Abstract
The objective of this study was to evaluate the feasibility of combined modular processes to selectively remove Sb from mine-impacted waters in an Arctic environment in order to fulfil local environmental criteria for discharged waters. Novel ion exchange, selective extraction and ultrafiltration, electrocoagulation, and dissolved air flotation technologies were investigated, individually or in combination, from the laboratory- to pilot-demonstration scale. Laboratory-scale testing using Fe2(SO4)3 precipitation, ion exchange resin, selective ion extraction and ultrafiltration, and electrocoagulation with or without subsequent dissolved air flotation indicated that any of the methods are potentially applicable to Sb removal from mine water. The observed differences between Sb and As removal efficiency by ion exchange resin illustrated the need for Sb-specific removal and recovery technologies. Techno-economic analyses showed that treatment of mine water using electrocoagulation-dissolved air flotation yields the lowest comparative life-cycle cost of examined technologies. Results demonstrated increased Sb attenuation efficiency using either electrocoagulation-dissolved air flotation or selective extraction and ultrafiltration, even when treating only 50% of the mine-impacted water, compared with conventional Fe2(SO4)3 precipitation from mine water. Additional investigation is necessary to characterize the long-term stability of the mineral phases in Sb-containing solid residues and to inform selection of Sb recovery methods and utilisation or final disposal options for the residual materials.
Zusammenfassung
Das Ziel dieser Studie war die Bewertung der Machbarkeit von Verfahren zur selektiven Entfernung von Sb aus bergbaubeeinflussten Wässern in arktischer Umgebung, um lokale Umweltauflagen für einzuleitende Wässer zu erfüllen. Techniken zum Ionenaustausch, zur selektiven Extraktion und Ultrafiltration, zur Elektrokoagulation und Flotation wurden einzeln oder in Kombination vom Labor- bis zum Pilot- und Demonstrationsmaßstab untersucht. Labortests mit Fe2(SO4)3-Ausfällung, Ionenaustauscherharz, selektiver Extraktion und Ultrafiltration sowie Elektrokoagulation mit oder ohne Flotation deuteten darauf hin, dass einige der Methoden zur Entfernung von Sb aus Bergbauwässern potentiell geeignet sind. Die beobachteten Unterschiede bei der Entfernung von Sb und As durch Ionenaustauscherharz zeigten die Notwendigkeit von Sb-spezifischen Reinigungs- und Rückgewinnungstechnologien. Eine wirtschaftliche Bewertung ergab, dass die Kombination von Elektrokoagulation und Flotation die niedrigsten Kosten verursacht. Die Sb-Reinigung von Grubenwässern bei Anwendung der Elektrokoagulation mit Flotation bzw. selektiver Extraktion und Ultrafiltration war höher im Vergleich zur konventionellen Fe2(SO4)3-Ausfällung. Weitere Untersuchungen sind notwendig, um die Langzeitstabilität der Mineralphasen in Sb-haltigen Feststoffrückständen zu bewerten und die Auswahl der Sb-Rückgewinnungsmethoden sowie die Verwendungs- bzw. Entsorgungsmöglichkeiten der Reststoffe zu bestimmen.
Resumen
El objetivo de este estudio fue evaluar la viabilidad de procesos modulares combinados para eliminar selectivamente Sb de las aguas impactadas por la actividad minera en un entorno ártico con el fin de cumplir con los criterios ambientales locales para las aguas vertidas. Se investigaron nuevas tecnologías como las de intercambio iónico, extracción selectiva y ultrafiltración, electrocoagulación y flotación por aire disuelto, utilizadas en forma individual o en combinación, desde la escala de laboratorio hasta la de piloto. Las pruebas a escala de laboratorio que utilizan: precipitación con Fe2(SO4)3, intercambio iónico, extracción selectiva de iones y ultrafiltración y electrocoagulación con o sin subsiguiente flotación con aire disuelto indicaron que cualquiera de los métodos es potencialmente aplicable a la remoción de Sb del agua de la mina. Las diferencias observadas entre la eficacia de eliminación de Sb y As por intercambio iónico demostraron la necesidad de tecnologías de eliminación y recuperación específicas de Sb. Los análisis técnico-económicos mostraron que el tratamiento del agua de la mina usando flotación por aire disuelto por electrocoagulación tiene el menor costo entre las tecnologías examinadas. Los resultados demostraron una mayor eficiencia de atenuación de Sb mediante la flotación con aire disuelto por electrocoagulación o la extracción selectiva y la ultrafiltración, incluso cuando se trata solo el 50% del agua impactada, en comparación con la precipitación convencional con Fe2(SO4)3. Es necesario realizar investigaciones adicionales para caracterizar la estabilidad a largo plazo de las fases minerales en los residuos sólidos que contienen Sb y para informar la selección de los métodos de recuperación de Sb y las opciones de utilización o disposición final para los materiales residuales.
抽象
为满足当地水环境要求的废水排放标准要求,研究旨在评估在北极环境从受采矿影响的废水中选择性去除锑的组合工艺的可行性。从实验室至小型演示试验、以单独或组合试验方式,研究了离子交换、选择性萃取与超滤、电凝聚与溶解空气浮选等锑去除与回收新技术。室内试验包括Fe2(SO4)3沉淀、离子交换树脂、选择性离子萃取与超滤、跟或不跟溶解气体浮选的电凝聚等,它们都适于去除矿井废水中的锑。离子交换树脂对锑和砷去除效率的差异显示出研究特定的锑去除和回收技术的必要。试验技术经济分析表明,电凝聚-溶解气体浮选处理矿井废水的寿命成本最低。结果表明,与传统的Fe2(SO4)3沉淀处理矿井废水相比,哪怕仅处理50%的矿井废水,电凝聚-溶解气体浮选或者选择性萃取与超滤都显示出增高的锑去除效率。我们还需要进一步研究含锑固体残渣中矿物相的长期稳定性,发现更好的锑回收与利用方法以及残渣最终处置方式。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimony is a common trace element contaminant of mine-impacted water, as it is a component of more than 100 different natural minerals (Anderson 2012). The element Sb typically occurs with S, Cu, Pb, As, and Ag. Wastes containing Sb and Sb compounds are designated by the Basel Convention (United Nations Environment Programme 1999) as hazardous. The Council of the European Union Drinking Water Directive 98/83/EC (1998) further establishes a maximum admissible concentration of 5 µg/L Sb in drinking water. Surface waters receiving mining and industrial effluent discharges frequently exhibit Sb concentrations substantially greater than 5 µg/L Sb. Although the 1976 Council of the European Communities Directive 76/464/EEC identified Sb as a substance with potential to have deleterious effects on the aquatic environment, permissible concentrations for Sb have not been determined (Council of the European Communities 1976). Despite its widespread use in commercial and consumer products, the present understanding of Sb geochemistry in the aquatic environment and its ecotoxicological characteristics remain incomplete (Obiakor et al. 2017).
Estimated global mine production of Sb was 130,000 t in 2016, down from 142,000 t in 2015 (USGS 2017). Primary Sb production is currently limited to only a few countries, of which China was the leading producer, generating 77% of the global total (100,000 and 110,000 t, respectively) in 2015 and 2016 (USGS 2017). The unequal distribution of the world’s Sb resources, low recycling inputs, and a declining trend in the recovery of antimonial lead by the battery industry (Filella et al. 2002) are indicative of a potential supply risk. These factors, concomitant with its low substitutability index, highlight the status of Sb as a critical strategic resource at risk of a supply shortage in Europe (European Commission 2017).
