp Med. 2024;262(2):63–74.
Sun L, et al. Construction and significance of a breast cancer prognostic model based on cuproptosis-related genotyping and lncRNAs. J Formos Med Assoc, 2024.
Wang K, et al. Long non-coding RNAs in ferroptosis and cuproptosis impact on prognosis and treatment in hepatocellular carcinoma. Clin Exp Med. 2024;24(1):135.
Zhang D, et al. Comprehensive analysis of a cuproptosis-related ceRNA network implicates a potential endocrine therapy resistance mechanism in ER-positive breast cancer. BMC Med Genomics. 2023;16(1):96.
Chen W, et al. Clinical significance of non-coding RNA regulation of programmed cell death in hepatocellular Carcinoma. Cancers (Basel). 2023;15(16):4187.
Baba SK, et al. Long non-coding RNAs modulate tumor microenvironment to promote metastasis: novel avenue for therapeutic intervention. Front Cell Dev Biol. 2023;11:1164301.
Riquelme I, et al. Long non-coding RNAs (lncRNAs) as Regulators of the PI3K/AKT/mTOR pathway in gastric Carcinoma. Int J Mol Sci. 2023;24(7):6294.
Liu J, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3.
Lin S, et al. Roles of Wnt/β-catenin signaling pathway regulatory long non-coding RNAs in the pathogenesis of non-small cell lung cancer. Cancer Manag Res. 2020;12:4181–91.
Zhang J, et al. MALAT1 inhibits the Wnt/β-catenin signaling pathway in colon cancer cells and affects cell proliferation and apoptosis. Bosn J Basic Med Sci. 2020;20(3):357–64.
Wang H, et al. Targeting p53 pathways: mechanisms, structures, and advances in therapy. Signal Transduct Target Ther. 2023;8(1):92.
Lin T, et al. Emerging roles of p53 related lncRNAs in cancer progression: A systematic review. Int J Biol Sci. 2019;15(6):1287–98.
Shi Y, Norberg E, Vakifahmetoglu-Norberg H. Mutant p53 as a regulator and target of autophagy. Front Oncol. 2020;10:607149.
Li A, et al. Mitochondrial autophagy: molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022;13(5):444.
Akram M, et al. Awareness and current knowledge of breast cancer. Biol Res. 2017;50(1):33.
Katsura C, et al. Breast cancer: presentation, investigation and management. Br J Hosp Med (Lond). 2022;83(2):1–7.
Kolak A, et al. Primary and secondary prevention of breast cancer. Ann Agric Environ Med. 2017;24(4):549–53.
Li Z, et al. The role of progesterone receptors in breast cancer. Drug Des Devel Ther. 2022;16:305–14.
Maughan KL, Lutterbie MA, Ham PS. Treatment of breast cancer. Am Fam Physician. 2010;81(11):1339–46.
Bonilla JM, Tabanera MT, Mendoza LR. Breast cancer in the 21st century: from early detection to new therapies. Radiologia. 2017;59(5):368–79.
Veronesi U, et al. Breast cancer. Lancet. 2005;365(9472):1727–41.
Ballantyne D, Scheid P. Central chemosensitivity of respiration: a brief overview. Respir Physiol. 2001;129(1–2):5–12.
Delmas D, et al. Silymarin and cancer: A dual strategy in both in chemoprevention and chemosensitivity. Molecules. 2020;25(9):2009.
Huckstepp RT, Dale N. Redefining the components of central CO2 chemosensitivity–towards a better understanding of mechanism. J Physiol. 2011;589(Pt 23):5561–79.
Li Y, et al. Lovastatin enhances chemosensitivity of paclitaxel-resistant prostate cancer cells through inhibition of CYP2C8. Biochem Biophys Res Commun. 2022;589:85–91.
Loeschcke HH. Respiratory chemosensitivity in the medulla oblongata. Acta Neurobiol Exp (Wars). 1973;33(1):97–112.
Wu J-H, et al. Identification of cuproptosis-related gene SLC31A1 and upstream LncRNA-miRNA regulatory axis in breast cancer. Sci Rep. 2023;13(1):18390.
Kamenov K, et al. The efficacy of psychotherapy, pharmacotherapy and their combination on functioning and quality of life in depression: a meta-analysis. Psychol Med. 2017;47(3):414–25.
Salleh MR. Life event, stress and illness. Malays J Med Sci. 2008;15(4):9–18.
