Abstract
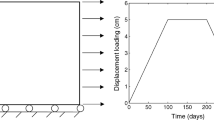
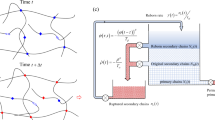
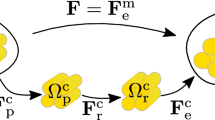
Soft biological tissues, such as arterial tissue, have the ability to grow and remodel in response to damage. Computational method plays a critical role in understanding the underlying mechanisms of tissue damage and healing. However, the existing healing model often requires huge computation time and it is inconvenient to implement finite element simulation. In this paper, we propose a computationally efficient gradient-enhanced healing model that combines the advantages of the gradient-enhanced damage model, the homeostatic-driven turnover remodeling model, and the damage-induced growth model. In the proposed model, the evolution of healing-related parameters can be solved explicitly. Additionally, an adaptive time increment method is used to further reduce computation time. The proposed model can be easily implemented in Abaqus, requiring only a user subroutine UMAT. The effectiveness of proposed model is verified through a semi-analytical example, and the influence of the variables in the proposed model is investigated using uniaxial tension and open-hole plate tests. Finally, the long-term development of aneurysms is simulated to demonstrate the potential applications of the proposed model in real biomechanical problems.



















Similar content being viewed by others
References
Abaqus 6.14 documentation (2014)
Braeu F, Seitz A, Aydin R, Cyron C (2017) Homogenized constrained mixture models for anisotropic volumetric growth and remodeling. Biomech Model Mechanobiol 16:889–906
Braeu FA, Aydin RC, Cyron CJ (2019) Anisotropic stiffness and tensional homeostasis induce a natural anisotropy of volumetric growth and remodeling in soft biological tissues. Biomech Model Mechanobiol 18:327–345
Comellas E, Gasser TC, Bellomo FJ, Oller S (2016) A homeostatic-driven turnover remodelling constitutive model for healing in soft tissues. J R Soc Interface 13:20151081
Cyron CJ, Humphrey JD (2017) Growth and remodeling of load-bearing biological soft tissues. Meccanica 52:645–664
Cyron CJ, Aydin RC, Humphrey JD (2016) A homogenized constrained mixture (and mechanical analog) model for growth and remodeling of soft tissue. Biomech Model Mechanobiol 15:1–15
Dimitrijevic B, Hackl K (2008) A method for gradient enhancement of continuum damage models. Tech Mech 28:43–52
Dimitrijevic B, Hackl K (2011) A regularization framework for damage-plasticity models via gradient enhancement of the free energy. Int J Numer Methods Biomed Eng 27:1199–1210
Ebinger T, Diebels S, Steeb H (2007) Numerical homogenization techniques applied to growth and remodelling phenomena. Comput Mech 39:815–830
Eichinger JF, Haeusel LJ, Paukner D, Aydin RC, Humphrey JD, Cyron CJ (2021) Mechanical homeostasis in tissue equivalents: a review. Biomech Model Mechanobiol 20:833–850
Fereidoonnezhad B, Naghdabadi R, Sohrabpour S, Holzapfel GA (2017) A mechanobiological model for damage-induced growth in arterial tissue with application to in-stent restenosis. J Mech Phys Solids 101:311–327
Frank CB, Shrive NG, Hiraoka H, Nakamura N, Kaneda Y, Hart DA (1999) Optimisation of the biology of soft tissue repair. J Sci Med Sport 2:190–210
Frank CB, Hart DA, Shrive NG (1999) Molecular biology and biomechanics of normal and healing ligaments—a review. Osteoarthr Cartil 7:130–140
Fung YC (1995) Stress, strain, growth, and remodeling of living organisms. In: Casey J, Crochet MJ (eds) Theoretical, experimental, and numerical contributions to the mechanics of fluids and solids: a collection of papers in honor of Paul M. Naghdi. Springer, pp 469–482
Ganghoffer JF, Boubaker MB (2016) Micromechanical analysis of volumetric growth in the context of open systems thermodynamics and configurational mechanics. application to tumor growth. Contin Mech Thermodyn 29:1–27
