Abstract
To determine the antibody levels at 6 months in SARS-CoV-2 vaccinated individuals in COVID-recovered versus non-infected groups to determine the need to administer booster COVID vaccine in each group. Prospective longitudinal study. Pathology Department, Combined Military Hospital, Lahore for a period of eight months from July 2021 to February 2022. Two hundred and thirty three study participants in both COVID recovered and non-infected groups (105 participants in infected group, 128 participants in non-infected group) were subjected to blood sampling at 6 months post-vaccination. Anti-SARS-CoV-2 IgG antibody test was done using Chemiluminescence method. Comparison of antibody levels between COVID-recovered and non-infected groups was made. Results were compiled and statistically analyzed using SPSS version 21. Out of 233 study participants, males were 183 (78%) while females were 50 (22%), mean age being 35.93 years ± 8.298. Mean Anti-SARS-CoV-2 S IgG levels among COVID-recovered group was 1342 U/ml and among non-infected group was 828 U/ml at 6 months post-vaccination. Mean antibody titers in COVID-19 recovered group are higher than in non-infected group at 6 months post-vaccination in both groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) is an enveloped, single-stranded RNA Beta-coronavirus, now a recognized causative agent of Coronavirus disease-19 (COVID-19). SARS-CoV-2 originated from Wuhan city of China in early December 2019. All seven human coronaviruses have a zoonotic origin, among them those with high pathogenicity include Severe acute respiratory syndrome coronavirus (SARS-CoV), Middle east respiratory syndrome coronavirus (MERS-CoV) and Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) while others such HCoV-229E, HCoV-OC43 cause seasonal and usually mild respiratory tract infections. The transmission of SARS-CoV-2 is mainly from human to human via respiratory droplets and aerosols. Clinically SARS-CoV-2 has a variable disease severity ranging from asymptomatic illness or mildly symptomatic to severe disease and a fatal outcome. It has posed a considerable risk to the vulnerable population, especially the elderly, immunocompromised population and exposed segments of the society such as health care workers and law enforcement agents [1].
During the early phase of COVID-19 pandemic, there was no scientific evidence of whether and how long patients would take to develop an immune response against SARS-CoV-2, both in case of infection and later on post-vaccination. Initially studies were conducted to define B-cell response and to determine how long antibodies mediated B cell immunity would last and provide protection following infection. It was found that antibodies formed against SARS-CoV-2 in an infected patient have strong neutralizing capacity against receptor binding domain (RBD) of Spike (S) structural protein of the virus [1]. It was identified that SARS-CoV-2 gains entry to human cell through binding of RBD of S protein to angiotensin-converting enzyme 2 (ACE2) receptor. ACE2 receptor is present on the surface of many cell types including alveolar type II cells in lung and epithelial cells present in the oral mucosa [1]. Later, the importance of T-cell mediated immunity following COVID-19 also became the focus of investigation. However, similar B and T cell responses are expected following vaccination and now the scientific community is probing as to what role COVID-19 vaccines might play in developing protective and durable immune response to SARS-CoV-2 [2].
During COVID-19 pandemic, the immune response following SARS-CoV-2 Emergency use authorization (EUA) vaccines has been studied in clinical trials. A lot of vaccines being developed for COVID-19 have focused on initiating an immune response to RBD of S protein. Various studies have been carried out to demonstrate the duration of immune response and the effectiveness of immunity following vaccination against SARS-CoV-2 [3, 4].There are numerous reports on immune response following vaccines based on messenger RNA (mRNA) technique that include BNT162b2 (Pfizer-BioNtech) and mRNA-1273 (Moderna) vaccines [5, 6]. The reports have shown that these two mRNA vaccines are almost 90% effective in the prevention of COVID-19 illness. Data on outcome of immune response following vaccines which have primarily been rolled out in less developed countries is still deficient. These vaccines mainly include rAd26-rAd5 (Sputnik V), ChAdOx1 (AstraZeneca/Oxford), and BBIBP-CorV (Sinopharm). These 3 vaccines had a dosage schedule of 2 administered at a minimum of three weeks interval. Clinical Trials have been conducted to determine the efficacy and safety profile of these vaccines [7,8,9]. A study conducted in elderly population of Faisalabad district of Pakistan showed that BBIBP-CorV (Sinopharm) vaccine was effective in reducing the risk of COVID-19, hospitalizations and mortality by 94.3%, 60.5% and 98.6%, respectively [10]. A randomized, double -blind, controlled phase 1/2 trial was conducted in participants younger than 18 years to demonstrate the safety of BBIBP-CoV vaccine. It was found that BBIBP-CorV was safe and well tolerated at all tested levels of vaccine in study participants and the reported adverse reactions were mild to moderate in severity. The most common local and systemic adverse reactions reported were pain at injection site and fever, respectively, among all study participants [11].
