Abstract
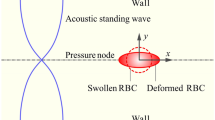
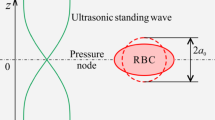
Under the influence of acoustic radiation force, particles can be trapped and deformed at the pressure node in a microfluidic channel. Based on this principle, the elastic modulus of biological cells can be estimated. In this study, a numerical framework, consisting of a boundary element model for acoustic field and an axisymmetric shell model, is developed to simulate the cell deformation under acoustic radiation force. The boundary element model is used to calculate the radiation traction exerted on the cell surface. The cell membrane deformation due to this traction is simulated by using the axisymmetric shell model. The Young’s moduli of algae and red blood cell membranes are then estimated by comparing the experimental observation with the simulated membrane deformation. It is found that the value of Young’s modulus of the red blood cell membrane is lower than that of algae cell membrane. Furthermore, for both cells, the estimated Young’s moduli are negligible compared to the bulk moduli of the cells reported in the previous studies.



















Similar content being viewed by others
Abbreviations
- \({\mathbf {\Pi }}\) :
-
Radiation stress tensor
- \({\mathbf T} _{\mathrm{ac}}\) :
-
Acoustic radiation traction
- \({\mathbf v} _1\) :
-
First-order acoustic velocity
- A :
-
Coefficient matrix of the final system of equations
- b :
-
Known vector of the final system of equations
- C :
-
Solid angle matrix
- H and G :
-
Coefficient matrices in the boundary element method
- I :
-
Identity matrix
- n :
-
Unit normal vector of the cell surface
- x :
-
Solution vector of the final system of equations
- \(\alpha\) :
-
Cell aspect ratio obtained from experiment
- \(\alpha ^*\) :
-
Cell aspect ratio obtained from simulation
- \(\beta\) :
-
Sensitivity factor
- \(\chi\) :
-
Rotation of the normal of the cell membrane
- \(\Delta a\) :
-
Change in the nominal radius of the core–shell structure
- \(\Delta V\) :
-
Change in the cell volume
- \(\dfrac{\partial \phi _{\mathrm{in}}}{\partial n}\) :
-
Incident wave velocity in the normal direction
- \(\dfrac{\partial \phi _{i}}{\partial n}\) :
-
Transmitted wave velocity in the normal direction
- \(\dfrac{\partial \phi _{\mathrm{sc}}}{\partial n}\) :
-
Scattered wave velocity in the normal direction
- \(\epsilon _v\) :
-
Volumetric strain of the core–shell structure
- \(\epsilon _{\theta }\) :
-
Tangential strain of the core–shell structure
- \(\gamma\) :
-
Acoustic contrast factor
- \(\gamma _{\mathrm{algae}}\) :
-
Acoustic contrast factor of the algae cell
- \(\gamma _{\mathrm{rbc}}\) :
-
Acoustic contrast factor of the red blood cell
- \(\kappa\) :
-
Fluid compressibility
- \(\kappa _0\) :
-
Compressibility of fluid outside of the cell membrane
- \(\kappa _i\) :
-
Compressibility of fluid inside of the cell membrane
- \(\lambda\) :
-
Wavelength
- \(\langle ..\rangle\) :
-
Time-averaging operator
- \(\omega\) :
-
Angular frequency
- \(\phi\) :
-
Total wave potential
- \(\phi _i\) :
-
Transmitted wave potential
- \(\phi _{\mathrm{in}}\) :
-
Incident wave potential
- \(\phi _{\mathrm{sc}}\) :
-
Scattered wave potential
- \(\rho\) :
-
Fluid mass density
- \(\rho _0\) :
-
Mass density of surrounding fluid
- \(\rho _i\) :
-
Mass density of interior fluid
- \(\sigma _0\) :
-
Magnitude of the radial surface stress (Guck et al. 2001)
- \(\sigma _r\) :
-
Radial surface stress due to optical force (Guck et al. 2001)
- \(\sigma _{\theta }\) :
-
Hoop stress acting on the core–shell structure
- \(\upsilon\) :
-
Poisson’s ratio of the cell membrane
- \(\varepsilon\) :
-
Iteration tolerance
- \(\xi\) :
-
Local coordinate of cell membrane in the normal direction
- a :
-
Nominal radius of the core–shell structure