Co-occurrence in mining and industrial emissions, along with similarities between the s2p3 outer orbital configuration of Sb and As, have resulted in the two metalloids frequently being considered analogues of one another with respect to their environmental behaviour. However, differences in Sb and As ionic radii and charge density yield differences in oxygen coordination and speciation as a function of redox potential and pH (Wilson et al. 2010). Antimony, like As, is known to occur in two main oxidation states in natural waters: Sb(V) is the dominant species in oxygenated systems, but multiple studies have documented Sb(III) in marine water, fresh surface water, groundwater, and rainwater (Filella et al. 2002). Similarly, Sb(V) has been observed in anoxic waters (Filella et al. 2002). The mechanism(s) enabling persistence of thermodynamically unstable Sb species may be related to the formation kinetics of secondary Sb minerals. Majzlan et al. (2016) report relatively rapid formation of soluble Sb minerals, followed by kinetically slow formation of the stable Sb mineral tripuhyrite (FeSbO4), on a time scale of years to decades. The solubility of initially formed Sb minerals and the slow formation rate of tripuhyrite has clear implications for Sb recovery from water.
Antimony Treatment and Recovery Technologies
Current treatment technologies for Sb removal from water include chemical precipitation, adsorption, ion exchange, membrane separation, and electrochemical techniques, either as single-unit operations or in combination with one another. Coagulation, typically using Fe or Al salts, has been used for decades to remove Sb and other oxyanions from contaminated waters through a combination of adsorption and precipitation reactions. Antimony is more effectively removed from solution using Fe salts than with Al salts, and FeCl3 has been shown to attenuate aqueous As and Sb species in the order As(V) > Sb(III) > As(III) > Sb(V) (Guo et al. 2009; Kang et al. 2003). Removal of both Sb(III) and Sb(V) was limited at pH < 4; however, whilst Sb(III) coagulation by FeCl3 was highly efficient between pH 4 and 10, Sb(V) exhibited a narrow optimum pH range for removal from pH 4.5–5.5 (Guo et al. 2009). In addition, Sb(V) attenuation by FeCl3 coagulation is substantially reduced in the presence of competing ions such as phosphate (PO43−), bicarbonate (HCO3−), sulphate (SO42−), and humic acid, whereas Sb(III) removal by FeCl3 coagulation is not significantly affected by competing ions (Guo et al. 2009; Wu et al. 2010).
The question arose whether similarities between Sb and As speciation and fate in solution could be exploited to effectively attenuate Sb-polluted mine-impacted waters. Ion exchange resins are widely used to treat As-contaminated waters and several commercially-available ion exchangers can be used to remove Sb from aqueous solution, although efficient Sb removal via ion exchange can be problematic due to differences in Sb speciation (McKevitt and Dreisinger 2009; Riveros et al. 2008). The removal of Sb and As oxyanions by conventional strong-base ion exchangers may be significantly hampered by the presence of other anions, such as sulphate, chloride, nitrate, and others, which compete for available binding sites on the resin (Sylvester et al. 2007; Ungureanu et al. 2015). The effectiveness of iron oxide impregnated resins for As(III) and As(V) removal from various sources of contaminated water sources, containing other anions at concentrations many orders of magnitude greater than As, is well documented (Koseoglu et al. 2011; Sarkar et al. 2007; Sylvester et al. 2007). These and other studies have demonstrated that iron oxide impregnated resins can be effectively regenerated and reused in sequential adsorption cycles with minimal loss in capacity. Limited information is available regarding the removal of Sb from water using iron oxide impregnated resins. Miao et al. (2014) showed a decrease in solution concentration of Sb(V) from 30 to 5 µg/L with hydrated ferric oxide supported by polymeric resin beads; however, there remains a dearth of information concerning the removal of Sb from water in the presence of As using iron oxide impregnated resins.
Studies of Sb removal from solution by membrane separation are somewhat limited but indicate differences in removal efficiency among Sb species. Reverse osmosis (RO), a high-pressure membrane separation process increasingly used in wastewater treatment, has shown preferential removal of Sb(V) compared with Sb(III) across the pH range of 3–10 with little solution pH effect (Kang et al. 2000). Hollow-fibre membranes with ligands specific for Sb adsorption have also shown potential for Sb recovery across a wide pH range. A chelating hollow-fibre membrane containing an iminodiethanol (IDE) group as the chelator effectively removed Sb from pH 4 aqueous solution prepared using an Sb(III) oxide, with an equilibrium adsorption capacity of ca. 15 mg Sb/g membrane (Nishiyama et al. 2003). Antimony was subsequently quantitatively eluted from the IDE fibre membrane. Antimony has similarly been removed from alkaline (pH > 11) solution prepared from Sb(III) oxide using an N-methylglucamine (NMG)-containing porous hollow fibre membrane (Saito et al. 2004). The NMG-fibre membrane was also able to adsorb Sb(V), with a maximum recovery of 130 mg Sb/g membrane at pH 3 (Kawakita et al. 2006). A hybrid process, which used membrane separation in combination with coagulation and flocculation treatment techniques, i.e. coagulation-flocculation-ultrafiltration, demonstrated nearly complete removal of Sb(III) from natural surface waters containing 30–150 µg/L Sb(III) (Du et al. 2014).
A great deal of research has been devoted to low-cost/low-input technologies for treatment of mining and industrial waters. Wetlands and similar systems that combine physical, chemical, and biological processes to remove contaminants have long been recognized as a cost-effective means of attenuating diffuse water pollution, particularly in remote areas. These treatment systems are generally purpose-built and designed to address specific water quality concerns for a known or estimated volume of water. Some natural wetlands located near mining facilities have been employed for water treatment, such as natural peatlands used to purify mine-impacted waters. A recent study showed that although effective for Sb, As, and Ni removal, mine-impacted water treatment using natural peatlands is unsustainable due to metal/metalloid accumulation within peat soils to levels exceeding guideline limits for contaminated soils (Palmer et al. 2015). Water purification systems that employ natural processes are also constrained by cold temperatures and short growing seasons in arctic and sub-arctic environments.
Electrocoagulation is a promising technology for the attenuation of metals in water as it is largely temperature-independent and the operating costs and energy consumption of the treatment process are generally low (Kuokkanen et al. 2013). Electrocoagulation employs the same concept as conventional coagulation techniques but with the coagulant generated in situ via electrolytic oxidation of anode materials. Electrocoagulation has demonstrated high rates of removal for many different contaminants from a range of different waters. Optimal operating conditions vary depending on contaminant characteristics. Although electrocoagulation generally performs optimally at near-neutral pH (Kuokkanen et al. 2013), electrode materials, current density, and treatment time can be altered as necessary to maximize contaminant removal. For example, one study of Sb removal from mine-impacted water using electrocoagulation showed 97–98% Sb attenuation from solution at pH 2 using a system with Al electrodes operated for 60 min at 166.7 A/m2 (Zhu et al. 2011). In contrast, hybrid Fe-Al electrodes operated at low current density of ca. 14 A/m2 for 40 min removed > 99% of Sb from pH 5–7 contaminated surface waters via adsorption onto Fe/Al (oxy)hydroxide minerals (Song et al. 2014). Optimized operating conditions, which facilitated 99% Sb removal from solution with an initial Sb concentration ca. 500 µg Sb/L using the Fe/Al hybrid electrode system included a solution pH of 5.2, current density 25.8 A/m2, and treatment time ≈ 90 min (Song et al. 2015).
Flotation, which has been used for many years in mineral ore processing for selective solid/solid separation, can be employed to separate Sb-containing solid particles from solution. Dissolved air flotation (DAF), or microflotation, is a well-known technique for the separation of solid particles from liquid using air dispersed into the liquid phase as micro-sized (< 100 µm) bubbles. These microbubbles attach to solid particles and lift them to the surface to form a sludge layer. The sludge can be scraped from the surface and thus separated from clarified water. A key consideration in DAF is the avoidance of high shear rates to protect fragile aggregates during flotation. The addition of flocculants to the water prior to DAF can improve floc formation and solid–liquid separation. Dosing of chemicals is typically adjusted in proportion to the incoming feed flow.