Ahmad A, Imran M, Ahsan H. Biomarkers as biomedical bioindicators: approaches and techniques for the detection, analysis, and validation of novel biomarkers of diseases. Pharmaceutics. 2023;15(6):1630.
Faith LA, Hillis-Mascia JD, Wiesepape CN. How does individual psychotherapy promote recovery for persons with psychosis? A systematic review of qualitative studies to understand the patient’s experience. Behav Sci. 2024;14(6):460.
Wu X, et al. Cuproptosis-related lncRNAs potentially predict prognosis and therapy sensitivity of breast cancer. Front Pharmacol. 2023;14:1199883.
Darvin P, et al. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11.
Mogilenko DA, Shchukina I, Artyomov MN. Immune ageing at single-cell resolution. Nat Rev Immunol. 2022;22(8):484–98.
Mou P, et al. Research progress on the immune microenvironment and immunotherapy in gastric cancer. Front Immunol. 2023;14:1291117.
Jiang ZR, et al. Identification of novel cuproptosis-related lncRNA signatures to predict the prognosis and immune microenvironment of breast cancer patients. Front Oncol. 2022;12:988680.
Xu QT, et al. A novel cuproptosis-related prognostic 2-lncRNAs signature in breast cancer. Front Pharmacol. 2022;13:1115608.
Pan Y, et al. Prognostic and immune microenvironment analysis of cuproptosis-related LncRNAs in breast cancer. Funct Integr Genomics. 2023;23(1):38.
Abril-Rodriguez G, Ribas A. SnapShot: Immune checkpoint inhibitors. Cancer Cell. 2017;31(6):848-848.e1.
Barroso-Sousa R, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173–82.
Galluzzi L, et al. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17(12):725–41.
Yu H, et al. A signature of cuproptosis-related lncRNAs predicts prognosis and provides basis for future anti-tumor drug development in breast cancer. Trans Cancer Res. 2023;12(6):1392–410.
Jiang B, et al. Database mining detected a cuproptosis-related prognostic signature and a related regulatory axis in breast cancer. Dis Markers. 2022;2022(1):9004830.
Abu Rous F, et al. Lung cancer treatment advances in 2022. Cancer Invest. 2023;41(1):12–24.
Bade BC, Dela Cruz CS. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin Chest Med. 2020;41(1):1–24.
Collins LG, et al. Lung cancer: diagnosis and management. Am Fam Physician. 2007;75(1):56–63.
de Sousa VML, Carvalho L. Heterogeneity in lung cancer. Pathobiology. 2018;85(1–2):96–107.
Jones GS, Baldwin DR. Recent advances in the management of lung cancer. Clin Med (Lond). 2018;18(Suppl 2):s41–6.
Lee E, Kazerooni EA. Lung cancer screening. Semin Respir Crit Care Med. 2022;43(6):839–50.
Mao Y, et al. Epidemiology of lung cancer. Surg Oncol Clin N Am. 2016;25(3):439–45.
Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am. 2019;103(3):463–73.
Rodriguez-Canales J, Parra-Cuentas E, Wistuba II. Diagnosis and molecular classification of lung cancer. Cancer Treat Res. 2016;170:25–46.
Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–40.
Cerna D, Camphausen K, Tofilon PJ. Histone deacetylation as a target for radiosensitization. Curr Top Dev Biol. 2006;73:173–204.
Cui L, et al. Radiosensitization by gold nanoparticles: Will they ever make it to the clinic? Radiother Oncol. 2017;124(3):344–56.
Dwarakanath BS. Cytotoxicity, radiosensitization, and chemosensitization of tumor cells by 2-deoxy-D-glucose in vitro. J Cancer Res Ther. 2009;5(Suppl 1):S27-31.
Xu Q, Liu T, Wang J. Radiosensitization-related cuproptosis LncRNA signature in non-small cell lung cancer. Genes. 2022;13(11):2080.
Yu S, et al. A cuproptosis-related lncRNA signature for predicting prognosis and immunotherapy response of lung adenocarcinoma. Hereditas. 2023;160(1):31.
Dong H, et al. Integrated bioinformatic analysis reveals the underlying molecular mechanism of and potential drugs for pulmonary arterial hypertension. Aging (Albany NY). 2021;13(10):14234–57.
Kleino I, et al. Computational solutions for spatial transcriptomics. Comput Struct Biotechnol J. 2022;20:4870–84.
Wang X, Jing H, Li H. A novel cuproptosis-related lncRNA signature to predict prognosis and immune landscape of lung adenocarcinoma. Transl Lung Cancer Rese. 2023;12(2):230–46.
