Abstract
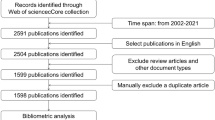
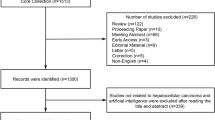
In the past 20 years, targeted therapy for cholangiocarcinoma has attracted certain attention. There is a significant upward in papers focusing on this field. In this study, we used bibliometric and visual methods to explore the current status and future directions in cholangiocarcinoma-targeted therapy research. A total of 1057 papers published in English from 2003 to 2022 were extracted from the Web of Science Core Collection SCI-expanded database. Furthermore, Citespace, Vosviewer, and Excel 2016 were utilized to conduct bibliometric and visual analysis. The volume of annual publications has steadily increased over the past two decades. The USA has published the largest number of publications, and the Mayo Clinic acted as the dominant institution. Cancers, Frontiers in Oncology, and Hepatology were the prolific resources in this research field. Moreover, the co-cited reference analysis uncovered the landmark paper in this field. With regard to research hotspots and frontiers, the burst keywords analysis showed that growth factor receptors and pathogenesis might become the hot topics of future research. To sum up, our study displays the current research status and future directions in the targeted therapy for cholangiocarcinoma. More comprehensive and in-depth investigations should focus on critical genetic mutations and their molecular mechanisms to prompt the molecular-targeted therapy.








Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Change history
03 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10238-023-01138-6
References
Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in Western countries. J Hepatol. 2022;77(6):1690–8. https://doi.org/10.1016/j.jhep.2022.07.022.
Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet (London, England). 2014;383(9935):2168–79. https://doi.org/10.1016/s0140-6736(13)61903-0.
Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma—evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111. https://doi.org/10.1038/nrclinonc.2017.157.
Esnaola NF, Meyer JE, Karachristos A, et al. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer. 2016;122(9):1349–69. https://doi.org/10.1002/cncr.29692.
Bressler L, Bath N, Manne A, Miller E, Cloyd JM (2023) Management of locally advanced intrahepatic cholangiocarcinoma: a narrative review. Chin Clin Oncol, 12(2):15. https://doi.org/10.21037/cco-22-115.
Zhao LM, Shi AD, Yang Y, et al. Advances in molecular and cell therapy for immunotherapy of cholangiocarcinoma. Front Oncol. 2023;13:1140103. https://doi.org/10.3389/fonc.2023.1140103.
Rizzo A, Brandi G. First-line chemotherapy in advanced biliary tract cancer ten years after the ABC-02 Trial: "and yet it moves!". Cancer treatment and research communications 2021, 27:100335. https://doi.org/10.1016/j.ctarc.2021.100335.
Ricci AD, Rizzo A, Brandi G. Immunotherapy in biliary tract cancer: worthy of a second look. Cancer Control J Moffitt Cancer Center. 2020;27(3):1073274820948047. https://doi.org/10.1177/1073274820948047.
Santoni M, Rizzo A, Kucharz J, et al. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the MOUSEION-03 meta-analysis. Cancer Immunol Immunother. 2023;72(6):1365–79. https://doi.org/10.1007/s00262-022-03349-4.
Kelley RK, Ueno M, Yoo C et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England) 2023. https://doi.org/10.1016/s0140-6736(23)00727-4.
Cho SM, Esmail A, Raza A, Dacha S, Abdelrahim M. Timeline of FDA-approved targeted therapy for cholangiocarcinoma. Cancers. 2022. https://doi.org/10.3390/cancers14112641.
Li N, Zeng A, Wang Q, et al. Regulatory function of DNA methylation mediated lncRNAs in gastric cancer. Cancer Cell Int. 2022;22(1):227. https://doi.org/10.1186/s12935-022-02648-1.
Dabney RS, Khalife M, Shahid K, Phan AT. Molecular pathways and targeted therapy in cholangiocarcinoma. Clin Adv Hematol Oncol. 2019;17(11):630–7.
Ricci AD, Rizzo A, Brandi G. The DNA damage repair (DDR) pathway in biliary tract cancer (BTC): A new Pandora’s box? ESMO open. 2020;5(5):e001042. https://doi.org/10.1136/esmoopen-2020-001042.
Lavacchi D, Caliman E, Rossi G, et al. Ivosidenib in IDH1-mutated cholangiocarcinoma: clinical evaluation and future directions. Pharmacol Ther. 2022;237:108170. https://doi.org/10.1016/j.pharmthera.2022.108170.
Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807. https://doi.org/10.1016/s1470-2045(20)30157-1.
Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–84. https://doi.org/10.1016/s1470-2045(20)30109-1.
Zhang Y, Liu C, Wu C, Song L. Natural peptides for immunological regulation in cancer therapy: mechanism, facts and perspectives. Biomed Pharmacother. 2023;159:114257. https://doi.org/10.1016/j.biopha.2023.114257.
Kam AE, Masood A, Shroff RT. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol Hepatol. 2021;6(11):956–69. https://doi.org/10.1016/s2468-1253(21)00171-0.
Ninkov A, Frank JR, Maggio LA. Bibliometrics: methods for studying academic publishing. Perspect Med Educ. 2022;11(3):173–6. https://doi.org/10.1007/s40037-021-00695-4.