Study Objectives
The goal of the present study was to assess the feasibility of using combined modular processes to selectively remove Sb from mine-impacted waters in an Arctic environment. The target application of the optimized Sb removal process was the treatment of neutral to alkaline (pH ca. 7.5–8.5) water from a Fenno-Scandic minesite containing ca. 200–500 µg/L Sb. Simple Fe coagulation using Fe2(SO4)3 flocculant PIX-105 (Kemira Oyj), and gravimetric settling is presently used to treat mine-impacted waters at the site. Challenges specific to the site include an arctic climate and remote working conditions. An alternative treatment process capable of handling up to 400 m3/h water was sought that could consistently and economically achieve Sb concentrations < 300 µg/L in the discharged water, as required by applicable permits. During the present study, up to 300 m3 of water containing 0.20–0.47 mg/L Sb was discharged hourly from the case study site, which had a total annual discharge allowance of 700 kg Sb. Thus, the Sb concentration for continuous discharge targeted in the present study was ≤ 0.26 mg/L Sb to meet both load-based and concentration-based environmental targets.
Materials and Methods
All mine-impacted waters examined herein were previously subject to simple Fe coagulation using Fe2(SO4)3 flocculant PIX-105 (Kemira Oyj) and gravimetric settling, as it was not possible to interrupt minesite operations to test technologies at a pilot scale. The characteristics of mine-impacted waters from the Arctic minesite shown in Table 1 and detailed elsewhere represent mine-impacted water quality post-Fe2(SO4)3 treatment. Reported improvements in water quality represent analyte removal not attributable to Fe coagulation using Fe2(SO4)3 flocculant.
Antimony and Arsenic Removal from Solution Via Ion Exchange Resin
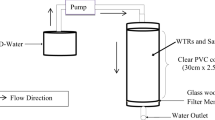
Synthetic mine-impacted water was prepared by dissolving KSb(OH)6, Na2HAsO4·7H2O, MnSO4·xH2O, MgSO4, Na2SO4, (NH4)2SO4, K2HPO4, NaCl, and NaNO3 in high purity deionized water (18 MΩ resistivity). All chemicals and reagents were of analytical grade and used without further purification. Batch adsorption tests were conducted to determine Sb(V) and As(V) equilibrium loading onto Lewatit® FO36 (Lanxess) iron oxide impregnated resin as a function of solution equilibrium concentration. Synthetic Sb- and As-bearing water was contacted with the resin at solution-to-resin ratios (v/v) ranging from 10 to 10,000 under ambient conditions. An equilibrium time of 24 h was allowed for complete diffusion of the Sb and As oxy-anions through the resin beads and subsequent adsorption onto the surfaces of the impregnated iron oxides. On completion of the tests, the loaded resin samples were separated from the barren solutions using a suitably sized screen and thoroughly washed with deionized water. Aqueous samples were preserved in a 2% HNO3 matrix, and the total Sb and As concentrations in solution were analysed by inductively coupled plasma-optical emission spectrometry (ICP-OES) or ICP-mass spectrometry (ICP-MS) within 24 h. The limit of detection by ICP-MS was 0.01 mg/L (10 ppb) for Sb and As, respectively.
Adsorption of Sb(V) and As(V) onto iron oxide impregnated resin was examined in a flow-through system using synthetic mine-impacted water containing ≈ 0.1 mg/L Sb and 0.33 mg/L As (Table 1). Two identical glass columns, with internal diameter 1 cm and 30 cm length, were packed with 11 mL of wet-settled resin using deionized water. The water was passed through the columns in a down-flow mode at volumetric flow rates of 31 BV/h and 10 BV/h (ca. 341 and 110 mL/h, respectively) using peristaltic pumps (Watson Marlow 101U/R). Column effluent samples were regularly collected and analysed for Sb and As content via ICP-MS. The columns were operated continuously for 4–6 weeks. Breakthrough was defined as the point at which effluent concentration of Sb or As, respectively, reached 0.05 mg/L. Saturation was defined as the point at which effluent Sb and As concentrations, respectively, were equal to influent concentrations. The height of the mass transfer zone (MTZ) was calculated by:
where BV1 is throughput volume at breakthrough, BV2 is throughput volume at 80% breakthrough, and h is the total height of the resin bed.
Selective Sb Extraction from Mine-Impacted Waters
The selective extraction of Sb from Fenno-Scandic mine-impacted quarry water was examined at laboratory scale using SELMEXT™ selective metal extraction-ultrafiltration technology. Subsamples of ca. pH 8.0 mine-impacted waters containing 0.12–0.23 mg/L Sb (Table 1) were treated with SELMEXT™ chemical PJ01 (Chromafora AB), then immediately filtered using an ultrafiltration membrane to separate the Sb-PJ01 complex from the aqueous phase. Remaining Sb in permeate was quantified by ICP-MS. Antimony removal using selective extraction technology was further optimized via in-situ testing at the same minesite operating under Arctic climate conditions. The SELMEXT™ onsite installation included a single insulated building housing the pilot-scale 0.9 m2 ultrafiltration membrane purification unit. Onsite operation was initiated in February 2013 and continued through November 2015. The influent mine-impacted water contained 0.22 mg Sb/L. The temperature of the mine-impacted waters entering the selective extraction system was maintained at 5–6 °C using a heat exchanger. Metal attenuation was evaluated by ICP-MS analysis of treated effluent. The retentate (sludge) was air-dried to a constant mass and digested using aqua regia (1:3 mixture of ultrapure HNO3 and HCl). The elemental composition of the digestate was determined by ICP-MS.
Electrochemical Water Treatment
Metal-contaminated Fenno-Scandic mine-impacted water (pH ca. 8.0, 0.37 mg/L Sb, 0.20 mg/L As, 0.08 mg/L Ni, 0.36 mg/L Mn, 0.10 mg/L Al, 0.10 mg/L Fe) was used to compare conventional metal removal via Fe precipitation with electrochemical treatment at the laboratory scale. Conventional metal precipitation was achieved using Fe2(SO4)3 and a 1 h reaction time, with Fe2(SO4)3 dosage yielding Fe:Sb ratios between 20:1 and 60:1. Laboratory-based electrochemical water treatment experiments were conducted in flow-through mode using a single 0.54 L laboratory-scale electrochemical water treatment (EWT) module. The operating current density was 155–780 A/m2 and the electrochemical treatment time ranged from 40 to 90 s, equivalent to 1.05–7.82 kWh/m3 energy consumption. Samples were collected directly from effluent lines and immediately passed through a 0.45 µm syringe filter. Metal removal was quantified by ICP-MS analysis of EWT effluents within 6 h of sample collection. The limits of detection for selected cations by ICP-MS were 0.01 mg/L for Sb, As, Ni, and Mn, and 0.2 mg/L for Al and Fe.
Electrocoagulation-Dissolved Air Flotation
A laboratory-scale 1.0 L (85 × 85 × 140 mm) acrylic plastic electrocoagulation-dissolved air flotation (E-DAF) unit was used to examine total suspended solids (TSS) attenuation using waters containing 320–720 mg/L TSS (iron oxyhydroxide sludge of unknown mineralogical character, denoted herein as “FeOH TSS”). Superfloc® A110 anionic polyacrylamide flocculant (Kemira Oyj) was diluted to 0.2% prior to dosing of 1 L sample volumes at 0, 0.5, 2.0, and 5.0 mg/L. Polymer addition was followed by 30 s of rapid mixing, then gentle mixing for 5 min. After flocculation, samples were gently poured into the E-DAF unit to avoid disruption of formed flocs. The E-DAF unit was operated in batch mode at 7.5–15 A current. The electrochemical treatment time was 60 s, after which the sample was gently floated for 5 min. The TSS in the treated water was measured by filtration to 1.5 µm.
Removal of FeOH TSS from mine-impacted water was further investigated using a pilot-scale unit (Flootech Oy FlooDaf B13), which had an effective surface area of 1.5 m2, an integrated flocculation chamber, a complete dispersion water system, and a control panel. Influent water was distributed evenly across the DAF pilot unit basin width prior to the addition of dispersion water. The dispersion water was prepared by saturating clarified water with air in the unit’s integrated pressurized dispersion vessel. Release of excess air pressure resulted in formation of 20–100 µm diameter air bubbles in the forepart of the basin. Approximately 15–25% of the dispersion water was recirculated. Sludge formed on the surface was moved with sludge rollers to a sludge compartment. The water level in the flotation basin was managed to optimize surface sludge dryness by the control system, consisting of level measurement and a control valve/pump at the outlet pipe. Initial water TSS concentration was 500–1460 mg/L. The first two test runs were conducted without any chemical addition whilst the two following test runs included polymer dosing. Superfloc® A110 solution (0.2%) was added to water to achieve final concentrations of 4 and 10 mg Superfloc® A110/L, respectively, in the third and fourth test runs. The DAF pilot unit was operated in continuous flow mode with an influent flow rate 2–4 m3/h. The dispersion water influent rate was 1.56 m3/h at 0.5 Mpa pressure. TSS was quantified by filtration to 1.5 µm.