Nakayama J, et al. Inhibition of the proliferation of a malignant peripheral nerve sheath tumor cell line by gamma interferon gene transfection. J Dermatol. 2003;30(12):879–85.
Wang Y, et al. PD-L1 regulates tumor proliferation and T-cell function in NF2-associated meningiomas. CNS Neurosci Ther. 2024;30(6): e14784.
Wu J, et al. Predictive value of cyst/tumor volume ratio of pituitary adenoma for tumor cell proliferation. BMC Med Imaging. 2024;24(1):69.
Thapa R, et al. Unlocking the potential of mesoporous silica nanoparticles in breast cancer treatment. J Nanopart Res. 2023;25(8):169.
Zhao H, et al. A five-cuproptosis-related LncRNA Signature: predicting prognosis, assessing immune function & drug sensitivity in lung squamous cell carcinoma. J Cancer. 2023;14(9):1499–514.
Balzar S. Continuous dual resetting of the immune repertoire as a basic principle of the immune system function. J Immunol Res. 2017;2017:3760238.
Balzar S. Self-centered function of adaptive immunity in regulation of immune responses and in tolerance. J Immunol Res. 2021;2021:7507459.
Bettigole SE, Glimcher LH. Endoplasmic reticulum stress in immunity. Annu Rev Immunol. 2015;33:107–38.
Gleeson M, Nieman DC, Pedersen BK. Exercise, nutrition and immune function. J Sports Sci. 2004;22(1):115–25.
An Y, et al. The role of copper homeostasis in brain disease. Int J Mol Sci. 2022;23(22):13580.
Chen X, et al. Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death Dis. 2023;14(2):105.
Chen Z, Li YY, Liu X. Copper homeostasis and copper-induced cell death: Novel targeting for intervention in the pathogenesis of vascular aging. Biomed Pharmacother. 2023;169:115839.
Ma C, et al. Crosstalk between copper homeostasis and cuproptosis reveals a lncRNA signature to prognosis prediction, immunotherapy personalization, and agent selection for patients with lung adenocarcinoma. Aging (Albany NY). 2023;15(22):13504–41.
Kossaï M, et al. Ovarian cancer: A heterogeneous disease. Pathobiology. 2018;85(1–2):41–9.
Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol. 2018;131(5):909–27.
Morand S, et al. Ovarian cancer immunotherapy and personalized medicine. Int J Mol Sci. 2021;22(12):6532.
Penny SM. Ovarian cancer: An overview. Radiol Technol. 2020;91(6):561–75.
Roett MA, Evans P. Ovarian cancer: An overview. Am Fam Phys. 2009;80(6):609–16.
Wang Y, et al. Cuproptosis-related lncRNAs ovarian cancer: Multi-omics analysis of molecular mechanisms and potential therapeutic targets. Environ Toxicol. 2024;39(3):1650–65.
Ahmed Khalil A, et al. Recent developments and anticancer therapeutics of paclitaxel: An update. Curr Pharm Des. 2022;28(41):3363–73.
Alqahtani FY, et al. Paclitaxel. Profiles Drug Subst Excip Relat Methodol. 2019;44:205–38.
Hoyer KA. Paclitaxel. Clin J Oncol Nurs. 2000;4(1):51–2.
Kohler DR, Goldspiel BR. Paclitaxel (taxol). Pharmacotherapy. 1994;14(1):3–34.
Li N, et al. Molecular characterization of cuproptosis-related lncRNAs: Defining molecular subtypes and a prognostic signature of ovarian cancer. Biol Trace Elem Res. 2024;202(4):1428–45.
Kciuk M, et al. Recent advances in molecular mechanisms of cancer immunotherapy. Cancers (Basel). 2023;15(10):2721.
Liu L, et al. Developing four cuproptosis-related lncRNAs signature to predict prognosis and immune activity in ovarian cancer. J Ovarian Res. 2023;16(1):88.
Sha D, et al. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020;10(12):1808–25.
Chia NY, Tan P. Molecular classification of gastric cancer. Ann Oncol. 2016;27(5):763–9.
Correa P. Gastric cancer: overview. Gastroenterol Clin North Am. 2013;42(2):211–7.
Douda L, Cyrany J, Tachecí I. Early gastric cancer. Vnitr Lek. 2022;68(6):371–5.
Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107(3):230–6.
Karimi P, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–13.
López MJ, et al. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841.
Petryszyn P, Chapelle N, Matysiak-Budnik T. Gastric cancer: Where are we heading? Dig Dis. 2020;38(4):280–5.