We have conducted this study to demonstrate the antibody titers in Pakistani population at six months following vaccination with BBIBP-CorV among both COVID-recovered and non-infected groups. The rationale of this comparison is to observe whether durable and persistent antibody response persists in either or both of the groups. This comparison will enable to determine the need of booster vaccine in either or both of the groups.
Patients and methods
The study protocol was approved by Research Review Board Combined Military Hospital Lahore (IRB number 350/2021). Informed consent was taken from study participants. It was a prospective longitudinal study which was carried out on 233 study participants who were vaccinated with BBIBP-CorV, they were followed up for 6 months and their serum samples were collected 6 months after vaccination.
Study population
A total of 400 study participants who were health care workers in Combined Military Hospital Lahore were initially enrolled in the study. Out of 400 participants, 100 participants who got booster dose/third dose of COVID vaccine during follow-up of 6 months and 67 participants who got infected after two doses (break-through cases) were excluded from study. As a result, 233 study participants were divided into two groups, 105 subjects in COVID-recovered group and 128 subjects in non-infected group. COVID-recovered participants included those who had a history of COVID-19 in their medical record. Patient data such as age, gender, previous medical history and severity of COVID-19 symptoms were endorsed on study instrument pro forma. Individuals of both genders fulfilling the criteria were included. A blood sample was drawn from 233 participants and sampling was done for a period of three months from December 2021 to February 2022.
Anti-SARS-CoV-2 S protein electro-chemiluminescence immunoassay
Serum samples of study participants were analyzed by using Elecsys® Anti-SARS-CoV-2 S protein RBD immunoassay with Cobas®analyzer. Elecsys® Anti-SARS-CoV-2-S immunoassay is used for the quantitative determination of antibodies (including IgG) to the Spike protein (S) receptor binding domain (RBD) of SARS-CoV-2 in human serum. The test was performed according to manufacturer’s protocol. This immunoassay incorporates a recombinant protein which represents RBD of S protein of SARS-CoV-2. The immunoassay is based on double-antigen sandwich assay format which favors the detection of antibodies with high binding affinity against SARS-CoV-2 S protein. This assay is used to assess the patient’s humoral immunity against SARS-CoV-2 S protein. The linear range of this immunoassay is 0.4–250 U/ml, and with 10× dilution the antibody levels upto 2500 U/ml were measured. As per manufacturer’s protocol, antibodies level < 0.8 U/ml was considered non-reactive and > 0.8 U/ml was taken as reactive. Two hundred and fifty U/ml was taken as a cutoff for its association with significant neutralization levels of Anti-SARS-CoV-2 IgG antibodies [12].
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, NY, USA). Mean antibody levels among COVID-recovered and non-infected group were determined using independent Samples T test. Correlations between mean antibody levels and severity of symptoms in infected group were analyzed using chi-Square Test. The association of age groups (< 35, 35–55, > 55 years) with mean antibody levels and with cutoff of 250 U/ml among both COVID-recovered and non-infected participants was analyzed using the chi-square test. A p value of < 0.05 was considered statistically significant.
Results
Mean age of 233 study participants was 35 ± 8.298 years. One hundred and eighty-three (78%) of the study participants were males and 50 (22%) were females. Baseline characteristics of study population are given in Table 1.
