Abstract
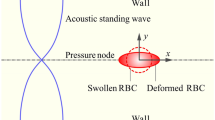
A computational model is developed to investigate the nonlinear static deformation of a spherical (osmotically swollen) red blood cell (RBC) induced by ultrasonic standing wave. The ultrasonic standing wave can generate steady acoustic radiation stress to deform the cell, and in turn, the deformed cell reshapes the acoustic field. This is a real-time coupling problem between the acoustic field and the mechanical field. In the computational model, the acoustic radiation stress acting on the RBC membrane is modeled by adopting the nonviscous momentum flux theory. The RBC membrane is modeled as a hyperelastic shell considering the in-plane elasticity, bending elasticity, and surface tension of the membrane. The volume conservation constraint of the membrane sealing fluid is applied to ensure the osmotic balance of the membrane. To address this real-time coupling problem, the computational model is implemented by a finite element method algorithm. The numerical results are compared with the existing theoretical model and experimental data, and the strain hardening trend of the experimental data is successfully predicted, which verifies the accuracy and effectiveness of the computational model. The computational model can accurately extract the mechanical properties of cells from acoustic deformation experiments, which is helpful for the diagnosis of some human diseases.














Similar content being viewed by others
References
Abraham EH, Vos P, Kahn J, Grubman SA, Jefferson DM, Ding I, Okunieff P (1996) Cystic fibrosis hetero- and homozygosity is associated with inhibition of breast cancer growth. Nat Med 2(5):593–596
Andrade MAB, Marzo A (2019) Numerical and experimental investigation of the stability of a drop in a single-axis acoustic levitator. Phys Fluids 31(11):117101
Augustsson P, Karlsen JT, Su HW, Bruus H, Voldman J (2016) Iso-acoustic focusing of cells for size-insensitive acousto-mechanical phenotyping. Nat Commun 7:11556
Baasch T, Dual J (2020) Acoustic radiation force on a spherical fluid or solid elastic particle placed close to a fluid or solid elastic half-space. Phys Rev Appl 14(2):024052
Bansch E, Gotz M (2018) Numerical study of droplet evaporation in an acoustic levitator. Phys Fluids 30(3):037103
Bazilevs Y, Calo VM, Hughes TJR, Zhang Y (2008) Isogeometric fluid-structure interaction: theory, algorithms, and computations. Comput Mech 43(1):3–37
Bernard I, Doinikov AA, Marmottant P, Rabaud D, Poulain C, Thibault P (2017) Controlled rotation and translation of spherical particles or living cells by surface acoustic waves. Lab Chip 17(14):2470–2480
Chowdhury F, Na S, Li D, Poh YC, Tanaka TS, Wang F, Wang N (2010) Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater 9(1):82–88
COMSOL (2018) COMSOL multiphysics reference manual, COMSOL multiphysics version 5.4. https://doc.comsol.com/5.4/doc/com.comsol.help.comsol/COMSOL_ReferenceManual.pdf. Accessed 23 Oct 2021
Dao M, Lim CT, Suresh S (2005) Mechanics of the human red blood cell deformed by optical tweezers. J Mech Phys Solids 53(2):493–494
Ding XY, Peng ZL, Lin SCS, Geri M, Li SX, Li P, Chen YC, Dao M, Suresh S, Huang TJ (2014) Cell separation using tilted-angle standing surface acoustic waves. Proc Natl Acad Sci U S A 111(36):12992–12997
Doinikov AA, Gerlt MS, Dual J (2020) Acoustic radiation forces produced by sharp-edge structures in microfluidic systems. Phys Rev Lett 124(15):154501
Duong TX, Roohbakhshan F, Sauer RA (2017) A new rotation-free isogeometric thin shell formulation and a corresponding continuity constraint for patch boundaries. Comput Methods Appl Mech Eng 316:43–83
Elliott CM, Stinner B (2010) Modeling and computation of two phase geometric biomembranes using surface finite elements. J Comput Phys 229(18):6585–6612
Feng F, Klug WS (2006) Finite element modeling of lipid bilayer membranes. J Comput Phys 220(1):394–408
Feng Z, Waugh RE, Peng ZL (2020) Constitutive model of erythrocyte membranes with distributions of spectrin orientations and lengths. Biophys J 119(11):2190–2204
Gao X, Huang ZP, Qu JM, Fang DN (2014) A curvature-dependent interfacial energy-based interface stress theory and its applications to nano-structured materials: (I) general theory. J Mech Phys Solids 66:59–77
Gao X, Huang ZP, Fang DN (2017) Curvature-dependent interfacial energy and its effects on the elastic properties of nanomaterials. Int J Solids Struct 113–114:100–107
Gossett DR, Tse HTK, Lee SA, Ying Y, Lindgren AG, Yang OO, Rao JY, Clark AT, Di Carlo D (2012) Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc Natl Acad Sci U S A 109(20):7630–7635