Smyth EC, et al. Gastric cancer. Lancet. 2020;396(10251):635–48.
Song Z, et al. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39(7):1010428317714626.
Zhao B, et al. Prediction model of clinical prognosis and immunotherapy efficacy of gastric cancer based on level of expression of cuproptosis-related genes. Heliyon. 2023;9(8):e19035.
Bilotta MT, et al. Liver X receptors: Regulators of cholesterol metabolism, inflammation, autoimmunity, and cancer. Front Immunol. 2020;11:584303.
Cardoso D, Perucha E. Cholesterol metabolism: A new molecular switch to control inflammation. Clin Sci (Lond). 2021;135(11):1389–408.
Cortes VA, et al. Physiological and pathological implications of cholesterol. Front Biosci (Landmark Ed). 2014;19(3):416–28.
Ho WY, Hartmann H, Ling SC. Central nervous system cholesterol metabolism in health and disease. IUBMB Life. 2022;74(8):826–41.
Feng A, et al. A novel cuproptosis-related lncRNA nomogram to improve the prognosis prediction of gastric cancer. Front Oncol. 2022;12:957966.
Berg M, et al. DNA sequence profiles of the colorectal cancer critical gene set KRAS-BRAF-PIK3CA-PTEN-TP53 related to age at disease onset. PLoS ONE. 2010;5(11):e13978.
Sahraeian SME, et al. Deep convolutional neural networks for accurate somatic mutation detection. Nat Commun. 2019;10(1):1041.
Thapa R, et al. Role of synbiotics in reproductive disorders, in Synbiotics in human health: Biology to drug delivery. Springer Nature: Singapore; 2024. p. 169–94.
Vijg J. Somatic mutations, genome mosaicism, cancer and aging. Curr Opin Genet Dev. 2014;26:141–9.
Yin C, et al. Development and evaluation of a novel cuproptosis-related lncRNA signature for gastric cancer prognosis. Comput Math Methods Med. 2023;2023:6354212.
Abdel-Razek AS, et al. Microbial natural products in drug discovery. Processes. 2020;8(4):470.
Chadwick A, et al. Understanding the psychological, physiological, and genetic factors affecting precision pain medicine: A narrative review. J Pain Res. 2021;14:3145–61.
Song X, et al. Metal-dependent programmed cell death-related lncRNA prognostic signatures and natural drug sensitivity prediction for gastric cancer. Front Pharmacol. 2022;13:1039499.
Arneth B. Tumor microenvironment. Medicina (Kaunas). 2019;56(1):15.
Du T, et al. Pyroptosis, metabolism, and tumor immune microenvironment. Clin Transl Med. 2021;11(8):e492.
Elhanani O, Ben-Uri R, Keren L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell. 2023;41(3):404–20.
Jarosz-Biej M, et al. Tumor microenvironment as a “Game Changer” in cancer radiotherapy. Int J Mol Sci. 2019;20(13):3212.
Chen L, et al. TFEB regulates cellular labile iron and prevents ferroptosis in a TfR1-dependent manner. Free Radical Biol Med. 2023;208:445–57.
Huang J, et al. Distinct tumor microenvironment landscapes in gastric cancer classified by cuproptosis-related lncRNAs. J Cancer. 2022;13(15):3687–700.
Yang W, et al. Exosomes from young healthy human plasma promote functional recovery from intracerebral hemorrhage via counteracting ferroptotic injury. Bioactive Mater. 2023;27:1–14.
Fazel SS, et al. Barriers and facilitators for the safe handling of antineoplastic drugs. J Oncol Pharm Pract. 2022;28(8):1709–21.
Guichard N, et al. Antineoplastic drugs and their analysis: a state of the art review. Analyst. 2017;142(13):2273–321.
Leso V, et al. Exposure to antineoplastic drugs in occupational settings: A systematic review of biological monitoring data. Int J Environ Res Public Health. 2022;19(6):3737.
Lima HRS, et al. Electrochemical sensors and biosensors for the analysis of antineoplastic drugs. Biosens Bioelectron. 2018;108:27–37.
Ge H, et al. Targeting ASIC1a promotes neural progenitor cell migration and neurogenesis in ischemic stroke. Research. 2023;6:0105.
Tu H, et al. Cuproptosis-Related lncRNA gene signature establishes a prognostic model of gastric adenocarcinoma and evaluate the effect of antineoplastic drugs. Genes (Basel). 2022;13(12):2214.