In Situ Electrocoagulation-Dissolved Air Flotation
A modular EWT system (cPlant EWT-40, Outotec Oy) coupled with a DAF microflotation treatment unit (FlooDaf, Flootech) was installed to demonstrate in situ metal removal from Fenno-Scandic mine-impacted water under Arctic climate conditions using electrocoagulation-dissolved air flotation. Influent water composition varied during the in situ testing within the following ranges: 0.22–0.23 mg/L Sb, 0.005–0.049 mg/L As, 0.10–0.12 mg/L Ni, 0.9–1.0 mg/L Mn, and 0.01–0.048 mg/L Fe. The EWT system was comprised of two 40′ HC sea containers including the EWT module, 72 kW DC power supply, feed pump, discharge pump, instrumentation, piping, and lifting equipment. Onsite operation of the EWT plant was initiated on 15 September 2016 and continued for 5 days through 19 September 2016. External and sea container internal air temperatures were 2–12 °C and 8–16 °C, respectively, during the testing. The temperature of mine-impacted waters ranged from 8 to 11 °C. The influent flow rate used in the piloting was 5–15 m3/h. The operating current density was 10.6–21.2 A/m2 and electrochemical treatment time 60–210 s, equivalent to 0.14–0.76 kWh/m3 energy consumption. Effluent samples were filtered to 0.45 µm and preserved with ultrapure HNO3 (0.5 mL HNO3 per 100 mL EWT effluent). Metal attenuation was evaluated by ICP-MS analysis of system effluents.
The elemental composition of solid residues generated via electrocoagulation was determined by X-ray fluorescence (XRF). Semi-quantitative XRF analysis was performed using a Panalytical Axios mAX 3 kW—X-ray fluorescence spectrometer with the semi-quantitative Omnian-programme. The quantification limit of the method was approximately 0.01%. The leachability of elements within solid EWT residues was investigated using the two-stage CEN batch leaching test (EN 12457-3). The solid residue sample was agitated with demineralized water for 6 h at a liquid:solid ratio of 2, and then the eluate and solid were separated by filtration. The solid residue was subsequently agitated for a further 18 h with demineralized water at a liquid:solid ratio of 8, after which the eluate and solid were again separated by filtration. The leaching test eluates were analysed for As, Ba, Cd, Cr, Cu, Mo, Ni, Pb, Sb, Se, and Zn by ICP-MS. Chloride, F−, and SO42− in the eluates were determined using ion chromatography (IC). The concentration of dissolved organic carbon (DOC) in the eluates was determined by infrared detector using the Finnish Standard Association (SFS) standard method EN 1484:1997.
Between 5 and 15 m3/h EWT effluent was discharged to a receiving surface water body and 5 m3/h of EWT-40 effluent was pumped to the DAF pilot unit for further separation of EWT-charged microflocs. The DAF inlet water TSS concentration was 120–250 mg/L. Initial testing was carried out without flocculant addition. Further in situ tests used the anionic polymer DrewFloc® 260 (Ashland) at a rate of ≈ 5 mg/L. The DAF pilot unit was operated at a continuous 5.0 m3/h influent flow. The dispersion water flow rate was 1.8 m3/h and the pressure in the dispersion water tank was 0.3 Mpa. Concentration of TSS in the samples was determined by filtration, using standard method SFS EN872.
Results
Antimony and Arsenic Removal from Solution with Ion Exchange
The composition of synthetic mine-impacted water used in batch and column ion exchange experiments is shown in Table 1 and compared with mine-impacted water from the Arctic minesite. The Sb and As were expected to be present in synthetic mine-impacted waters as Sb(OH)6− and HAsO42−, respectively.
Antimony and As oxyanions were effectively removed from solution by the ion exchange resin (Fig. 1). Equilibrium adsorption data for Sb were fit using the Freundlich isotherm model (Eq. 2), whilst experimental equilibrium data for As adsorption fit using the Langmuir isotherm model (Eq. 3) (Helfferich 1962; Xi et al. 2011):
where Kd is the distribution coefficient, q is the quantity of adsorbate per unit mass adsorbent, C is the equilibrium concentration of adsorptive in solution, n is a correction factor, k is a constant related to binding strength, and b is the maximum quantity of absorptive that can be adsorbed assuming monolayer coverage.
The steep slope of the As equilibrium adsorption curve at low As solution concentrations illustrated the high affinity and selectivity of the ion exchange resin for As (Fig. 1). From the Langmuir model, a maximum As loading capacity of approximately 5500 mg/L resin (≈ 15 mg/g) was estimated. Conversely, the resin exhibited unfavourable uptake of Sb under the experimental conditions used, which may indicate poor adsorption selectivity (Fig. 1). Antimony uptake by the resin was most efficient at greater equilibrium solution concentration. For example, the Sb loading capacity for the resin was approximately 562 mg/L (≈ 1.5 mg/g) at an equilibrium Sb solution concentration of 1.3 mg/L.
Simultaneous removal of Sb and As by the ion exchange resin from the aqueous solution containing 0.10 mg/L Sb and 0.33 mg/L As was further examined via continuous adsorption in a fixed-bed column. The ion exchange resin capacity for Sb and As removal was determined as the quantity of As and Sb, respectively, adsorbed onto the resin when the column effluent concentrations of both contaminants reached 0.05 mg/L (Supplemental Table S-1). Although both Sb and As were removed from influent solution to concentrations < 0.05 mg/L (Fig. 2), the resin showed greater affinity for As than Sb. At a flow rate of 341 mL/h (31 BV/h), nearly 11.8 L (1073 BV) of water passed through the column prior to Sb breakthrough, whereas 61.5 L (5590 BV) of water was treated before As broke through (Fig. 2). The corresponding breakthrough capacities were determined as 79 mg/L for Sb (≈ 0.22 mg/g) and 1682 mg/L for As (≈ 4.6 mg/g) (Supplemental Table S-1). A slower flow rate of 110 mL/h (10 BV/h) retarded Sb and As breakthrough, with Sb breakthrough observed at approximately 18.8 L (1713 BV) and As breakthrough at ca. 69.5 L (6322 BV), respectively (Fig. 2). Equivalent breakthrough capacities were ≈ 130 mg/L (≈ 0.38 mg/g) and 1801 mg/L (≈ 5.3 mg/g) for Sb and As, respectively.
The total loading capacities of the ion exchange resin at saturation points, calculated by integrating the area below the breakthrough curves, were approximately 165 mg/L for Sb (≈ 0.48 mg/g) and 3047 mg/L (≈ 8.3 mg/g) for As, respectively. These values correspond reasonably well with the loading capacities estimated from equilibrium adsorption isotherms (Fig. 1). The Langmuir isotherm model predicted an equilibrium As loading capacity of 2987 mg/L where initial solution concentration was 0.33 mg/L. The Freundlich model, which estimated nil equilibrium saturation with 0.1 mg/L Sb in the initial solution, predicted a lesser Sb loading capacity; however, this is likely attributable to the poor fit of the Freundlich model at low initial Sb concentration.
Selective Extraction of Sb
Laboratory screening trials indicated that the addition of Sb-selective PJ01 reagent to pH ca. 8.0 mine-impacted water from an Arctic minesite (Table 1) followed by membrane filtration reduced aqueous Sb concentration by as much as 96% (Fig. 3), from 123 to 4.3 µg/L. The Sb-selective reagent had minimal impact on other ions in mine-impacted water, including As, selectively promoting coagulation of Sb. The addition of selective Sb extraction reagent PJ01 to mine-impacted water did not alter the solution pH.
