Abstract
As part of the Kushiro Wetland Restoration Project, we evaluated the effect of the tree-cutting (February 2003) on soil water chemistry for 3 years in an alder (Alnus japonica) shrubland on the peat soil in the Kushiro Wetland, the largest wetland in Japan. The alder stand was divided into two types; low stature and high stature stands, mean heights being 1.5 and 2.6 m, respectively. The treatment plot with tree cutting and the reference plot measuring 25 m × 25 m each were established in both stands, and a soil incubation experiment was also conducted by trenching the root zone of wetland vegetation in the treatment plots in both stands in the summer of 2004. The tree cutting did not substantially increase the concentrations of ammonium ion (NH4 +) and nitrate ion (NO3 −) in the soil water, although a gradual and slight increase of NO3 − concentration was found after the tree-cutting only in the high stature stand (<10 μmol/l). This increase in the NO3 − concentration in the high stature stand was probably due to the rhizosphere oxidation of wetland herbaceous vegetation, and was accompanied by the increase of Ca2+ concentration. The soil incubation experiment showed no significant change of the nitrogen concentration, suggesting that inorganic nitrogen was immobilized and NO3 − was denitrified intensively in this wetland peat soil. The results of this study suggested that this wetland ecosystem had a high potential to stabilize the soil water chemistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Kushiro Wetland, the largest wetland in Japan, has shrunk or deteriorated by various disturbances caused by human activities (Nakamura et al. 2002). The deteriorative problems in the Kushiro Wetland include land-use development, the load of suspended sediment and nutrients contributing to eutrophication (Nakamura et al. 2004a), stream channelization (Nakamura et al. 1997), expansion of non-native vegetation, decreases of endangered species populations and expansion of abandoned fields. The Kushiro Wetland Restoration Project was started in 2002 to assess these deteriorative problems (Nakamura and Ahn 2006). In the Kushiro Wetland, expansion of the distribution area of alder (Alnus japonica), a nitrogen-fixing species, has been one of the major problems (Fujimura et al., 2008). Human activity caused environmental changes in the Kushiro Wetland, leading to a change in vegetative composition through the expansion of the alder (Nakamura and Ahn 2006). Zedler and Kercher (2004) suggested the impact of invasive plants on nutrient cycling, including nitrogen processing, in the wetland ecosystem, although impacts on ecosystem function are site- and species-specific. Ehrenfeld (2003) suggested large effects of an invasive nitrogen-fixing species on nitrogen cycling. Therefore, direct removal (e.g., cutting) of alder may be an effective method to restore the original vegetation in the Kushiro Wetland. However, establishment of alder forest has constructed a peculiar wetland environment for several decades, and alder cutting may have serious influences on the current wetland ecosystems. Then, tentative experiments have been conducted in parts of the Kushiro Wetland to evaluate the influence of the alder cutting from many aspects: the changes of the vegetation, microclimate and chemistry of soil water and groundwater.
Tree-cutting experiments have been conducted in many upland forests (e.g., Bormann and Likens 1979; Matson et al. 1987; Swank et al. 2001; Van Miegroet and Cole 1984). These studies suggested a change of the nutrient dynamics in forest soil and increased losses of nitrate ion and base cations from the forest soil, although time length taken for the nutrient losses after the tree-cutting varied among forest ecosystems. Tree-cutting in the wetlands has been conducted, and influences of not only the vegetation (Batzer et al. 2000; Gale et al. 1998; Spencer et al. 2001) and hydrology (Sun et al. 2000; Xu et al. 2002), but also the chemical characteristics of soil and soil water (McLaughlin et al. 1994, 1996, 2000; Westbrook and Devito 2004) have been evaluated. However, these tree-cutting studies on soil chemistry in wetlands were limited and were mainly conducted for the evaluation of soil fertility in managed systems. Other than for the managed systems, the cutting of the invasive and/or expanding tree species may be necessary for the restoration of the original vegetation in a wetland ecosystem. Therefore, the evaluation for the effect of tree-cutting on nutrient dynamics in the wetland ecosystem is important to acquire basic information for the conservation of wetland ecosystems.