Wang Y, et al. A novel risk model construction and immune landscape analysis of gastric cancer based on cuproptosis-related long noncoding RNAs. Front Oncol. 2022;12:1015235.
Wang H, et al. MST1 mediates neuronal loss and cognitive deficits: A novel therapeutic target for Alzheimer’s disease. Prog Neurobiol. 2022;214:102280.
Ansari D, et al. Pancreatic cancer: yesterday, today and tomorrow. Future Oncol. 2016;12(16):1929–46.
Cai J, et al. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021;520:1–11.
Jairajpuri DS, et al. Identification of high-affinity inhibitors of TANK-binding Kinase 1 (TBK1): A promising frontier for controlling inflammatory signaling in cancer. Discov Med. 2024;36(180):129–39.
Goral V. Pancreatic cancer: Pathogenesis and diagnosis. Asian Pac J Cancer Prev. 2015;16(14):5619–24.
Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694–705.
Klatte DCF, et al. Hereditary pancreatic cancer. Best Pract Res Clin Gastroenterol. 2022;58–59:101783.
Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18(7):493–502.
Feng F, et al. Lactylome analysis unveils lactylation‐dependent mechanisms of stemness remodeling in the liver cancer stem cells. Adv Sci, 2024: p. 2405975.
Qin C, et al. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol Cancer. 2020;19(1):50.
Bau DT, et al. Genetic susceptibility to prostate cancer in Taiwan: A genome-wide association study. Mol Carcinog. 2024;63(4):617–28.
Thapa R, et al. Recent developments in the role of protocatechuic acid in neurodegenerative disorders. EXCLI J. 2023;22:595.
Cui Z, et al. Association between lncRNA CASC8 polymorphisms and the risk of cancer: a meta-analysis. Cancer Manag Res. 2018;10:3141–8.
Haerian MS, et al. Lack of association of CASC8 rs1447295 with colorectal cancer in Iranian population: A multicenter case-control study. Gene. 2017;634:74–6.
Qian F-C, et al. SEanalysis 2.0: a comprehensive super-enhancer regulatory network analysis tool for human and mouse. Nucleic Acids Res. 2023;51(W1):W520–7.
Hu R, et al. Long noncoding RNA cancer susceptibility candidate 8 suppresses the proliferation of bladder cancer cells via regulating glycolysis. DNA Cell Biol. 2017;36(9):767–74.
Yao HF, et al. Analysis of cuproptosis-related lncRNA signature for predicting prognosis and tumor immune microenvironment in pancreatic cancer. Apoptosis. 2023;28(7–8):1090–112.
Jiang W, et al. Construction of a prognostic model based on cuproptosis-related lncRNA signatures in pancreatic cancer. Can J Gastroenterol Hepatol. 2022;2022:4661929.
Chen L, et al. The roles and mechanism of m6A RNA methylation regulators in cancer immunity. Biomed Pharmacother. 2023;163:114839.
Sun Y, et al. Development and validation of cuproptosis-related lncRNAs associated with pancreatic cancer immune microenvironment based on single-cell. Front Immunol. 2023;14:1220760.
Akram M. Mini-review on glycolysis and cancer. J Cancer Educ. 2013;28(3):454–7.
Fernie AR, Carrari F, Sweetlove LJ. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol. 2004;7(3):254–61.
Chen L, et al. Systemic analyses of cuproptosis-related lncRNAs in pancreatic adenocarcinoma, with a focus on the molecular mechanism of LINC00853. Int J Mol Sci. 2023;24(9):7923.
Lou J-S, et al. Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. Phytomedicine. 2021;80:153370.
Romero-Garcia S, et al. Tumor cell metabolism: an integral view. Cancer Biol Ther. 2011;12(11):939–48.
DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2(5):e1600200.
Liu M, et al. The trajectory of oral mucositis in head and neck cancer patients undergoing radiotherapy and its influencing factors. Ear Nose Throat J, 2024: 01455613241228211.
Zhu A, Lee D, Shim H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Semin Oncol. 2011;38(1):55–69.
Yu L, et al. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908–26.
Pérez-Tomás R, Pérez-Guillén I. Lactate in the tumor microenvironment: An essential molecule in cancer progression and treatment. Cancers (Basel). 2020;12(11):3244.
Li B, et al. Aggregation-induced emission-based macrophage-like nanoparticles for targeted photothermal therapy and virus transmission blockage in Monkeypox. Adv Mater. 2024;36(9):2305378.
Wang Y, et al. Cuproptosis-related lncRNAs are correlated with tumour metabolism and immune microenvironment and predict prognosis in pancreatic cancer patients. IET Syst Biol. 2023;17(4):174–86.