Haase K, Pelling AE (2015) Investigating cell mechanics with atomic force microscopy. J R Soc Interface 12(104):20140970
Hochmuth RM (2000) Micropipette aspiration of living cells. J Biomech 33(1):15–22
Jakobsson O, Antfolk M, Laurell T (2014) Continuous flow two-dimensional acoustic orientation of nonspherical cells. Anal Chem 86(12):6111–6114
Lautenschlager F, Paschke S, Schinkinger S, Bruel A, Beil M, Guck J (2009) The regulatory role of cell mechanics for migration of differentiating myeloid cells. Proc Natl Acad Sci U S A 106(37):15696–15701
Lee CP, Wang TG (1993) Acoustic radiation pressure. J Acoust Soc Am 94(2):1099–1109
Link A, Franke T (2020) Acoustic erythrocytometer for mechanically probing cell viscoelasticity. Lab Chip 20(11):1991–1998
Liu GT, Lei JJ, Cheng F, Li KM, Ji XR, Huang ZG, Guo ZN (2021) Ultrasonic particle manipulation in glass capillaries: a concise review. Micromachines 12(8):876
Marston PL, Goosby SG (1985) Ultrasonically stimulated low-frequency oscillation and breakup of immiscible liquid drops: photographs. Phys Fluids 28(5):1233–1242
Mietke A, Otto O, Girardo S, Rosendahl P, Taubenberger A, Golfier S, Ulbricht E, Aland S, Guck J, Fischer-Friedrich E (2015) Extracting cell stiffness from real-time deformability cytometry: theory and experiment. Biophys J 109(10):2023–2036
Mishra P, Hill M, Glynne-Jones P (2014) Deformation of red blood cells using acoustic radiation forces. Biomicrofluidics 8(3):034109
Nama N, Aguirre M, Humphrey JD, Figueroa CA (2020) A nonlinear rotation-free shell formulation with prestressing for vascular biomechanics. Sci Rep 10(1):17528
Nodargi NA, Bisegna P, Caselli F (2017) Effective computational modeling of erythrocyte electro-deformation. Meccanica 52(3):613–631
Nodargi NA, Kiendl J, Bisegna P, Caselli F, De Lorenzis L (2018) An isogeometric analysis formulation for red blood cell electro-deformation modeling. Comput Methods Appl Mech Eng 338:392–411
Omori T, Ishikawa T, Barthes-Biesel D, Salsac AV, Imai Y, Yamaguchi T (2012) Tension of red blood cell membrane in simple shear flow. Phys Rev E 86(5):056321
Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE (2007) Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci U S A 104(40):15619–15624
Park Y, Best CA, Kuriabova T, Henle ML, Feld MS, Levine AJ, Popescu G (2011) Measurement of the nonlinear elasticity of red blood cell membranes. Phys Rev E 83(5):051925
Peng XJ, He W, Xin FX, Genin GM, Lu TJ (2020) Standing surface acoustic waves, and the mechanics of acoustic tweezer manipulation of eukaryotic cells. J Mech Phys Solids 145:104134
Pozrikidis C (2001) Effect of membrane bending stiffness on the deformation of capsules in simple shear flow. J Fluid Mech 440:269–291
Rapaport E, Fontaine J (1989) Anticancer activities of adenine-nucleotides in mice are mediated through expression of erythrocyte ATP pools. Proc Natl Acad Sci U S A 86(5):1662–1666
Sarvazyan AP, Rudenko OV, Nyborg WL (2010) Biomedical applications of radiation force of ultrasound: historical roots and physical basis. Ultrasound Med Biol 36(9):1379–1394
Sauer RA, Duong TX (2017) On the theoretical foundations of thin solid and liquid shells. Math Mech Solids 22(3):343–371
Schollhammer D, Fries TP (2019) Kirchhoff-Love shell theory based on tangential differential calculus. Comput Mech 64(1):113–131
Seifert U (1997) Configurations of fluid membranes and vesicles. Adv Phys 46(1):13–137
Silva GT, Tian LF, Franklin A, Wang XJ, Han XJ, Mann S, Drinkwater BW (2019) Acoustic deformation for the extraction of mechanical properties of lipid vesicle populations. Phys Rev E 99(6):063002
Sprague RS, Olearczyk JJ, Spence DM, Stephenson AH, Sprung RW, Lonigro AJ (2003) Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am J Physiol Heart Circul Physiol 285(2):693–700
Suresh S, Spatz J, Mills JP, Micoulet A, Dao M, Lim CT, Beil M, Seufferlein T (2005) Connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria. Acta Biomater 1(1):15–30
Tachev KD, Angarska JK, Danov KD, Kralchevsky PA (2000) Erythrocyte attachment to substrates: determination of membrane tension and adhesion energy. Colloid Surf B Biointerfaces 19(1):61–80
Tan YH, Sun D, Wang JZ, Huang WH (2010) Mechanical characterization of human red blood cells under different osmotic conditions by robotic manipulation with optical tweezers. IEEE Trans Biomed Eng 57(7):1816–1825
Vlahovska PM, Gracia RS, Aranda-Espinoza S, Dimova R (2009) Electrohydrodynamic model of vesicle deformation in alternating electric fields. Biophys J 96(12):4789–4803