In general, in wetland soil that is under anaerobic conditions, ammonification occurs, while the nitrification does not occur easily (Bowden 1987). Moreover, uptake by plant root and microbial immobilization of inorganic nitrogen generally and intensively occurs in the wetland soil, and denitrification also occurs (Hedin et al. 1998; McLaughlin et al. 2000; Zhu and Ehrenfeld 2000; Westbrook and Devito 2004). Especially in the peat wetlands in cold regions, slow decomposition of organic matter and consequent slow release of inorganic nitrogen lead to the intense competition between plant roots and soil microbes for the inorganic nitrogen. (Mitsch and Gosselink 2000). Therefore, the ‘net’ change of the inorganic nitrogen dynamics after tree cutting will be difficult to find in the soil and soil water under stable waterlogged conditions. This will be the case for cutting of nitrogen-fixing trees such as alder. The purpose of this study was to evaluate the effect of tree-cutting on the nitrogen concentrations in the soil water in a wetland ecosystem in combination with the soil incubation experiment to assess nitrogen mineralization and with the observation of other nutrient concentrations. Some implications for wetland restoration and the future prospects will be suggested.
Materials and methods
Study site
We studied the effect of tree-cutting on the soil water chemistry in the Hirosato area (N43°03′, E144°24′, Fig. 1a) in the Kushiro Wetland. Our study area was surrounded by the Kyu-Setsuri River, the Junigou-shisen River and a dike that was constructed along the Shin-Kushiro River (Fig. 1b). The Kyu-Setsuri River and the Junigou-shisen River have been cut off by the Shin-Kushiro River and the dike since the river and dike were newly constructed during 1921–1931. The present study area was located at the east side of the Shin-Kushiro River about 6.0–8.5 km upstream from an estuary facing the North Pacific. Abandoned farmland with a ca. 80-ha spread along the Kyu-Setsuri River as well as the remaining area of ca. 160 ha was a mire undisturbed artificially. Over the mire, Japanese alder (Alnus japonica) was widely distributed. The distribution area of the alder was divided into two types of stands: low stature (L) and high stature (H). The L stand had a mean height of 1.5 m and the H stand a mean height of 2.6 m (Nakamura et al. 2004b). Most alders grew on hummocks in both stands. In each stand, alder was cut 5 cm above the ground with a handsaw in an area of 25 m × 25 m (treatment plot, T) in February 2003. Stumps were left, and the cut trees were immediately transported to the abandoned farmland. The sprouts regrew from the stumps that were left, and then they were harvested in late August every year. On the other hand, a reference plot (R) having the same area was also established in each stand.
Study site. a Location of the study site; b picture of the study site; c plot outline. Open squares are the plots established. “H” and “L” mean low stature and high stature of the alder stand, respectively. “R” and “T” mean reference and treatment plot, respectively. Arrows denote the direction of river flow. In the plot outline c, the closed circle and closed square are the points of soil water sampling and field soil incubation, respectively
At the Kushiro Local Meteorological Observation station, about 4 km south-southwest from the present study site, the annual mean temperature and mean annual precipitation (1986–2005) were 6.2°C and 1030 mm, respectively (Kushiro Local Meteorological Observation, http://www.jma-net.go.jp/kushiro/). The mean of maximum snowpack depth during the same period was 36 cm, and mean monthly temperatures from December to March were below −1°C. The soil freezes in winter every year, from November–January (varied with years and places) to April in the present site (K. Okada and H. Yamada, unpublished data); alder was cut on the frozen soil.
The altitude of the alder stand with low stature (LT and LR) was 2.3 m above sea level and was highest in the Hirosato area. Altitudes of HT and HR were 2.1 and 2.2 m, respectively. These altitudes were on hollows. The groundwater table was usually within ca. 5 cm below the soil surface. The soil type of the alder stand was Fibric Histosols (FAO/Unesco 1988) derived from a peat layer of ca. 3 m, composed mainly of decomposing reed and sedge (Yamada et al. 2004).