The effect of filtration membrane pore size on Sb removal from mine-impacted water containing 0.23 mg/L Sb and 0.09 mg/L As, respectively, was examined at the laboratory scale in a tangential flow filtration unit, in combination with PJ01 reagent addition to promote particulate coagulation. Permeate flux was 200 L/h using a larger pore size membrane and decreased to 60 L/h when using a membrane with a smaller pore size in an experimental coagulation-filtration unit with a membrane area of 0.9 m2. Results showed that the larger pore size and greater permeate flux capacity was suitable for removal of both Sb and As from mine-impacted water (Supplemental Fig. S-1).
In situ pilot scale trials at the Arctic minesite further demonstrated effective simultaneous removal of Sb and As from mine-impacted water via selective coagulation and filtration (Fig. 4). At the influent pH ca. 8.0, and the mine-impacted water containing 0.23 mg/L Sb, the mass of Sb in the effluent (permeate) was reduced by > 65%. The Sb removed via coagulation-filtration was concentrated within approximately 10% of the original water volume (retentate), yielding a permeate with a Sb concentration of 0.09 mg/L and a retentate with ca. 1.5 mg/L Sb (Fig. 4). Arsenic was similarly concentrated within the retentate. More than 99% of the As in the influent mine-impacted water was retained; the As concentration in the permeate was reduced to 0.005 mg/L, resulting in a retentate concentration ca. 0.9 mg/L As (Fig. 4).
Analysis of dried retentate (sludge) showed that the retained solid material was dominated by Fe and Ca phases, which accounted for 28% and 3% of the dry mass, respectively (Supplemental Table S-2). Dried selective extraction-ultrafiltration retentate also contained minor quantities of Mg (0.60 wt%) and Na (0.44 wt%), and trace K (0.07 wt%). The Sb content of the dried retentate was 0.05 wt%. Calculations based on pilot-scale testing indicated that the selective extraction coagulation-filtration system would generate 440 kg/day or 161 t/year of dry sludge assuming annual treatment of 300 m3 mine-impacted water.
Conventional Precipitation Versus Electrocoagulation of Sb
Comparison between Sb and As removal from mine-impacted water using conventional precipitation by Fe2(SO4)3 with a 1 h reaction time, and using electrocoagulation with 40–90 s reaction time, showed that electrochemical water treatment achieved superior Sb and As removal and required significantly less treatment time (Fig. 5). Rates of Fe2(SO4)3 addition were selected to correspond with iron coagulant dosages used in conventional water treatment, and were equivalent to Fe:Sb ratios of 20:1 to 60:1. Iron precipitation reduced the Sb concentration of the mine-impacted water by up to 22% at an Fe2(SO4)3 addition rate equivalent to an Fe:Sb ratio of 60:1. The same rate of Fe2(SO4)3 addition removed ca. 97% of As from solution. Electrochemical water treatment removed 65–83% of the Sb and ≥ 95% of the As from the pH ca. 8.0 mine-impacted water (Fig. 5). The theoretical quantity of Fe generated by electrochemical water treatment was defined by operating parameters, and was approximately an order of magnitude greater than the quantity of Fe added as Fe2(SO4)3 in conventional iron precipitation.
Electrocoagulation-Dissolved Air Microflotation
Laboratory-scale E-DAF experiments were undertaken to define operating parameters for removal of Sb-containing flocs from the mine-impacted water. E-DAF treatment of waters containing FeOH TSS without polymer addition yielded 43 ppm residual TSS. Addition of Superfloc® A110 coagulant to FeOH TSS-containing mine-impacted water at 0.5, 2.0, and 5.0 mg/L Superfloc® A110 dosages resulted in a TSS below detection limits in the treated waters (Supplemental Table S-3).
Further investigation of FeOH solids removal from mine-impacted water using a pilot-scale DAF unit with an effective surface area of 1.5 m2 demonstrated 69–98% removal of solids (Table 2). Mine-impacted water containing FeOH TSS was introduced to the DAF pilot unit following electrocoagulation. In these trials with a continuous flow of water, the addition of coagulant did not substantially increase FeOH TSS removal efficiency.
In Situ Electrocoagulation-Dissolved Air Microflotation at Arctic Minesite
In situ testing of the pilot-scale EWT unit demonstrated effective removal of Sb and As from mine-impacted water using low levels of energy input (Fig. 6). The Sb concentration decreased by nearly 50% following electrochemical treatment at 0.14 kWh/m3, whilst As concentrations was concomitantly reduced by more than 75%. At the time of pilot-scale electrocoagulation testing, the Sb concentration of the mine-impacted water was 0.23 mg/L prior to treatment, below the desired maximum concentration of 0.26 mg/L for discharged waters. Analysis of dried electrocoagulation residues (sludge) showed that the residual solid material was strongly dominated by Fe phases, which accounted for 64% of the dry mass (Supplemental Table S-2). Dried electrocoagulation residues contained minor Si (1.8 wt%), Ca (1.0 wt%) phases, with lesser quantities of S (0.53 wt%) and Mg (0.48 wt%), and trace Al (0.12 wt%). The Sb content of the dried electrocoagulation residue was 0.06 wt.%, similar to the 0.05 wt% Sb of dried selective extraction-ultrafiltration retentate. The electrocoagulation residue content of most trace elements was less than the 0.01% limit of detection by XRF on a dry weight basis.
Characteristics of deionised water leachates of air-dried electrocoagulation residues (Table 3) were compared with European criteria for non-hazardous landfill waste (2003/33/EC: Council Decision of 19 December 2002 establishing criteria and procedures for the acceptance of waste at landfills pursuant to Article 16 of and Annex II to Directive 1999/31/EC). The comparison indicated that the electrocoagulation residues could potentially be disposed of as non-hazardous landfill waste. All analytes in electrocoagulation residue leachates were present at concentrations substantially less than the European limits for non-hazardous landfill waste.
Following electrocoagulation, mine water influent to the pilot-scale DAF unit contained between 125 and 250 mg/L TSS. Approximately 70% TSS reduction was achieved by DAF at a continuous flow rate of 5 m3/h and in the absence of coagulant addition. The addition of approximately 5 mg/L anionic polymer enhanced TSS removal, achieving 80–93% TSS removal and treated effluent with mean TSS 25 mg/L. The decreased solids removal efficiency in situ compared with pilot-scale trials is likely related to the size of flocs formed in the electrocoagulation system and low on-site operating temperatures. The flocs formed in during electrocoagulation were of small size and sensitive to shear forces, thus the flocs and sludge layer were easily broken down. In addition, the low temperature caused challenges in polymer dissolution and dosing.
Discussion
Antimony Removal from Mine-Impacted Water
Individual laboratory-scale investigations of Sb removal from mine-impacted water using Fe2(SO4)3 precipitation, ion exchange resin, selective ion extraction and ultrafiltration, and electrocoagulation with or without subsequent DAF show that any of these methods are potentially applicable to Sb removal from mine-impacted water (Table 4). Comparison between Sb and As removal by the ion exchange resin clearly demonstrated differences in adsorption selectivity between Sb and As. The ion exchange resin demonstrated high affinity and selectivity for As, and greater breakthrough (qe) and saturation (qs) capacities for As compared with Sb. The observed differences between Sb and As removal efficiency by ion exchange resin illustrate the need for Sb-specific remediation technologies. Despite removal of Sb from aqueous solution to < 0.05 mg/L, the relatively low adsorption capacity of the ion exchange resin for Sb (qs = 0.42–0.48 g Sb/kg resin) may limit the applicability of this technology to situations requiring near-complete removal of Sb coupled with the potential for recovery of the adsorbed Sb.
The selective ion extraction and ultrafiltration technique selectively promoted Sb coagulation in laboratory trials, removing 96% of the Sb from solution. Antimony-containing flocs were resistant to breakdown and were effectively removed from solution using a relatively large pore size ultrafiltration membrane, thereby enabling treatment of up to 200 L/h. In situ pilot-scale selective extraction trials demonstrated ca. 65% reduction of Sb concentration in mine-impacted water. The level of Sb removal achieved in situ by selective extraction coagulation and ultrafiltration was well below the target Sb concentration to meet water quality objectives for discharged waters. Thus, depending on the targeted environmental criteria, it may be possible to treat only a portion of the mine-impacted water using the selective extraction technique.