- \(B_{\mathrm{core}}\) :
-
Core bulk modulus
- \(B_{\mathrm{eff}}\) :
-
Effective bulk modulus of the core–shell structure
- \(B_{\mathrm{shell}}\) :
-
Shell bulk modulus
- C :
-
Solid angle
- E :
-
Young’s modulus of the cell membrane
- e :
-
Difference between the simulated aspect ratio and that from experiment
- \(G_k\) :
-
Green’s function of Helmholtz equation
- h :
-
Cell membrane thickness
- k :
-
Wave number
- \(k_0\) :
-
Wave number in the exterior domain
- \(k_i\) :
-
Wave number in the interior domain
- l :
-
Load point
- \(M_s\) :
-
Membrane moment resultant
- N :
-
Number of experimental data points
- \(N_s\) :
-
Membrane stress resultant in the s-direction
- p :
-
Uniform internal pressure on the core–shell structure
- \(p_1\) :
-
First-order acoustic pressure
- \(p_2\) :
-
Second-order acoustic pressure
- \(P_o\) :
-
Pressure amplitude of incident wave
- \(P_{\mathrm{core}}\) :
-
Internal pressure
- \(P_{\mathrm{in}}\) :
-
Incident standing wave
- \(P_{\mathrm{sc}}\) :
-
Scattered acoustic pressure
- Q :
-
Field point
- \(Q_s\) :
-
Membrane stress resultant in the \(\xi\)-direction
- \(r, \theta\), and z :
-
Polar cylindrical coordinates of the cell membrane
- \(R_{1}\) :
-
Principal radius of curvature in the meridian direction
- \(R_{2}\) :
-
Principal radius of curvature in the latitude direction
- S :
-
Cell surface
- s :
-
Local coordinate of cell membrane in the tangential direction
- \(S_e\) :
-
Sum of \(e^2\)
- \({\mathrm{std}}(\alpha )\) :
-
Standard deviation of cell aspect ratio
- \({\mathrm{std}}(E)\) :
-
Standard deviation of Young’s modulus
- \(T_\xi\) :
-
Membrane traction in the \(\xi\)-direction
- \(T_s\) :
-
Membrane traction in the s-direction
- u :
-
Displacement of middle surface of the cell membrane in the s-direction
- \(u_z\) :
-
Displacement of the pole in the z-direction (Guck et al. 2001)
- V :
-
Cell volume
- w :
-
Displacement of middle surface of the cell membrane in the \(\xi\)-direction
References
Barsanti L, Gualtieri P (2014) Algae: anatomy, biochemistry, and biotechnology. CRC Press, London
Bushell GR, Cahill C, Clarke FM, Gibson CT, Myhra S, Watson GS (1999) Imaging and force-distance analysis of human fibroblasts in vitro by atomic force microscopy. Cytometry 36(3):254–264
Daily B, Elson EL, Zahalak GI (1984) Cell poking. determination of the elastic area compressibility modulus of the erythrocyte membrane. Biophys J 45(4):671
Discher DE, Mohandas N (1996) Kinematics of red cell aspiration by fluorescence-imaged microdeformation. Biophys J 71(4):1680
Doinikov AA (1994) Acoustic radiation pressure on a compressible sphere in a viscous fluid. J Fluid Mech 267:1–22
Dulińska I, Targosz M, Strojny W, Lekka M, Czuba P, Balwierz W, Szymoński M (2006) Stiffness of normal and pathological erythrocytes studied by means of atomic force microscopy. J Biochem Biophys Methods 66(1):1–11
Flügge Wilhelm (1973) Stress in shells. Spring, Berlin
Gomez Hincapie E (2014) Development of a continuous flow ultrasonic harvesting system for microalgae. Ph.D. thesis, Colorado State University
Gonzalez-Delgado AD, Kafarov V (2011) Microalgae based biorefinery: issues to consider. CTF-Ciencia, Tecnologia y Futuro 4(4):5–22
Guck J, Ananthakrishnan R, Mahmood H, Moon TJ, Cunningham CC, Käs J (2001) The optical stretcher: a novel laser tool to micromanipulate cells. Biophys J 81(2):767–784
Gunerken E, D’Hondt E, Eppink MHM, Garcia-Gonzalez L, Elst K, Wijffels RH (2015) Cell disruption for microalgae biorefineries. Biotechnol Adv 33(2):243–260
Hartono D, Liu Y, Tan PL, Then XYS, Yung LYL, Lim KM (2011) On-chip measurements of cell compressibility via acoustic radiation. Lab Chip 11(23):4072–4080
Kim Y, Kim K, Park Y (2012) Measurement techniques for red blood cell deformability: recent advances. INTECH Open Access Publisher