Tree density of alder, which was estimated from the census for the belt transect of 5 m × 25 m, was 11440 and 8720 trees/ha in the L and H stand, respectively. Percentage of canopy occupancy, which was calculated from the tree crown projection by the hand-writing in the same belt transects, was 72 and 82% in the L and H stands, respectively; however, foliar density was thin. Other tree species were seldom found in the present study site. Herbaceous vegetation in both alder stands was composed of Carex lasiocarpa, var. occultans, Phragmites australis (Nakamura et al. 2004b). The floor vegetation in the L stand was often occupied by some Sphagnum species, Sphagnum imbricatum and S. subfulvum. In the high stature, however, Sphagnum spp. substantially lessened, and occupancy of Carex lasiocarpa increased.
Sampling methods
Soil water was sampled at a depth of 10 cm (usually below the groundwater table) using a porous ceramic cup at five points of hollows in each plot (closed circle in Fig. 1c). This sampling was conducted two or three times a year during the no-snow season: seven times from 2003 to 2005 (Table 1). In winter, soil water was not sampled because of the soil freezing. Frequent field observation was not permitted to protect the endangered species. Groundwater was also sampled at depths of 50, 100 and 150 cm at the same point as soil water in August and November 2003 using a hand pump and the PVC tube with a diameter of 5 cm screened between the respective depths ±5 cm. The sampling point for the groundwater was the hollow.
Chemistry of bulk precipitation, throughfall and stemflow was obtained to compare the influence on the soil water chemistry between treatment and reference plots. Bulk precipitation was sampled with a funnel 20 cm in diameter and a container at an open field in the abandoned farmland. Throughfall was sampled at five points in HR using a bucket with an opening 23 cm in diameter. Stemflow was sampled from five target trees in HR attaching the vinyl tube in spiral. Bulk precipitation, throughfall and stemflow were sampled when rainfall exceeded 20 mm/event in 2003. Stemflow was converted into “mm” using the tree crown projection described above.
Field soil incubation
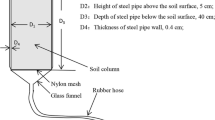
There are various methods of soil incubation to assess the nitrogen dynamics in the soil water. We attached importance to (1) minimum disturbance of the wetland soil and (2) semi-natural condition of the hydrological regimes in the surface soil, except for the transpiration by the vegetation. Especially for (2), an oxidative-reductive condition related to hydrological regime is important for the nitrogen dynamics in the wetland soil, especially in the habitat of alder, an actinorhizal nitrogen-fixing species (Hurd and Raynal 2004). Therefore, we chose the method of trenching the root zone (Vitousek et al. 1982; Yamashita et al. 2004) of the wetland vegetation, allowing the influence of rainfall and deep groundwater.
In mid-July 2004, plastic frames that were 25 cm × 25 cm with a height of 25 cm were inserted into the soil to 20 cm depth at five hollows in LT and HT to trench the root zone of vegetation (Fig. 1c); 5 cm was above the ground. Then, herbaceous vegetation within the frame was cut at the ground surface, and shed sheets (vinylon meshed cloth) were covered on the frame doubly to intercept 76% of the light. The plastic frame and shed sheet were black to minimize photosynthetic growth. In mid-August 2004, the vegetation regrown within the frames was removed again. Soil water was sampled in the same way as described above immediately before the installation of the frame and after 9 weeks (mid-September 2004) within and out of the frame. At the same time, the pH and oxidation reduction potential (ORP) of the soil were also measured.
Chemical analyses
The water sample was passed through the glass fiber filter with a pore size of 1.0 μm after the pH measurements and stored in the dark below 4°C until chemical analyses. The pH was determined using a glass electrode (TOA, HM-12P). Total dissolved nitrogen was analyzed by ultraviolet absorption spectrometry after digestion with potassium peroxodisulfate. NO3 −, Cl−, NH4 +, K+ and Ca2+ were analyzed by an ion chromatography (Dionex, DX-500) after passage through the 0.20-μm membrane filter. Total dissolved carbon (DOC) was measured with a total organic carbon analyzer (Shimadzu Inc., TOC-5000) for the soil water of field soil incubation. The C/N ratio (mass ratio) in soil water was determined by dividing the DOC concentration by total nitrogen concentration. In the field soil incubation experiment, in situ pH and ORP in the soil (at a depth of 10 cm) were measured with the glass electrode of a portable meter (TOA-DKK, HM-20P and RM-20P, respectively) rifting the soil and then inserting the electrodes into the rift. The tips of the electrodes were below the groundwater table. ORP was converted to the value at pH 7 (ORP7).