Ganghoffer JF, Boubaker MB (2017) Micromechanical analysis of volumetric growth in the context of open systems thermodynamics and configurational mechanics. application to tumor growth. Continuum Mech Thermodyn 29:429–455
Ganghoffer J-F, Rahouadj R (2017) Thermodynamic formulations of continuum growth of solid bodies. Math Mech Solids 22:1027–1046
Gasser TC (2017) Damage in vascular tissues and its modeling. In: Avril S, Evans S (eds) Material parameter identification and inverse problems in soft tissue biomechanics, pp 85–118
Gierig M, Wriggers P, Marino M (2023) Arterial tissues and their inflammatory response to collagen damage: a continuum in silico model coupling nonlinear mechanics, molecular pathways, and cell behavior. Comput Biol Med 158:106811
Himpel G (2007) Computational modeling of biomechanical phenomena—remodeling, growth and reorientation. Cambridge University Press
Horvat N, Virag L, Holzapfel GA, Sori J, Karaj I (2019) A finite element implementation of a growth and remodeling model for soft biological tissues: verification and application to abdominal aortic aneurysms. Comput Methods Appl Mech Eng 352:586–605
Horvat N, Virag L, Holzapfel GA, Sorić J, Karšaj I (2019) A finite element implementation of a growth and remodeling model for soft biological tissues: verification and application to abdominal aortic aneurysms. Comput Methods Appl Mech Eng 352:586–605
Humphrey JD (2003) Continuum biomechanics of soft biological tissues. Proc R Soc Lond Ser A Math Phys Eng Sci 459:3–46
Humphrey J (2021) Constrained mixture models of soft tissue growth and remodeling-twenty years after. J Elast 145:49–75
Humphrey JD, Rajagopal K (2002) A constrained mixture model for growth and remodeling of soft tissues. Math Models Methods Appl Sci 12:407–430
Kuhl E, Menzel A, Steinmann P (2003) Computational modeling of growth. Comput Mech 32:71–88
Latorre M, Humphrey JD (2018) Critical roles of time-scales in soft tissue growth and remodeling. APL Bioeng 2:026108
Latorre M, Humphrey JD (2020) Numerical knockouts-in silico assessment of factors predisposing to thoracic aortic aneurysms. PLoS Comput Biol 16:e1008273
Loerakker S, Ristori T (2020) Computational modeling for cardiovascular tissue engineering: the importance of including cell behavior in growth and remodeling algorithms. Curr Opin Biomed Eng 15:1–9
Lubliner J (1984) A maximum-dissipation principle in generalized plasticity. Acta Mech 52:225–237
Nolan DR, Gower AL, Destrade M, Ogden RW, McGarry J (2014) A robust anisotropic hyperelastic formulation for the modelling of soft tissue. J Mech Behav Biomed Mater 39:48–60
Ostwald R, Kuhl E, Menzel A (2019) On the implementation of finite deformation gradient-enhanced damage models. Comput Mech 64:847–877
Rao IJ (2011) Modeling of growth and remodeling in soft biological tissues with multiple constituents. Mech Res Commun 38:24–28
Schulte R, Ostwald R, Menzel A (2020) Gradient-enhanced modelling of damage for rate-dependent material behaviour—a parameter identification framework. Materials 13:3156
Simo JC, Ju J (1987) Strain-and stress-based continuum damage models—I. Formulation. Int J Solids Struct 23:821–840
Skalak R (1981) Growth as a finite displacement field. In: Proceedings of the IUTAM symposium on finite elasticity. Springer, pp 347–355
Waffenschmidt T, Polindara C, Menzel A, Blanco S (2014) A gradient-enhanced large-deformation continuum damage model for fibre-reinforced materials. Comput Methods Appl Mech Eng 268:801–842
Acknowledgements
The research leading to this paper is funded by Department of Education of Liaoning Province [JYTQN2023010] and Dalian Science and Technology Innovation Fund [2023R063].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zuo, D., Zhu, M., Chen, D. et al. A computationally efficient gradient-enhanced healing model for soft biological tissues. Biomech Model Mechanobiol (2024). https://doi.org/10.1007/s10237-024-01851-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10237-024-01851-5