Chen H, et al. Cuproptosis-related LncRNAs signature as biomarker of prognosis and immune infiltration in pancreatic cancer. Front Genet. 2023;14:1049454.
Li X, et al. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer. 2021;21(9):541–57.
Salazar J, Le A. The heterogeneity of liver cancer metabolism. Adv Exp Med Biol. 2021;1311:127–36.
Sun JH, et al. Liver cancer stem cell markers: Progression and therapeutic implications. World J Gastroenterol. 2016;22(13):3547–57.
Yamashita T, Kaneko S. Liver cancer. Rinsho Byori. 2016;64(7):787–96.
Zheng Y, et al. The progress of immune checkpoint therapy in primary liver cancer. Biochim Biophys Acta Rev Cancer. 2021;1876(2):188638.
Liu Y, et al. Development and validation of cuproptosis-related gene signature in the prognostic prediction of liver cancer. Front Oncol. 2022;12:985484.
Liang J, et al. Long noncoding RNA CYTOR in cancer: A TCGA data review. Clin Chim Acta. 2018;483:227–33.
Moradi MT, Hatami R, Rahimi Z. Circulating CYTOR as a potential biomarker in breast cancer. Int J Mol Cell Med. 2020;9(1):83–90.
Ou C, et al. lncRNA cytoskeleton regulator RNA (CYTOR): Diverse functions in metabolism, inflammation and tumorigenesis, and potential applications in precision oncology. Genes Dis. 2023;10(2):415–29.
Chen Q, et al. Cuproptosis-related LncRNA signature for predicting prognosis of hepatocellular carcinoma: A comprehensive analysis. Dis Markers. 2022;2022:3265212.
Llovet JM, et al. Hepatocellular carcinoma. Nature Rev Disease Primers. 2021;7(1):6.
Guan MC, et al. Early diagnosis and therapeutic strategies for hepatocellular carcinoma: From bench to bedside. World J Gastrointest Oncol. 2021;13(4):197–215.
Thapa R, et al. ncRNAs and their impact on dopaminergic neurons: Autophagy pathways in Parkinson's disease. Ageing Res Rev, 2024: 102327.
Wang S, et al. A cuproptosis-related LncRNA risk model for predicting prognosis and immunotherapeutic efficacy in patients with hepatocellular carcinoma. Biochem Genet. 2024;62(3):2332–51.
de Bree R, et al. Response assessment after induction chemotherapy for head and neck squamous cell carcinoma: From physical examination to modern imaging techniques and beyond. Head Neck. 2017;39(11):2329–49.
Oliveira LC, et al, Neuroimaging markers of patient-reported outcome measures in acute ischemic stroke. medRxiv, 2023.
Montrezor LH. Performance in physiology evaluation: possible improvement by active learning strategies. Adv Physiol Educ. 2016;40(4):454–7.
Liu Y, Jiang J. A novel cuproptosis-related lncRNA signature predicts the prognosis and immunotherapy for hepatocellular carcinoma. Cancer Biomark. 2023;37(1):13–26.
Hadian K, Stockwell BR. The therapeutic potential of targeting regulated non-apoptotic cell death. Nat Rev Drug Discov. 2023;22(9):723–42.
Jan R, Chaudhry GE. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv Pharm Bull. 2019;9(2):205–18.
Wang X, et al. Non-apoptotic cell death-based cancer therapy: Molecular mechanism, pharmacological modulators, and nanomedicine. Acta Pharm Sin B. 2022;12(9):3567–93.
Zhang G, Sun J, Zhang X. A novel Cuproptosis-related LncRNA signature to predict prognosis in hepatocellular carcinoma. Sci Rep. 2022;12(1):11325.
Li J, et al. Targeting miR-30d reverses pathological cardiac hypertrophy. EBioMedicine. 2022;81:104108.
Xu Q, Liu T, Wang J. Radiosensitization-related cuproptosis LncRNA signature in non-small cell lung cancer. Genes (Basel). 2022;13(11):2080.
Qian Y, Shi L, Luo Z. Long non-coding RNAs in cancer: Implications for diagnosis, prognosis, and therapy. Front Med (Lausanne). 2020;7:612393.
Olivero CE, Dimitrova N. Identification and characterization of functional long noncoding RNAs in cancer. Faseb j. 2020;34(12):15630–46.
Li ZX, et al. MALAT1: a potential biomarker in cancer. Cancer Manag Res. 2018;10:6757–68.