In the present study, nitrogen had three species, dissolved organic nitrogen (Org-N), NH4 + and NO3 −. The concentration of Org-N was determined by subtracting the concentration of NH4 + plus NO3 − from that of total dissolved nitrogen. The source of Ca2+ in the soil water was divided into sea and non-sea sources (ss and nss, respectively) using the ratios to Cl− concentration in sea water, on the assumption that Cl− in the soil water and groundwater was derived from sea water as follows.
“Ca” and “Cl” denote the Ca2+ and Cl− concentration, respectively. Subscript “sw” denotes the concentration in soil water, and subscript “sea” denotes the concentration in sea water. In the same calculation K+, the sources of these ions could not be separated successfully because most nssK+ concentrations were negative.
Indices of climatic conditions
In the present study, in situ hourly precipitation and air temperature were observed from June to November. Soil water chemistry was expected to be influenced by the climatic condition on the sampling day and antecedent condition. Precipitation directly affects water chemistry through concentration or dilution, and temperature affects the activity of plant and soil microbes. Therefore, we used the sum of the antecedent precipitation and index of temperature for 1 week before the sampling (Table 1). The index of temperature consists of two types for warmness and coldness. Cumulative hourly temperatures in cases of >0 and <0°C were calculated by summing the temperature for 1 week before the sampling day in the respective cases.
Statistics
In each plot, the difference in soil water chemistry among sampling days was evaluated by one-way repeated measures ANOVA, and post hoc test was conducted by Bonferroni’s multiple comparison. For each sampling day, the difference in soil water chemistry between the treatment and reference plot in each stand was analyzed by the t test. In field soil incubation, the difference in soil water chemistry among treatments was evaluated by one-way ANOVA followed by Scheffe’s multiple comparison (p < 0.05).
Results
Vertical distribution of chemistry in soil water and groundwater
The dominant form of nitrogen species was Org-N, and NO3 − was a minor component in soil water and groundwater (Fig. 2a). Inorganic nitrogen, especially NO3 −, was often undetected, increasing the standard error. Org-N in HT in August and that in HR in August and November exhibited decreasing tendencies along the depth. In other cases, however, there was little change in nitrogen concentration along the depth.
In both the H and L stands, Cl− concentrations exhibited increasing tendencies along the depth, although the H stand had lower Cl− concentrations than the L stand (Fig. 2b). The same tendencies were found in Ca2+ (Fig. 2b) and K+ (not shown) concentrations. The mean concentration (min. and max. values) of Cl− in the soil water was 2368 (1029–5823), 2435 (785–6088), 1107 (513–2229) and 1574 (886–2938) μmolc/l in LT, LR, HT and HR, respectively. Similarly, pH in the soil water was 6.61 (6.18–7.86), 6.51 (6.05–7.48), 6.44 (5.93–7.53) and 6.51 (6.01–7.22) in LT, LR, HT and HR, respectively.
Seasonal change in soil water chemistry
NH4 + concentrations in the soil waters usually increased in July or August, although no significant seasonal change was found (Fig. 3; Table 2). The NO3 − concentrations in all plots increased in November 2005. NO3 − concentrations in HT increased from the beginning of the present study step by step. Org-N concentrations in all plots tended to decrease.
Seasonal change in chemistry of soil water after tree-cutting. Open circle LR, filled circle LT, open triangle HR, filled triangle HT. Vertical bar denotes SE. Characters denote the results of RM-ANOVA followed by Bonferroni’s multiple comparison (p < 0.05). Small character denotes the difference for the control plot and large character for the treatment plot. Values with the same character are not significantly different at the 5% level
Ca2+ concentrations in the soil water in all plots significantly fluctuated. The Ca2+ concentrations only in HT increased from the beginning of the present study step by step. K+ concentrations in all plots increased in November 2005. These patterns of the Ca2+ concentration in HT and K+ concentrations in all plots followed the seasonal changes in the respective NO3 − concentrations, although the change in NO3 − was smaller than that in base cations.