At the laboratory scale, electrocoagulation for 40–60 s removed 65–83% of the Sb from the mine-impacted water (Fig. 5). Like the selective extraction technology, electrocoagulation achieved Sb removal from mine-impacted waters to a concentration well below the water quality target concentration. Similarly, treatment of the entire water volume using electrocoagulation may not be necessary for discharged water to meet environmental criteria. The electrocoagulation technology was effectively up-scaled in in situ mine-impacted water treatment at the Arctic minesite. On-site testing showed ca. 50–75% removal of Sb at cold temperatures and at continuous flow rates up to 20 m3/h (Fig. 6). Individual electrocoagulation units can be connected in series, thereby eliminating any potential further up-scaling effects on electrocoagulation technology performance.
In combination with electrocoagulation, Sb-containing flocs can be floated and removed to yield water suitable for discharge with both low Sb concentration and low TSS, respectively. Laboratory trials using E-DAF to remove TSS from waters containing FeOH sludge showed > 85% removal of TSS without coagulant addition. All rates of coagulant addition during E-DAF resulted in TSS concentrations less than limits of detection (Supplemental Table S-3). Removal of TSS by E-DAF was also effective in controlled environment pilot-scale trials, with 93–98% reduction of TSS observed when the system was operated under continuous 2–4 m3/h flow conditions without coagulant addition. Polymer addition to promote particulate flocculation did not improve TSS removal efficiency in these trials (Table 2).
In situ E-DAF at the Arctic minesite treated up to 5 m3 water/h. The cold climate presented challenges for polymer use to promote coagulation in the DAF unit. Despite difficulties dissolving the anionic polymer in low-temperature mine-impacted waters (8–11 °C), up to 93% removal of TSS was achieved under continuous flow conditions. The mean TSS concentration of effluents from the E-DAF hybrid system was decreased to only 25 mg/L.
The purpose of the experiments described herein was to investigate the potential applicability and scalability of selected technologies for Sb removal and recovery from mine-impacted water. In situ pilot scale trials further examined the performance of selected technologies under challenging (Arctic) environmental conditions. Results obtained for Sb removal from mine-impacted water using selective ion extraction and ultrafiltration, and electrocoagulation with or without subsequent dissolved air flotation indicate that these methods show promise for Sb removal from mine-impacted water. Additional study of each technology at process scale is however required to comprehensively assess long-term performance.
Potential for Sb Recovery
With the exception of the anion exchange technology, all water treatment processes examined herein produced a Sb precipitate or sludge retentate requiring further processing, on-site storage, or disposal. Additional testing is necessary to design a process to recover metals of value and/or characterize the long-term stability of solid mineral phases in Sb precipitates/sludge and inform selection of a final disposal option. The leachate characterisation undertaken in the present study for electrocoagulation residues constitutes basic characterisation under European landfill waste testing procedures. Additional compliance testing and on-site verification testing of Sb-containing solid materials generated by each of the technologies investigated herein would be necessary for acceptance as non-hazardous waste at European landfill facilities. Numerous studies document the successful recovery of metals from ion-exchange resins and subsequent resin regeneration and reuse (e.g. McKevitt and Dreisinger 2009; Nikoloski and Ang 2014; Petruzzelli et al. 1995; Riveros et al. 2008; Sarkar et al. 2007; Selvi et al. 2004). In addition to Sb removal from aqueous solution to extremely low residual concentration, an advantage of ion exchange resin use for mine water purification is the relative ease of recovery of adsorbed ions. The relative selectivity of ion exchange resin for Sb compared with As indicates that additional purification steps would be necessary to recover a pure Sb phase; further research is needed to identify solutions for optimum Sb recovery from the ion exchange resin and for resin regeneration. In addition, detailed information regarding the temperature dependence of Sb adsorption to the ion exchange resin is required to determine the minimum hydraulic retention time and scaling of ion exchange resin-containing units for Sb removal and recovery from the mine-impacted water.
Recovery of Sb from the mine-impacted water using other technologies—selective extraction and ultrafiltration, electrocoagulation, and/or DAF—is possible but may require considerably more post-processing compared with resin stripping. For example, the Sb removed by selective extraction-ultrafiltration was concentrated ca. 10 × in retentate, which when dried contained 0.05 wt% Sb. Calculations indicated that for the Arctic mine case study described herein, the selective extraction-ultrafiltration system would generate 161 t/y of dry sludge, assuming annual treatment of 300 m3 water. Thus, sludge valorisation could yield ca. 80 kg of Sb/y. Entrained Sb could potentially be recovered by oxide volatilization wherein volatile Sb2O3 would be recovered following roasting at 1000 °C (Dupont et al. 2016). Oxide volatilization is a pyrometallurgical process used to recover Sb from low-grade sulfidic ores (5–25% Sb), typically generating commercial grade Sb2O3 of > 99% purity (Anderson 2012). Combustion of sulphide components of low-grade ores provides heat, thereby reducing fuel requirements for oxide volatilization. However, the energy cost for Sb recovery from mine-impacted water retentate sludge by oxide volatilization may outweigh the value of the recovered Sb, depending on the relative Sb and sulphide contents of the sludge.
The purity of recovered Sb is an additional concern as both PbO and As2O3 trace element contaminants are considered critical impurities of Sb2O3 (Binz and Friedrich 2015). In the present study, technologies for removal of Sb via selective extraction-ultrafiltration or electrocoagulation each yielded a solid residue with minimal (< 0.01 wt%) Pb (Supplemental Table S-2). The As content of dry selective extraction-ultrafiltration retentate was approximately 0.03 wt% whilst the retentate contained 0.05 wt% Sb. In contrast, dry solid residues of electrocoagulation (EWT) contained significantly greater Sb (0.06 wt.%) compared with As (< 0.01 wt%). The lesser As:Sb ratio of electrocoagulation residues compared with the As and Sb content of solid residues from selective extraction-ultrafiltration may indicate a greater potential for Sb recovery from electrocoagulation residues. Hydrometallurgical techniques for Sb recovery involve leaching, either with an alkaline sulphide or acidic chloride solution, followed by electrowinning. Alkaline sulphide leaching is favoured due to the minimal associated corrosion issues and has been successfully used to recover Sb from pyrometallurgical residues (Anderson 2001). Alkaline sulphide leaching of mineral processing residues by Anderson (2001) solubilized approximately equal proportions of Sb and As, necessitating additional processing to purify Sb. Additional study is necessary to determine whether the low As:Sb ratio of EWT residues facilitates simplified Sb recovery from the solid phase. Several multi-step leaching processes have been proposed for selective extraction of Sb from pyrometallurgical wastes and incinerator residues (see Dupont et al. 2016 and references therein). Some of these hydrometallurgical processes have effectively isolated Sb from a range of different wastes at the laboratory scale (Dupont et al. 2016); however, a single production-scale method for effective, cost-efficient Sb extraction from complex mixtures has yet to be widely accepted.
Life-Cycle Costs for Sb Removal from the Mine Water
The life-cycle costs of Sb separation from mine water using technologies evaluated in-situ at the Arctic minesite in the present study was assessed with respect to the Fe2(SO4)3 precipitation reference process (scenario #1, Supplemental Fig. S-2) combined with phase separation in a clarifier. In this analysis, scenario #2 (Supplemental Fig. S-3) used E-DAF whilst scenario #3 (Supplemental Fig. S-4) was based on selective Sb extraction and ultrafiltration.
The techno-economic assessment was based on technical data from experiments presented herein and supporting information obtained directly from the suppliers of these technologies. Key operating expenditures, including chemical and utility consumption, are summarised in Supplemental Table S-4. These values were used to estimate the variable operating costs of Sb removal for all three water treatment scenarios. Additional fixed operating expenditures considered were labour costs (one operator with annual personnel cost of 60,000 €) and maintenance costs (4% of total capital investments annually) (Supplemental Table S-5).