Hajibabaei S, et al. Targeting long non-coding RNA MALAT1 reverses cancerous phenotypes of breast cancer cells through microRNA-561-3p/TOP2A axis. Sci Rep. 2023;13(1):8652.
Lu J, et al. Long non-coding RNA MALAT1: A key player in liver diseases. Front Med (Lausanne). 2021;8:734643.
Zhong X, et al. A hypoxia-related lncRNA signature correlates with survival and tumor microenvironment in colorectal cancer. J Immunol Res. 2022;2022:9935705.
Maldonado V, Melendez-Zajgla J. The role of hypoxia-associated long non-coding RNAs in breast cancer. Cells. 2022;11(10):1679.
Hajjari M, Salavaty A. HOTAIR: an oncogenic long non-coding RNA in different cancers. Cancer Biol Med. 2015;12(1):1–9.
D’Angelo E, Agostini M. Long non-coding RNA and extracellular matrix: the hidden players in cancer-stroma cross-talk. Noncoding RNA Res. 2018;3(4):174–7.
Sun C, et al. Tumor-associated nonmyelinating Schwann cell-expressed PVT1 promotes pancreatic cancer kynurenine pathway and tumor immune exclusion. Sci Adv. 2023;9(5):eadd995.
Huang J, et al. Identification of a cuproptosis-related lncRNA signature to predict the prognosis and immune landscape of head and neck squamous cell carcinoma. Front Oncol. 2022;12:983956.
Gong H, et al. Identification of cuproptosis-related lncRNAs with the significance in prognosis and immunotherapy of oral squamous cell carcinoma. Comput Biol Med. 2024;171:108198.
Shi Y, et al. Cuproptosis-related lncRNAs predict prognosis and immune response of thyroid carcinoma. Front Genet. 2023;14:1100909.
Zhou L, et al. Cuproptosis-related LncRNAs are potential prognostic and immune response markers for patients with HNSCC via the integration of bioinformatics analysis and experimental validation. Front Oncol. 2022;12:1030802.
Badowski C, He B, Garmire LX. Blood-derived lncRNAs as biomarkers for cancer diagnosis: the Good, the Bad and the Beauty. NPJ Precis Oncol. 2022;6(1):40.
Arriaga-Canon C, et al. The clinical utility of lncRNAs and their application as molecular biomarkers in breast cancer. Int J Mol Sci. 2023;24(8):7426.
Onagoruwa OT, et al. Oncogenic role of PVT1 and therapeutic implications. Front Oncol. 2020;10:17.
Masrour M, et al. Long non-coding RNA as a potential diagnostic biomarker in head and neck squamous cell carcinoma: A systematic review and meta-analysis. PLoS ONE. 2023;18(9):e0291921.
Snyder M, et al. Discovery and validation of clinically relevant long non-coding RNAs in colorectal cancer. Cancers (Basel). 2022;14(16):3866.
Gao M, et al. GAE-LGA: integration of multi-omics data with graph autoencoders to identify lncRNA–PCG associations. Briefings Bioinform. 2022;23(6):bbac452.
Luo H-S, et al. Prediction of prognosis, immune escape and drug sensitivity of lung adenocarcinoma based on Cuproptosis-related LncRNA. Life Res. 2024;7:6–10.
Chen Y, et al. Long non-coding RNAs: From disease code to drug role. Acta Pharm Sin B. 2021;11(2):340–54.
Yao ZT, et al. New insights into the interplay between long non-coding RNAs and RNA-binding proteins in cancer. Cancer Commun (Lond). 2022;42(2):117–40.
Taniue K, Akimitsu N. The functions and unique features of LncRNAs in cancer development and tumorigenesis. Int J Mol Sci. 2021;22(2):632.
Aliperti V, Skonieczna J, Cerase A. Long non-coding RNA (lncRNA) roles in cell biology, neurodevelopment and neurological disorders. Noncoding RNA. 2021;7(2):36.
Winkle M, et al. Noncoding RNA therapeutics — challenges and potential solutions. Nat Rev Drug Discovery. 2021;20(8):629–51.
Hueso M, et al. ncRNAs in therapeutics: Challenges and limitations in nucleic acid-based drug delivery. Int J Mol Sci. 2021;22(21):11596.
Cui G, et al. Comprehensive analysis of the prognostic signature and tumor microenvironment infiltration characteristics of cuproptosis-related lncRNAs for patients with colon adenocarcinoma. Front Oncol. 2022;12:1007918.