A significant difference in soil water chemistry between the treatment and reference plot was found for Org-N in July 2003 (p < 0.01) and NO3 − in November 2005 (p < 0.05) in the H stand; the concentrations in HR were higher in HT in both cases.
The concentration of ssCa2+ and nssCa2+ in all plots except for ssCa2+ in HT significantly fluctuated (Fig. 4; Table 2); most of these fluctuations exhibited no obvious pattern. However, the concentration of nssCa2+ only in HT increased from the beginning of the present study step by step, except for the temporal increase in August 2003. This pattern of nssCa2+ in HT seemed to be similar to that of NO3 − in HT (Fig. 3).
Concentrations of sea source and non-sea source Ca2+ in the soil water. Closed squares denote the Ca2+ concentration of sea source (ssCa2+) and open squares Ca2+ of non-sea source (nssCa2+). Characters denote the results of RM-ANOVA followed by Bonferroni’s multiple comparison (p < 0.05). Small character denotes the difference for the non-sea source and large character for the sea source. Values with the same character are not significantly different at the 5% level
Comparison of chemistry among soil water, bulk precipitation, throughfall and stemflow
Bulk precipitation chemistry reflects nutrient input to the treatment plots. On the other hand, throughfall and stemflow chemistry reflects nutrient input to the reference plots, although the contribution of water amount in the stemflow ranged from 0.05 to 0.2% of bulk precipitation.
The concentrations of Org-N in the bulk precipitation, throughfall and stemflow were usually lower than that in the soil water, although high concentrations were occasionally found in the bulk precipitation and stemflow (Figs. 2, 5). The NO3 − concentration in the bulk precipitation was higher than in the soil water, and those in the throughfall and stemflow were usually similar to those in the soil water. The NH4 + concentrations in bulk precipitation were similar to those in the soil water, and those in throughfall and stemflow were in the lower range of those in the soil water. The concentrations of Ca2+ and Cl− in three precipitations were lower than those in the soil water. The K+ concentrations in bulk precipitation and throughfall were usually lower than those in the soil water, while the K+ concentration in stemflow had a wider range than those in the soil water (Fig. 5).
The range of solute concentration in bulk precipitation, throughfall and stemflow was the 10th, 25th, 50th, 75th and 90th percentile of the concentration distributed. Closed circles denote the mean concentrations. Bidirectional arrows denote the concentration range of respective solutes in the soil water in all the plots during the study period (see Fig. 2). For NH4 +, the concentration range in the soil water (0–104 μmolc/l) covered entirely the concentration range of three precipitations. For Cl− and Ca2+, the concentration range in the soil water (720–6088 and 68–469 μmolc/l, respectively) was over the concentration range of three precipitations
Effect of field soil incubation
The concentration of Org-N in the soil water in HT was significantly increased by incubation (Post, Fig. 6) and that in LT did not significantly increase during the incubation, although it increased without incubation (No Inc.). The NO3 − concentrations showed no significant change in either plot. The NH4 + concentrations in both plots significantly increased in September regardless of the incubation. The DOC concentration in both plots was significantly increased by incubation.
Soil water chemistry before and after the soil incubation and under no incubation. “Pre” means initial condition before incubation (in July). “No Inc.” means without incubation (in September). “Post” means after field incubation (in September). Left slash bar and right mesh bar denote the value in HT and LT, respectively. Solute concentrations are in the soil water, while pH and ORPs are measured in the soil into which the electrodes were inserted. Letters denote the results of analysis of variance followed by Scheffé’s test (p < 0.05). Lowercase letter denotes the difference in the HT and uppercase letter in the LT. Values with the same character are not significantly different at the 5% level. The upper right asterisk in the X-axis denotes a significant difference between HT and LT (t test, p < 0.05)
Values of pH in the soil showed no significant change, although the mean value decreased without incubation in both plots. ORP and ORP7 in LT significantly increased in September without incubation. However, these ORP values were maintained at the initial lower value (Pre) after the incubation. Those in HT showed no significant change, although the mean value was lower after the incubation. ORP and ORP7 under the initial condition (Pre) were lower in LT than in HT.