Wu HY, Liu T, Zhong T, et al. Research trends and hotspots of neoadjuvant therapy in pancreatic cancer: a bibliometric analysis based on the Web of Science Core Collection. Clin Exp Med. 2023. https://doi.org/10.1007/s10238-023-01013-4.
Yao L, Hui L, Yang Z, Chen X, Xiao A. Freshwater microplastics pollution: detecting and visualizing emerging trends based on Citespace II. Chemosphere. 2020;245:125627. https://doi.org/10.1016/j.chemosphere.2019.125627.
Arruda H, Silva ER, Lessa M, Proença D Jr, Bartholo R. VOSviewer and Bibliometrix. J Med Libr Assoc. 2022;110(3):392–5. https://doi.org/10.5195/jmla.2022.1434.
Yuan X, Chang C, Chen X, Li K. Emerging trends and focus of human gastrointestinal microbiome research from 2010–2021: a visualized study. J Transl Med. 2021;19(1):327. https://doi.org/10.1186/s12967-021-03009-8.
Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–29. https://doi.org/10.1053/j.gastro.2013.10.013.
Kipp BR, Voss JS, Kerr SE, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol. 2012;43(10):1552–8. https://doi.org/10.1016/j.humpath.2011.12.007.
Song L, Zhang J, Ma D, et al. A bibliometric and knowledge-map analysis of macrophage polarization in atherosclerosis from 2001 to 2021. Front Immunol. 2022;13:910444. https://doi.org/10.3389/fimmu.2022.910444.
Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47(9):1003–10. https://doi.org/10.1038/ng.3375.
Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45(12):1470–3. https://doi.org/10.1038/ng.2813.
Ross JS, Wang K, Gay L, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist. 2014;19(3):235–42. https://doi.org/10.1634/theoncologist.2013-0352.
Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17(1):72–9. https://doi.org/10.1634/theoncologist.2011-0386.
Churi CR, Shroff R, Wang Y, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS ONE. 2014;9(12):e115383. https://doi.org/10.1371/journal.pone.0115383.
Javle M, Lowery M, Shroff RT, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol. 2018;36(3):276–82. https://doi.org/10.1200/jco.2017.75.5009.
Javle M, Roychowdhury S, Kelley RK, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol. 2021;6(10):803–15. https://doi.org/10.1016/s2468-1253(21)00196-5.
Arai Y, Totoki Y, Hosoda F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59(4):1427–34. https://doi.org/10.1002/hep.26890.
Jusakul A, Cutcutache I, Yong CH, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017;7(10):1116–35. https://doi.org/10.1158/2159-8290.Cd-17-0368.
Liang S, Guo H, Ma K, et al. A PLCB1-PI3K-AKT signaling axis activates EMT to promote cholangiocarcinoma progression. Can Res. 2021;81(23):5889–903. https://doi.org/10.1158/0008-5472.Can-21-1538.
Bekaii-Saab TS, Bridgewater J, Normanno N. Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann Oncol Off J Eur Soc Med Oncol. 2021;32(9):1111–26. https://doi.org/10.1016/j.annonc.2021.04.012.
Farha N, Dima D, Ullah F, Kamath S. Precision oncology targets in biliary tract cancer. Cancers. 2023. https://doi.org/10.3390/cancers15072105.
Bridgewater JA, Goodman KA, Kalyan A, Mulcahy MF. Biliary tract cancer: epidemiology, radiotherapy, and molecular profiling. Am Soc Clin Oncol Edu Book Am Soc Clin Oncol Annu Meet. 2016;35:e194-203. https://doi.org/10.1200/edbk_160831.
Sun Q, Wang H, Xiao B, Xue D, Wang G. Development and validation of a 6-gene hypoxia-related prognostic signature for cholangiocarcinoma. Front Oncol. 2022;12:954366. https://doi.org/10.3389/fonc.2022.954366.
Seubwai W, Kraiklang R, Wongkham C, Wongkham S. Hypoxia enhances aggressiveness of cholangiocarcinoma cells. Asian Pacific journal of cancer prevention : APJCP. 2012;13(Suppl):53–8.
Sugihara T, Isomoto H, Gores G, Smoot R. YAP and the Hippo pathway in cholangiocarcinoma. J Gastroenterol. 2019;54(6):485–91. https://doi.org/10.1007/s00535-019-01563-z.
Funding
This research was supported by Science & Technology Department of Sichuan Province Funding Project (2022YFSY0027) and Science & Technology Department of Sichuan Province Funding Project (2022NSFSC1348).
Author information
Authors and Affiliations
Contributions
Conceptualization was contributed by QL; methodology was contributed by PH; formal analysis and investigation were contributed by PH and FW; writing—original draft preparation was contributed by PH and QJ.W; writing—review and editing were contributed by QL and PF.Z; funding acquisition was contributed by QL and PF.Z; supervision was contributed by QL. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, P., Wen, F., Wu, Q. et al. Research trends of targeted therapy for cholangiocarcinoma from 2003 to 2022: a bibliometric and visual analysis. Clin Exp Med 23, 3981–3994 (2023). https://doi.org/10.1007/s10238-023-01110-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01110-4