Kirkup SM (2007) The boundary element method in acoustics. Integrated Sound Software
Leibacher I, Dietze W, Hahn P, Wang J, Schmitt S, Dual J (2014) Acoustophoresis of hollow and core-shell particles in two-dimensional resonance modes. Microfluid Nanofluidics 16(3):513–524
Lekka M, Fornal M, Pyka-Fosciak G, Lebed K, Wizner B, Grodzicki T, Styczen J (2005) Erythrocyte stiffness probed using atomic force microscope. Biorheology 42(4):307–318
Marston Philip L (1980) Shape oscillation and static deformation of drops and bubbles driven by modulated radiation stresses–theory. J Acoust Soc Am 67(1):15–26
Mathworks (2015) Matlab the language of technical computing. http://www.mathworks.com/products/matlab
Mishra P, Hill M, Glynne-Jones P (2014) Deformation of red blood cells using acoustic radiation forces. Biomicrofluidics 8(3):034109
Mohandas N, Evans E (1994) Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu Rev Biophys Biomol Struct 23(1):787–818
Nava G, Bragheri F, Yang T, Minzioni P, Osellame R, Cristiani I, Berg-Sørensen K (2015) All-silica microfluidic optical stretcher with acoustophoretic prefocusing. Microfluid Nanofluidics 19(4):837–844
Nikon (2013) Nikon NIS Elements BR scientific image processing software. http://www.nikoninstruments.com/Products/Software/NIS-Elements-Basic-Research
Rahnama S Sepehri, Lim K-M, Chau FS (2013) Numerical calculation of acoustic radiation force and moment on rigid spheres and ellipsoids in a standing wave. In: Proceedings of the 2013 international congress on ultrasonics (ICU, 2013), pp 814–819
Settnes M, Bruus H (2012) Forces acting on a small particle in an acoustical field in a viscous fluid. Phys Rev E 85(1):016327
Sleep J, Wilson D, Simmons R, Gratzer W (1999) Elasticity of the red cell membrane and its relation to hemolytic disorders: an optical tweezers study. Biophys J 77(6):3085–3095
Wu HW, Kuhn T, Moy VT (1998) Mechanical properties of l929 cells measured by atomic force microscopy: effects of anticytoskeletal drugs and membrane crosslinking. Scanning 20(5):389–397
Yosioka K, Kawasima Y (1955) Acoustic radiation pressure on a compressible sphere. Acta Acust United Acust 5(3):167–173
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
As discussed in Sect. 2.1, for each acoustic domain, a system of equations that is similar to Eq. (6) can be formulated. For the exterior domain, the system of equations is given by
It is noted that \({\mathbf H} ^-_{0}\) and \({\mathbf G} _{0}\) are evaluated by using Eqs. (7) and (8) based on \(k_0\) and \({\mathbf n} ^-\). Matrix \({\mathbf C} ^-\) is a diagonal matrix that contains the solid angles of the surface elements with respect to the exterior domain. Similarly, for the interior domain, the system of equations is given by
where \({\mathbf C} ^+\) is the diagonal matrix that contains the solid angles of the surface elements with respect to the interior domain. It is noted that the incident wave potential \(\phi _{\mathrm{in}}\) satisfies the boundary integral equation for the interior domain if \(k_i = k_0\). Hence, the following system of equations can be formulated.
By substituting Eqs. (9), (37) and (10)–(36), we obtain
In the limit of \(\dfrac{\rho _{0}}{\rho _{i}}\rightarrow 0\), Eq. (38) reduces to
which is the boundary condition for a rigid immovable particle. The final system of equations, Eq. (11), is given by the combination of Eqs. (35) and (38).
Rights and permissions
About this article
Cite this article
Wijaya, F.B., Mohapatra, A.R., Sepehrirahnama, S. et al. Coupled acoustic-shell model for experimental study of cell stiffness under acoustophoresis. Microfluid Nanofluid 20, 69 (2016). https://doi.org/10.1007/s10404-016-1734-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-016-1734-1