The Cl− concentration in HT showed no significant change, while that in LT significantly decreased during incubation. Before incubation and without incubation, Cl− concentrations were higher in LT than in HT, although no difference between the two plots was found after the incubation. At the end of the field soil incubation, i.e., September 2004, soil water had been subject to more rainfall than at other sampling days (Table 1).
Discussion
Effects of the tree-cutting on nitrogen concentrations in the wetland soil water
In this study, the inorganic nitrogen concentrations in the soil water in the treatment plot were not significantly higher than in the reference plot in either stand. In general, soil disturbance accompanied with tree-cutting stimulates the soil microbial activity, leading to enhancement of mineralization, nitrification and mobilization of NO3 − and base cations (Bormann and Likens 1979; van Miegroet and Cole 1984). This is because the soil temperature and moisture are increased, and soil disturbance supplies oxygen to the soil microbes (Bormann and Likens 1979). However, the tree-cutting in the present study was conducted on frozen soil and on snowpack of 20–40 cm. In this study, therefore, the soil was likely little disturbed by the tree-cutting treatment, suggesting little relation of soil disturbance to the change of the inorganic nitrogen concentration in the soil water.
The increases in the NH4 + concentrations in summer in all plots were probably caused by the enhanced ammonification owing to high temperature (Fig. 3). Very low concentrations of NO3 − in the soil water (<10 μmol/l) would be due to low nitrification and the following denitrification under water-saturated conditions (Hedin et al. 1998; Hefting et al. 2004), microbial immobilization (Westbrook and Devito 2004) and uptake by the plant root. The soil incubation experiment suggested that the uptake by the plant root was not a major process mitigating the enhancement of inorganic nitrogen in the soil water, because the inorganic nitrogen that was not taken up should have remained in the soil water within the incubation frame (Fig. 6). The incubation experiment in the present study not only inhibited nutrient uptake by the plant root, but also likely enhanced the soil temperature by using the black material. In warmed soil, microbial activity was likely stimulated. Thus, ammonification and nitrification (in the surface soil above the groundwater table) might also be stimulated in the present incubation experiment. However, an increase of DOC after the incubation would enhance the microbial immobilization of NH4 + and NO3 − (Hart et al. 1994). In addition to this DOC increase, the decrease of ORP stimulates denitrification (Korom 1992; Schlesinger 1997). At the soil ORP shown in Fig. 6, nitrification does not occur in the soil (Schlesinger 1997). Soil warming would stimulate the decomposition of organic matter above the water table, i.e., oxygen consumption by soil respiration, and the soil below the water table would be reduced severely. Below the water table, therefore, the aerobic decomposition of organic matter would be inhibited and dissolved organic substances would be released, resulting in the DOC increase. Therefore, microbial immobilization of inorganic nitrogen and heterotrophic denitrification would occur sufficiently to inhibit the substantial change in inorganic nitrogen concentrations in the soil water.
At the end of the incubation experiment in the present study (September 2004), the wetland soil received antecedent precipitation (Table 1). The present wetland soil, especially in the L stand, was subject to the influence of sea salts from deep groundwater (Fig. 2b), probably because of the leakage of salt wedge. Cl− concentration without incubation (No Inc.) in September 2004 in LT was similar to that before incubation (Fig. 6), because the soil water diluted with rainwater was rapidly diffused, and the Cl− concentration was enhanced by the deep groundwater. On the other hand, the soil water within the incubation frame was diffused insufficiently, and the Cl− concentration after incubation in LT remained low. Similarly, rainwater having higher NO3 − (Fig. 5) was supplied to the soil water and mostly would remain within the frame. However, the NO3 − supplied from the atmosphere would be almost completely consumed by soil microbes through immobilization and/or denitrification.