Ma C, et al. Prognosis and personalized treatment prediction in lung adenocarcinoma: An in silico and in vitro strategy adopting cuproptosis related lncRNA towards precision oncology. Front Pharmacol. 2023;14:1113808.
Ma C, et al. A novel defined risk signature of cuproptosis-related long non-coding RNA for predicting prognosis, immune infiltration, and immunotherapy response in lung adenocarcinoma. Front Pharmacol. 2023;14:1146840.
Zhong G, et al. Cuproptosis is involved in copper-induced hepatotoxicity in chickens. Sci Total Environ. 2023;866:161458.
Springer C, Humayun D, Skouta R. Cuproptosis: Unraveling the mechanisms of copper-induced cell death and its implication in cancer therapy. Cancers. 2024;16(3):647.
Sun X, et al. Characterization of cuproptosis-related lncRNA landscape for predicting the prognosis and aiding immunotherapy in lung adenocarcinoma patients. Am J Cancer Res. 2023;13(3):778–801.
Yang T, et al. Expression of the copper transporters hCtr1, ATP7A and ATP7B is associated with the response to chemotherapy and survival time in patients with resected non-small cell lung cancer. Oncol Lett. 2015;10(4):2584–90.
Han J, et al. Roles and mechanisms of copper homeostasis and cuproptosis in osteoarticular diseases. Biomed Pharmacother. 2024;174:116570.
Zhou H, Toan S. Pathological roles of mitochondrial oxidative stress and mitochondrial dynamics in cardiac microvascular Ischemia/Reperfusion injury. Biomolecules. 2020;10(1):85.
Gaur K, et al. Iron and copper intracellular chelation as an anticancer drug strategy. Inorganics. 2018;6(4):126.
Gaur K, et al. Iron and copper intracellular chelation as an anticancer drug strategy. Inorganics (Basel). 2018;6(4):126.
Wang Y, et al. Cuproptosis: A novel therapeutic target for overcoming cancer drug resistance. Drug Resist Updates. 2024;72: 101018.
Huang G, et al. Identification of cuproptosis-related long noncoding RNA signature for predicting prognosis and immunotherapy response in bladder cancer. Sci Rep. 2022;12(1):21386.
Pulumati A, et al. Technological advancements in cancer diagnostics: Improvements and limitations. Cancer Rep (Hoboken). 2023;6(2): e1764.
Li D, et al. Clinical significance and immune landscape of cuproptosis-related lncRNAs in kidney renal clear cell carcinoma: a bioinformatical analysis. Ann Transl Med. 2022;10(22):1235.
Pfeffer CM, Singh ATK. Apoptosis: A target for anticancer therapy. Int J Mol Sci. 2018;19(2):448.
Zhang X, et al. Copper-mediated novel cell death pathway in tumor cells and implications for innovative cancer therapies. Biomed Pharmacother. 2023;168:115730.
Zhong Y, et al. The effect of lipid metabolism on cuproptosis-inducing cancer therapy. Biomed Pharmacother. 2024;172:116247.
Baldari S, Di Rocco G, Toietta G. Current biomedical use of copper chelation therapy. Int J Mol Sci. 2020;21(3):1069.
Talib WH, et al. Targeting drug chemo-resistance in cancer using natural products. Biomedicines. 2021;9(10):1353.
Yang L, et al. Cuproptosis-related lncRNAs are biomarkers of prognosis and immune microenvironment in head and neck squamous cell carcinoma. Front Genet. 2022;13:947551.
Ganesh K, Massagué J. Targeting metastatic cancer. Nat Med. 2021;27(1):34–44.
Liao J, et al. Artificial intelligence assists precision medicine in cancer treatment. Front Oncol. 2022;12:998222.
Li L, Zhou H, Zhang C. Cuproptosis in cancer: biological implications and therapeutic opportunities. Cell Mol Biol Lett. 2024;29(1):91.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
AAB, MA, and EM contributed to conceptualization, methodology, visualization, writing—original draft, and writing—review and editing. RT, HA, and WHA contributed to conceptualization, methodology, visualization, and writing—review and editing. IK and SIA contributed to methodology, visualization, and writing—review and editing. GG and VS contributed to conceptualization, methodology, supervision, visualization, writing—original draft, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhat, A.A., Afzal, M., Moglad, E. et al. lncRNAs as prognostic markers and therapeutic targets in cuproptosis-mediated cancer. Clin Exp Med 24, 226 (2024). https://doi.org/10.1007/s10238-024-01491-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-024-01491-0