Wan JD, Ristenpart WD, Stone HA (2008) Dynamics of shear-induced ATP release from red blood cells. Proc Natl Acad Sci U S A 105(43):16432–16437
Wijaya FB, Mohapatra AR, Sepehrirahnama S, Lim KM (2016) Coupled acoustic-shell model for experimental study of cell stiffness under acoustophoresis. Microfluid Nanofluid 20(5):69
Xin FX, Lu TJ (2016) Acoustomechanical constitutive theory for soft materials. Acta Mech Sin 32(5):828–840
Xin FX, Lu TJ (2017) A nonlinear acoustomechanical field theory of polymeric gels. Int J Solids Struct 112:133–142
Yarin AL, Pfaffenlehner M, Tropea C (1998) On the acoustic levitation of droplets. J Fluid Mech 356:65–91
Ye T, Nhan PT, Khoo BC, Lim CT (2013) Stretching and relaxation of malaria-infected red blood cells. Biophys J 105(5):1103–1109
Yu M, Lira RB, Riske KA, Dimova R, Lin H (2015) Ellipsoidal relaxation of deformed vesicles. Phys Rev Lett 115(12):128303
Zheng Y, Nguyen J, Wei Y, Sun Y (2013) Recent advances in microfluidic techniques for single-cell biophysical characterization. Lab Chip 13(13):2464–2483
Zhong C, Helfrich W (1989) Bending energy of vesicle membranes: general expressions for the first, second, and third variation of the shape energy and applications to spheres and cylinders. Phys Rev A 39(10):5280–5288
Acknowledgements
This work was supported by the National Natural Science Foundation of China (11772248, 52075416, and 11761131003) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Numerical model for calculating the energy minimization shape of lipid membrane
Appendix: Numerical model for calculating the energy minimization shape of lipid membrane
According to Sect. 3, the weak form equations for the evolution velocity and curvature tensor, \(\left( {{\mathbf{v}},{\mathbf{b}}} \right)\), of the lipid membrane are given by
Here, \(\left( {\delta {\mathbf{v}},\delta {\mathbf{b}}} \right)\) are the test functions of \(\left( {{\mathbf{v}},{\mathbf{b}}} \right)\). \(\left( {\sigma ,\Delta p} \right)\) are two Lagrange multipliers to enforce the following area and volume constraints. The surface tension \(\sigma\) is now used as a Lagrange multiplier to enforce the surface area retention constraint
Here, \(\overline{A}\) is the desired surface area, which is considered to be consistent with the reference surface area, and \(A\) is the current surface area. Formally, they are given by
The pressure jump \(\Delta p\) is now used as a Lagrange multiplier to enforce the volume controller
The controller drives the current volume \(V\) to the desired volume \(\overline{V}\) in a characteristic time \(\tau_{V}\). \(\overline{V}\) is derived from \(\overline{A}\) according to the reduced volume \(v\) as
\(V\) and its time derivative \({{{\text{d}}V} \mathord{\left/ {\vphantom {{{\text{d}}V} {{\text{d}}\tau }}} \right. \kern-\nulldelimiterspace} {{\text{d}}\tau }}\) are given by
The parameters are set as: \(E_{b} = 1\;{\text{N/m}}\), \(\tau_{V} = 1{\text{s}}\), \(a_{0} = 1\;{\text{m}}\) and \(\gamma = 1\;{\text{Pa}} \cdot {\text{s}} \cdot {\text{m}}^{ - 1}\). The overall computational algorithm is similar to that described in Sect. 3 and Fig. 6: the acoustic module is de-activated, and the mechanical module is replaced with weak form equations (41)–(47) to solve the unknown field variables \(\left( {{\mathbf{v}},{\mathbf{b}}} \right)\) and global variables \(\left( {\sigma ,\Delta p} \right)\), while maintaining the moving mesh module. Taking reduced volume \(v = 0.58\) as an example, Fig.
The energy minimum shapes calculated from different initial shapes at reduce volume \(v = 0.58\): a the oblate spheroid with a/b = 1.5 and b the prolate spheroid with a/b = 0.44. For both cases, the membrane surface is discretized by 96 line elements. Note that the “moving mesh” module in COMSOL is not only supported in the boundary, so the computational domain of the moving mesh is extended to the internal domain defined by the membrane
15 shows the energy minimum shapes calculated from different initial shapes: (a) an oblate spheroid with three semi-axes \(\left( {1.5a_{0} ,1.5a_{0} ,a_{0} } \right)\) and (b) a prolate spheroid with three semi-axes \(\left( {2.25a_{0} ,a_{0} ,a_{0} } \right)\). It is observed that the initial oblate shape leads to a biconcave energy minimum shape, while the initial prolate shape leads to a peanut-shaped one.
Rights and permissions
About this article
Cite this article
Liu, Y., Xin, F. Nonlinear large deformation of a spherical red blood cell induced by ultrasonic standing wave. Biomech Model Mechanobiol 21, 589–604 (2022). https://doi.org/10.1007/s10237-021-01550-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-021-01550-5