In this analysis, the discharged volume of Sb-containing mine water was 400 m3/h. Because the observed efficiency of Sb removal via E-DAF (scenario #2) and selective extraction-ultrafiltration (scenario #3), respectively, were high, the present analysis assumed that 50% of the volume required treatment to reach the target Sb concentration of < 300 µg/Lin effluents. Annual operational time of the water treatment plant was 8000 h. Capital costs were calculated based on the main process units (reactors, separators and main pumps). The other capital costs (installation, piping, automation, civic, etc.) are provided here as a fraction of the total main process unit costs. The applied fraction for reference scenario #1 was 210%, and 170% for scenarios #2 and #3, respectively, as installation costs are less for mobile container type units (Supplemental Table S-6).
The life-cycle costs of water treatment facilities were calculated based on the respective break-even cost of treating 1 m3 of mine-impacted water. Here, the discounted cash flow analysis (DCF) earnings before interest and taxes (EBIT) basis was used. The analysis assumed a discount rate of 10% and plant lifetime of 10 years, with a 1-year installation process prior to commissioning when capital costs occur. The working capital was considered equivalent to 1 month of variable costs. Linear depreciation was applied at a rate of 10% of original investment per annum. The value of the treatment plant was considered zero after 10 years.
The cost to treat 1 m3 of mine-impacted water was 1.00 € for the Fe2(SO4)3 precipitation and clarification reference scenario. In contrast, the calculated treatment cost was 1.10 € for E-DAF (scenario #2) and 2.20 € for selective extraction and ultrafiltration (scenario #3). Both new mine water treatment concepts compare favourably with conventional Fe2(SO4)3 precipitation as only 50% of the water requires treatment in scenarios #2 and #3 to meet the desired mean Sb concentration and annual Sb load in discharged water. The different cost fractions for scenarios #1-#3 are illustrated in Fig. 7.
Breakdown of cost to treat 1 cubic meter of water containing 0.3 mg/L Sb for the Fe2(SO4)3 precipitation reference scenario (left) and for two new treatment concepts based on electrocoagulation and dissolved air flotation (scenario #2, centre) and selective extraction-ultrafiltration (scenario #3, right)
Consideration of life-cycle costs across 10 years of operation yields a net present value of cumulative cost of 17 million EUR for treatment of Sb-containing mine water by Fe2(SO4)3 precipitation (scenario #1), and 20 million EUR for treatment using selective extraction and ultrafiltration (scenario #3) (Fig. 8). Analyses showed that treatment of mine water using E-DAF yields the lowest comparative life-cycle cost of 10 million EUR. In both scenarios #2 and #3, it is possible to exceed the Sb attenuation efficiency of conventional Fe2(SO4)3 precipitation treatment, even where only 50% of the mine-impacted water is treated using the identified technologies.
The most economically feasible treatment process for Sb-containing mine waters was E-DAF based on life-cycle cost analysis. The superior Sb removal efficiency of this process compared with conventional Fe2(SO4)3 precipitation facilitates treatment of a relatively lesser proportion of mine-impacted water to meet the targeted mean concentration of Sb in discharged water and to comply with applicable legislation. Both new water treatment technologies analysed provide higher water purification efficiency than conventional Fe2(SO4)3 precipitation (scenario #1). However, these technologies are not entirely mature and additional uncertainties are involved. This conceptual level life-cycle cost analysis requires reassessment for particular cases and water quality requirements.
The lesser capital expenditures in scenario #2 compared to the reference scenario is in part due to the modular nature of the technology, e.g. on-site installation in shipping containers. In addition, the variable operating expenditures are less; however, the continuous replacement of proprietary electrodes and related costs may influence the economic feasibility of E-DAF technologies. Analyses showed that the mine water treatment technology based on selective metal extraction and ultrafiltration (scenario #3) yields life-cycle costs greater than the reference scenario. This is largely due to chemical and membrane costs. As in scenario #2, on-site installation of the selective metal extraction and ultrafiltration technology-based process in a shipping container reduced relative capital expenditure relative to the reference scenario. Likewise, continuous use of proprietary chemicals and related costs may additionally influence the long-term economic feasibility of selective metal extraction and ultrafiltration.
Conclusions
Critical evaluation of potential new technologies for Sb removal and recovery, coupled with life-cycle cost analysis based on 10 years of operation at an Arctic mine site discharging 400 m3 water per annum and targeting a maximum Sb concentration of 300 µg/L, indicated that E-DAF may be the most cost-effective solution. The applicability of the ion exchange resin examined herein is limited by its relatively low Sb adsorption capacity. However, use of resin-based ion exchange may be preferable to other technologies investigated here in scenarios requiring near-complete removal of Sb and the potential for rapid recovery of adsorbed Sb. Further investigation of Sb adsorption to ion exchange resins under varying conditions, including up-scaling and optimization of operating parameters via pilot-scale demonstration, is needed to accurately assess the potential for full-scale use of ion exchange resin-based technologies for Sb removal and recovery from mine-impacted water.
Both E-DAF and selective ion extraction-ultrafiltration techniques exhibited superior Sb removal from mine-impacted water relative to conventional Fe2(SO4)3 precipitation in in situ demonstrations. The increased Sb removal efficiency enables treatment of a proportion of mine-impacted water < 100%. Thus, despite relatively greater costs to treat a single unit of mine-impacted water using E-DAF or selective extraction-ultrafiltration compared with Fe2(SO4)3 precipitation, both novel technologies remain potentially cost-effective. Techno-economic analysis showed that E-DAF implementation involved a greater capital expenditure than extraction-ultrafiltration, but that estimated life-cycle costs associated with on-going operating expenses resulted in significantly lower life-cycle costs for E-DAF than selective extraction-ultrafiltration. Additional research is needed to quantify Sb recovery from exchange resin, Sb-containing precipitate, or sludge retentate. Further work is also required to examine the costs associated with processing of Sb-containing phases and the final treatment, recycling, and/or disposal of residual materials.