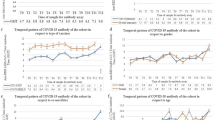
At 6 months post-vaccination in both groups, 100% of vaccine recipients had positive or detectable antibody response, i.e., > 0.8 U/ml. Mean Anti-SARS-CoV-2 S IgG levels among COVID-recovered group were 1342 U/ml ± 100.67, while among non-infected group was 828 U/ml ± 82.28 (p value: 0.000) as shown in Fig. 1.
Anti-SARS-CoV-2 Antibody level of 250 U/ml was taken as a cutoff for reported association with neutralization [10]. Among 105 COVID-recovered participants, 82 (77%) had > 250 U/ml while 24 (23%) had < 250 U/ml. Among the 128 non-infected participants 77 (61%) had > 250 U/ml while 50 (39%) had < 250 U/ml (p value: 0.001).
In COVID-19 recovered group (n: 105), males (n: 70, 66%) had mean antibody levels 1250 U/ml. Among males in recovered group, 53 (76%) had > 250 U/ml while 17 (24%) had < 250 U/ml. Females (n: 35, 34%) in the recovered group had mean antibody levels 1528 U/ml. Among females 29 (83%) had > 250 U/ml while 6 (17%) had < 250 U/ml.
In non-infected group (n: 128), males (n: 113, 88%) had mean antibody levels 851 U/l. Among males in non-infected group, 67 (59%) had > 250 U/ml while 46 (41%) had < 250 U/ml. Females (n: 15, 12%) in the non-infected group had mean antibody levels 659 U/ml. Among females in non-infected group, 10 (67%) had > 250 U/ml while 5 (33%) had < 250 U/ml (p value: 0.95).
Mean antibody levels correlate inversely with clinical grades, i.e., lower the grade higher the mean antibody level
Mean antibody levels were analyzed in clinical grades, i.e., mild, moderate and severe using Independent samples T test (95% CI) as shown in Table 1 and correlation between severity of symptoms and neutralizing antibodies cutoff of 250 U/ml by Pearson chi-Square test, and it was found statistically significant (p value: 0.000) as shown in Table 2.
Mean antibody levels correlate inversely with age groups
Mean antibody levels were analyzed in defined age groups, i.e., age group < 35 years, 35–55 and > 55 years using Independent samples T test (95% CI) as shown in Table 2 and correlation between age groups and neutralizing antibodies cutoff of 250 U/ml by Pearson chi-Square test, and it was found statistically significant (p value: 0.004) as shown in Table 3.
Discussion
We sought to determine the humoral antibody response after vaccination with two doses of BBIBP-CorV in both COVID- recovered and non-infected groups in order to find out the need of booster COVID vaccine in each group. We have found that mean IgG levels in COVID-recovered group is reasonably higher than in non-infected group. These findings are consistent with those of the comparison of humoral antibody response after being vaccinated with BNT162b2 and mRNA-1273 [13]. It was found that with both types of vaccines previously infected study participants had significantly higher antibody levels (mean titer, 9461U/ml {95% CI}) compared with previously non-infected study participants (mean titre,1613 U/ml {95% CI}) [13]. Our findings are also in concordance with a retrospective cohort study on humoral immune response to BBIBP-CorV in health care workers [14]. It was demonstrated that previously infected health care workers had a 10.9, 14.3 and 8.6 fold higher IgG antibody titer than previously non-infected at 21, 90 and 180 days post-vaccination, respectively [14]. In our study we have also observed a linear relationship between neutralizing antibodies level after SARS-CoV-2 vaccination in COVID-recovered versus non-infected groups showing clearly that COVID-recovered participants had more neutralizing antibodies levels than the non-infected groups. This has also been demonstrated by several studies [15, 16] which have also established that the neutralizing antibody response dynamics can accurately determine immune response longevity in COVID-19 recovered individuals.
We determined the difference in antibody response in both genders in our study. It was found that COVID-recovered females have higher antibody levels than COVID-recovered males. This finding is consistent with earlier evidence which tells that infected females generate a stronger humoral immune response and also generate a higher vaccine mediated immune response than males [17, 18]. However, in the non-infected group, males have higher mean antibody levels than females which is consistent with many studies [19]. This difference of mean antibody levels in both genders in the COVID-recovered and non-infected group was not found statistically significant.