Under the condition influenced by both the nitrogen-fixing species and frequent oxidizing events, such as large fluctuations of the water table and water mixing in a riparian wetland, nitrogen concentrations in the soil water might have changed substantially (Hurd and Raynal 2004). Owing to the soil characteristics such as a high and stable groundwater table, however, the cutting of alder probably had only a slight influence on the nitrogen concentrations in the soil water in this wetland ecosystem. Thus, the hollow soil in the present wetland had high potential to mitigate the change of nitrogen concentrations in the soil water, even if ammonification and nitrification were enhanced in the thin surface layer above the water table owing to the warmed condition after the removal of the alder.
Mobilization of base cations in the wetland soil water in relation to NO3 − concentrations
In the present study, we found significant and temporal fluctuations of NO3 − concentrations in the soil water, although the fluctuation ranges of NO3 − concentrations were small. In general, mobilization of NO3 − is accompanied by base cation mobilization in the soil after the tree-cutting (Bormann and Likens 1979; van Miegroet and Cole 1984).
The gradual increases of NO3 − (Fig. 3) and nssCa2+ (Fig. 4) in the soil water in HT after the tree-cutting probably resulted from two processes. The first process was probably the rhizosphere oxidation of herbaceous species, reed and sedge (Schlesinger 1997), flourishing after the alder removal, and the consequential nitrification (Hosomi 2000). The next process was probably the ion exchange between Ca2+ and H+ caused by nitrification (Bormann and Likens 1979; Van Miegroet and Cole 1984), although the electric equivalence of NO3 − was lower than that of Ca2+ in the soil water. Imbalance of the electric equivalence between Ca2+ and NO3 − was probably caused by the NO3 − consumption processes as below. The rhizosphere oxidation occurs not only for herbaceous species, but also for Japanese alders (Grosse et al. 1993). In the present site, however, most of the alder grew on the hummock, where the soil water was not sampled. After the removal of alder, the roots of herbaceous species consequentially would increase at hollows, resulting in an increased probability of the sampling the soil water having a high NO3 −. However, the oxidized rhizosphere is very thin, and the NO3 − diffused out of the oxidized rhizosphere is immediately denitrified (Hosomi 2000). Moreover, part of the NO3 − would be immobilized by soil microbes and taken up by plant roots. Therefore, the soil water sampled with a ceramic cup did not contain a large amount of NO3 −, and there was only a slight increase in NO3 − concentration; the NO3 − concentration in HT did not significantly increase more than in HR. The lack of gradual increase in the NO3 − concentration in the LT soil water was probably caused by the weak effect of rhizosphere oxidation on the soil water in LT and partly because the soil water in LT was probably influenced by reductive deep groundwater more than that in HT.
The increases in the NO3 − concentrations in all plots in November 2005 (Fig. 3) were irrelevant to the tree-cutting, and were the phenomena common to all the plots, such as a climatic event; they are perhaps due to the nitrification enhancement by a freeze-thaw cycle in the surface soil (a few centimeters) (Groffman et al. 2001; Fitzhugh et al. 2001, 2003). The low cumulative coldness at that time (Table 1) suggests the surface soil freezing at night and thawing in daytime. Soil surface temperature in the present site dropped under 0°C before the sampling day in November 2005, while it did not drop under 0°C before the sampling day in November 2004 (K. Okada and H. Yamada, unpublished data). Moreover, denitrification activity might decrease at a low temperature, especially near or below 0°C at night (Knowles 1982). The simultaneous increases in K+ concentration in the soil water (Fig. 3) also would be caused by the nitrification followed by the ion exchange with H+, although the electric equivalence of NO3 − was lower than that of K+ in the soil water. Fitzhugh et al. (2003) suggested that the simultaneous releases of NO3 − and K+ from a forested watershed in New Hampshire were due to the disruption of interactions among root, microbe and soil by the soil-freezing events. In November 2005 in the present study, the plant root probably took up few nutrients, and decomposition of the plant fine root hardly would proceed because of a cool condition. However, soil freezing might cause quick nitrification and simultaneous mobilization of K+ in the surface wetland soil in the present site.