References
Anderson CG (2001) Hydrometallurgically treating antimony-bearing industrial wastes. J Miner Metals Mater Soc 53:18–20. https://doi.org/10.1007/s11837-001-0156-y
Anderson CG (2012) The metallurgy of antimony. Chem Erde-Geochem 72(S4):3–8. https://doi.org/10.1016/j.chemer.2012.04.001
Binz F, Friedrich B (2015) Recovery of antimony trioxide flame retardants from lead refining residues by slag conditioning and fuming. Chem Ing Tech 87:1569–1579. https://doi.org/10.1002/cite.201500071
Council of the European Communities (1976) Council Directive 76/464/EEC of 4 May 1976 on pollution caused by certain dangerous substances discharged into the aquatic environment of the community. Off J L 129 (18/05/1976):23–29
Council of the European Union (1998) Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Offi J L 330:32–54
Du X, Qu F, Liang H, Li K, Yu H, Bai L, Li G (2014) Removal of antimony(III) from polluted surface water using a hybrid coagulation-flocculation-ultrafiltration (CF-UF) process. Chem Eng J 254:293–301. https://doi.org/10.1016/j.cej.2014.05.126
Dupont D, Arnout S, Jones PT, Binnemans K (2016) Antimony recovery from end-of-life products and industrial process residues. J Sustain Metall 2:79–103. https://doi.org/10.1007/s40831-016-0043-y
European Commission (2017) Communication from the Commission to the European Parliament, The Council, The European Economic and Social Committee and the Committee of the Regions on the 2017 list of Critical Raw Materials for the EU. COM(2017)490 final. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52017DC0490&from=EN. Accessed 24 March 2018
Filella M, Belzile N, Chen Y-W (2002) Antimony in the environment: a review focused on natural waters I. Occurrence. EarthSci Rev 57:125–176. https://doi.org/10.1016/S0012-8252(01)00070-8
Guo X, Wu Z, He M (2009) Removal of antimony(V) and antimony(III) from drinking water by coagulation-flocculation-sedimentation (CFS). Water Res 43:4327–4335. https://doi.org/10.1016/j.watres.2009.06.033
Helfferich F (1962) Ion exchange. McGraw-Hill, New York
Kang M, Kawasaki M, Tamada S, Kamei T, Magara Y (2000) Effect of pH on the removal of arsenic and antimony using reverse osmosis membranes. Desalination 131:293–298. https://doi.org/10.1016/S0011-9164(00)90027-4
Kang M, Kamei T, Magara Y (2003) Comparing polyaluminum chloride and ferric chloride for antimony removal. Water Res 37:4171–4179. https://doi.org/10.1016/S0043-1354(03)00351-8
Kawakita H, Uezu K, Tsuneda S, Saito K, Tamada M, Sugo T (2006) Recovery of Sb(V) using a functional-ligand-containing porous hollow-fiber membrane prepared by radiation-induced graft polymerization. Hydrometallurgy 81:190–196. https://doi.org/10.1016/j.hydromet.2005.12.010
Koseoglu P, Yoshizuka K, Nishihama S, Yuksel U, Kabay N (2011) Removal of boron and arsenic from geothermal water in Kyushu Island, Japan, by using selective ion exchange resins. Solvent Extr Ion Exc 29:440–457. https://doi.org/10.1080/07366299.2011.573448
Kuokkanen V, Kuokkanen T, Rämö J, Lassi U (2013) Recent applications of electrocoagulation in treatment of water and wastewater - A review. Green Sustain Chem 3:89–121. https://doi.org/10.4236/gsc.2013.32013
Majzlan J, Števko M, Lánczos T (2016) Soluble secondary minerals of antimony in Pezinok and Kremnica (Slovakia) and the question of mobility or immobility of antimony in mine waters. Environ Chem 13:927–935. https://doi.org/10.1071/EN16013
McKevitt B, Dreisinger D (2009) A comparison of various ion exchange resins for the removal of ferric ions from copper electrowinning solutions Part II: Electrolytes containing antimony and bismuth. Hydrometallurgy 98:122–127. https://doi.org/10.1016/j.hydromet.2009.04.007
Miao Y, Han F, Pan B, Niu Y, Nie G, Lv L (2014) Antimony(V) removal from water by hydrated ferric oxides supported by calcite sand and polymeric anion exchanger. J Environ Sci 26:307–314. https://doi.org/10.1016/S1001-0742(13)60418-0
Nikoloski AN, Ang K-L (2014) Review of the application of ion exchange resins for the recovery of platinum-group metals from hydrochloric acid solutions. Miner Process Extr M 35:369–389. https://doi.org/10.1080/08827508.2013.764875
Nishiyama S-Y, Saito K, Saito K, Sugita K, Sato K, Akiba M, Saito T, Tsuneda S, Hirata A, Tamada M, Sugo T (2003) High-speed recovery of antimony using chelating porous hollow-fiber membrane. J Membr Sci 214:275–281. https://doi.org/10.1016/S0376-7388(02)00558-6
Obiakor MO, Tighe M, Pereg L, Wilson SC (2017) Bioaccumulation, trophodynamics and ecotoxocity of antimony in environmental freshwater food webs. Crit Rev Environ Sci Technol 47:2208–2258. https://doi.org/10.1080/10643389.2017.1419790
Palmer K, Ronkanen A-K, Kløve B (2015) Efficient removal of arsenic, antimony and nickel from mine wastewaters in Northern treatment peatlands and potential risks in their long-term use. Ecol Eng 75:350–364. https://doi.org/10.1016/j.ecoleng.2014.11.045
Petruzzelli D, Passino R, Tiravanti G (1995) Ion exchange process for chromium removal and recovery from tannery wastes. Ind Eng Chem Res 34:2612–2617. https://doi.org/10.1021/ie00047a009
Riveros PA, Dutrizac JE, Lastra R (2008) A study of the ion exchange removal of antimony(III) and antimony(V) from copper electrolytes. Can Metall Q 47:307–316. https://doi.org/10.1179/cmq.2008.47.3.307
Saito T, Kawakita H, Uezu K, Tsuneda S, Hirata A, Saito K, Tamada M, Sugo T (2004) Structure of polyol-ligand-containing polymer brush on the porous membrane for antimony(III) binding. J Membr Sci 236:65–71. https://doi.org/10.1016/j.memsci.2004.02.012
Sarkar S, Blaney LM, Gupta A, Ghosh D, Sengupta AK (2007) Use of ArsenXnp, a hybrid anion exchanger, for arsenic removal in remote villages in the Indian subcontinent. React Funct Polym 67:1599–1611. https://doi.org/10.1016/j.reactfunctpolym.2007.07.047
Selvi P, Ramasami M, Samuel MHP, Adaikkalam P, Srinivasan GN (2004) Recovery of gallium from Bayer liquor using chelating resins in fixed-bed columns. Ind Eng Chem Res 43:2216–2221. https://doi.org/10.1021/ie030695n
Song P, Yang Z, Xu H, Huang J, Yang X, Wang L (2014) Investigation of influencing factors and mechanism of antimony and arsenic removal by electrocoagulation using Fe-Al electrodes. Ind Eng Chem Res 53:12911–12919. https://doi.org/10.1021/ie501727a
Song P, Yang Z, Zeng G, Yang X, Xu H, Huang J, Wang L (2015) Optimization, kinetics, isotherms and thermodynamics studies of antimony removal in electrocoagulation process. Water Air Soil Pollut 226:380–392. https://doi.org/10.1007/s11270-015-2615-z
Sylvester P, Westerhoff P, Möller T, Badruzzaman M, Boyd O (2007) A hybrid sorbent utilizing nanoparticles of hydrous iron oxide for arsenic removal from drinking water. Environ Eng Sci 24:104–112. https://doi.org/10.1089/ees.2007.24.104
Ungureanu G, Santos S, Boaventura R, Botelho C (2015) Arsenic and antimony in water and wastewater: overview of removal techniques with special reference to latest advances in adsorption. J Environ Manag 151:326–342. https://doi.org/10.1016/j.jenvman.2014.12.051
United Nations Environment Programme (1999) Basel convention on the control of transboundary movements of hazardous wastes and their disposal, SBC No. 99/001. UNEP, Geneva
United States Geological Survey, Summaries MC, January (2017) https://minerals.usgs.gov/minerals/pubs/commodity/antimony/mcs-2017-antim.pdf. Accessed 4 July 2017
Wilson SC, Lockwood PV, Ashley PM, Tighe M (2010) The chemistry and behavior of antimony in the soil environment with comparisons to arsenic: a critical review. Environ Pollut 158:1169–1181. https://doi.org/10.1016/j.envpol.2009.10.045
Wu Z, He M, Guo X, Zhou R (2010) Removal of antimony(III) and antimony(V) from drinking water by ferric chloride coagulation: Competing ion effect and the mechanism analysis. Sep Purif Technol 76:184–190. https://doi.org/10.1016/j.seppur.2010.10.006
Xi J, He M, Lin C (2011) Adsorption of antimony(III) and antimony(V) on bentonite: Kinetics, thermodynamics and anion competition. Microchem J 97:85–91. https://doi.org/10.1016/j.microc.2010.05.017
Zhu J, Wu F, Pan X, Guo J, Wen D (2011) Removal of antimony from antimony mine flotation wastewater by electrocoagulation with aluminum electrodes. J Environ Sci-China 23:1066–1071. https://doi.org/10.1016/S1001-0742(10)60550-5
Acknowledgements
Open access funding provided by Technical Research Centre of Finland (VTT). This Eureka Acqueau project MIWARE (E!4927) was supported by the Tekes—Finnish Funding Agency for Innovation, Vinnova—Sweden’s Innovation Agency, and the Department of Science and Technology, South Africa.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Arnold, M., Kangas, P., Mäkinen, A. et al. Mine Water as a Resource: Selective Removal and Recovery of Trace Antimony from Mine-Impacted Water. Mine Water Environ 38, 431–446 (2019). https://doi.org/10.1007/s10230-019-00597-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-019-00597-2