There is a positive correlation of antibody levels with age groups in infected participants being high in age group > 55 years than in 35–55 and < 35 years (p value: 0.004). We have found a negative correlation of mean antibody levels with age in previously non-infected participants (p value: 0.001), being high in age group younger than 35 years than in > 35–55 years age group which is in agreement with other studies [13].
There is scarcity of scientific evidence regarding the association of mean antibody levels with severity of COVID-19 symptoms in individuals after vaccination. We have demonstrated in our study the difference of mean antibody levels in infected group post-vaccination according to severity of symptoms at the time of infection. The clinical grading was done according to World Health Organization (WHO) clinical criteria for management of COVID-19 [20]. We found that post-vaccination study participants with mild symptoms during COVID-19 had higher antibody levels (Mean IgG level: 1650 IU/ml) than those with moderate symptoms (Mean IgG level: 1359 IU/ml) and severe symptoms (Mean IgG level: 954 IU/ml). This association of antibody levels with clinical grades in study participants post-vaccination compares variably with studies conducted in vaccinated infected individuals. Wolszczak-Biedrzycka B has demonstrated in his study that those with severe symptoms during the course of COVID-19 develop a higher antibody titer post-vaccination than those with mild and moderate symptoms [21]. The disagreement of our study with these studies might be due to the fact that different vaccine types are used in these studies. According to the immunopathogenesis of SARS-CoV-2 virus studied so far, both the functional and dysfunctional immune response in an individual determine the outcome of COVID-19. In both the responses IgG antibodies are produced in abundance; however, the high titer of neutralizing antibodies results in a mild disease whereas on the other hand, high levels of non-neutralizing antibodies are generally associated with severe disease [1, 22]. A study conducted to understand the biology of afucosylated immune complexes demonstrated that afucosylated non-neutralizing IgG antibodies specific to SARS-CoV-2 were associated with severe COVID-19. Non-neutralizing afucosylated IgG immune complexes isolated from COVID-19 patients with severe disease were shown to induce inflammatory cytokine production and robust infiltration of the lung by immune cells [22]. There is currently no gold standard assay available for comparing SARS-CoV-2 neutralizing as well as non-neutralizing antibody profiles [23] after COVID-19 as well as post-vaccination.
Our data have shown the antibody persistence through 6 months after vaccination in both infected and non-infected groups and support the use of this vaccine to combat the COVID-19 pandemic. However, further studies should be done to compare the immune response post-vaccination in two groups beyond 6 months to determine the need of booster dose accordingly. It is also not clear and yet to be established whether vaccine mediated antibody response among seropositive individuals will show longevity as compared to that in non-infected individuals [24].
The present study suggests that previously infected individuals after receiving two doses of COVID vaccine should be subjected to serological testing for IgG antibodies, in order to prioritize the use of booster vaccine doses to those individuals with no history of previous infection. This approach will accelerate the vaccine and its booster roll out in developing countries. Moreover, unnecessary booster doses may lead to increase in reactogenicity and an increase in vaccine hesitancy among recipients [21].
The limitation of our study was small sample size because167 study participants had to be excluded in follow up of initially enrolled 400 study participants post-vaccination. One hundred out of them got booster COVID vaccine and 67 got break-through infection after two doses of COVID vaccine. Moreover, the study was conducted on health care workers who were continuously exposed to SARS-CoV-2 in hospital environment, and there is a marked possibility of health care workers in non-infected group to have history of asymptomatic COVID-19 without having knowledge and documentation through positive RT-PCR. A future study at community level on a large scale will be planned to cover these limitations.
Conclusion
Our study has provided evidence that after the two doses of COVID vaccine, the humoral immune response against SARS-CoV-2 in individuals with a previous history of SARS-CoV-2 infection is greater than those in non-infected participants. The booster COVID vaccine should be prioritized for vaccinated population not previously infected with SARS-CoV-2 over those being vaccinated and infected with COVID-19.
Data availability
The data sets generated and analyzed during the current study are available.
Code availability
The data were analyzed using IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, NY, USA).
References
V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–70.
Sewell HF, Agius RM, Stewart M, Kendrick D. Cellular immune responses to COVID-19. BMJ. 2020;370:m3018.
Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063.
Goldberg Y, Mandel M, Woodbridge Y, et al. Protection of previous SARS-CoV-2 infection is similar to that of BNT162b2 vaccine protection. A three-month nationwide experience from Israel. medRxiv. 2021.
Baden LR, El Sahly HM, Essink B, et al. COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5);403–16.
Polack FP, Thomas SJ, Kitchin N, et al. C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–15.
Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Gam-COVID-Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomized controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–81.
Voysey M, Clemens SAC, Madhi SA, et al. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111.
Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35–45.
Nadeem I, Ul Munamm SA, Ur Rasool M, Fatimah M, Abu Bakr M, Rana ZK, et al. Safety and efficacy of Sinopharm Vaccine (BBIBP-CorV) in elderly population of Faisalabad district of Pakistan. Postgrad Med J. 2022; postgradmedj-2022-141649.
Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-Cor V, in people younger than 18 years: a randomized, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis. 2022;22(2):196–208.
Alejo JL, Mitchell J, Chang A, Chiang TPY, Massie AB, Segev DL, et al. Prevalence and durability of SARS-CoV-2 antibodies among unvaccinated US adults by history of COVID-19. JAMA. 2022;327:1085–7.
Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326(15):1533–5.
Gómez de laTorre JC, Cáceres-Del Aguila JA, Muro-Rojo C, De La Cruz-Escurra N, Copaja-Corzo C, Hueda-Zavaleta M, et al. Humoral immune response induced by the BBIBP-CorV Vaccine (Sinopharm) in healthcare workers: a Cohort Study. Trop Med Infect Dis. 2022;7(5):66.
Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–11.
Chia WN, Zhu F, Ong SWX, Young BE, Fong SW, Le Bert N, et al. Dynamics of SARS-CoV-2 neutralizing antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2(6):e240–9.
Wei J, Stoesser N, Matthews PC, Ayoubkhani D, Studley R, Bell I, et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021;6:1140–9.
Chang WH. A review of vaccine effects on women in light of the COVID-19 pandemic. Taiwan J Obstet Gynecol. 2020;59:812–20.
Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–38.
World Health Organization. Clinical management of COVID-19: Interim Guidance. World Health Organization. 2020: 13–15.
Wolszczak-Biedrzycka B, Bienkowska A, Dorf J. Assessment of post-vaccination antibody response eight months after the administration of BNT1622b2 vaccine to health care workers with particular emphasis on the impact of previous COVID-19 infection. Vaccines. 2021;9:1508.
Chakraborty S, Gonzalez JC, Sievers BL, Mallajosyula V, Chakraborty S, Dubey M, et al. Early non-neutralizing afucosylated antibody responses are associated with COVID-19 severity. Sci Transl Med. 2022;14(635):eabm 7853.
Chansaenroj J, Yorsaeng R, Posuwan N, Puenpa J, Sudhinaraset N, Chirathaworn C, et al. Detection of SARS-CoV-2 specific antibodies via rapid diagnostic immunoassays in COVID-19 patients. Viro J. 2021;18:52.
Manisty C, Otter AD, Treibel TA, McKnight A, Altmann DM, Brooks T, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2 infected individuals. Lancet. 2021;397(10279):1057–8.
Acknowledgements
The authors would like to express their gratitude to Lt. Gen Nigar Johar HI (M), Surg Gen/DGMS (IS) for her valuable assistance and esteemed patronage.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Dr. Saadiya Mushtaq, Dr. Muhammad Khalid Azam Khan and Dr. Muhammad Qaiser Alam Khan. The first draft of the manuscript was written by Dr. Saadiya Mushtaq. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study protocol was approved by Research Review Board Combined Military Hospital Lahore (IRB number 350/2021). The study was carried out after taking informed consent from study participants.
Informed consent
The study was carried out after taking informed consent from study participants for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mushtaq, S., Azam Khan, M., Alam Khan, M. et al. Comparison of immune response to SARS-COV-2 vaccine in COVID-recovered versus non-infected Individuals. Clin Exp Med 23, 2267–2273 (2023). https://doi.org/10.1007/s10238-023-01005-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01005-4