Conclusion
In the present study, the cutting of alder had only a slight influence on the nitrogen concentrations in the soil water in this wetland ecosystem, although some changes caused by the tree-cutting were found. Moreover, the subtle change of the soil water chemistry was caused not by the direct influence of soil disturbance and alder removal, but probably by an indirect influence through the rhizosphere oxidation of the herbaceous vegetation flourishing after the tree-cutting. The soil water chemistry in the present wetland ecosystem will be relatively stable in the future because the present wetland soil probably had a high potential to mitigate the change of nitrogen concentrations in the soil water.
Soil freezing might obscure the subtle change of the soil water chemistry in late autumn. However, tree-cutting on the frozen soil probably contributed to minimizing the soil disturbance in the tree-cutting and the change in soil water chemistry. The influence of physical disturbance of wetland soil on soil water chemistry is an important problem in itself. In the removal of plant species from a wetland ecosystem, the manipulation minimizing the disturbance of the soil environment may be important to minimize the influence on the chemical environment of the wetland soil.
References
Batzer DP, Jackson CR, Mosner M (2000) Influences of riparian logging on plants and invertebrates in small depressional wetlands of Georgia, USA. Hydrobiologia 441:123–132
Bormann FH, Likens GE (1979) Pattern and processes in a forested ecosystem. Springer, New York
Bowden WB (1987) The biogeochemistry of nitrogen in freshwater wetlands. Biogeochemistry 4:313–348
Ehrenfeld JG (2003) Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6:503–523
FAO/Unesco (1988) Revised legend of the FAO-Unesco soil map of the world. World Soil Resources Report 60. FAO, Rome
Fitzhugh RD, Driscoll CT, Groffman PM, Tierney GL, Fahey TJ, Hardy JP (2001) Effects of soil freezing disturbance on soil solution nitrogen, phosphorus, and carbon chemistry in a northern hardwood ecosystem. Biogeochemistry 56:215–238
Fitzhugh RD, Likens GE, Driscoll CT, Mitchell MJ, Groffman PM, Fahey TJ, Hardy JP (2003) Role of soil freezing events in interannual patterns of stream chemistry at the Hubbard Brook Experimental Forest, New Hampshire. Environ Sci Technol 37:1575–1580
Fujimura Y, Fujita H, Kato K, Yanagiya S (2008) Vegetation dynamics related to sediment accumulation in Kushiro Mire, northeastern Japan. Plant Ecol 199:115–124
Gale MR, McLaughlin JW, Jurgensen MF, Trettin CC, Soelsepp T, Lydon PO (1998) Plant community responses to harvesting and post-harvest manipulations in a Picea-Larix-Pinus wetland with a mineral substrate. Wetlands 18:150–159
Groffman PM, Driscoll CT, Fahey TJ, Hardy JP, Fitzhugh RD, Tierney GL (2001) Effects of mild winter freezing on soil nitrogen and carbon dynamics in a northern hardwood forest. Biogeochemistry 56:191–213
Grosse W, Schulte A, Fujita H (1993) Pressurized gas transport in two Japanese alder species in relation to their natural habitats. Ecol Res 8:151–158
Hart SC, Nason GE, Myrold DD, Perry DA (1994) Dynamics of gross nitrogen transformations in an old-growth forest: the carbon connection. Ecology 75:880–891
Hedin LO, von Fischer JC, Ostrom NE, Kennedy BP, Brown MG, Robertson GP (1998) Thermodynamics constraints on nitrogen transformations and other biogeochemical processes at soil-stream interfaces. Ecology 79:684–703
Hefting M, Clement JC, Dowrick D, Cosandey AC, Bernal S, Cimpian C, Tatur A, Burt TP, Pinay G (2004) Water table elevation controls on nitrogen cycling in riparian wetlands along a European climatic gradient. Biogeochemistry 67:113–134
Hosomi M (2000) Conservation and application of wetland ecosystems. In: Sudo R (ed) Eco-technology for environmental restoration (the title tentatively translated into Japanese). Kodansha Scientific, Tokyo, pp 167–190 (in Japanese)
Hurd TM, Raynal DJ (2004) Comparison of nitrogen solute concentrations within alder (Alnus incana ssp. rugosa) and non-alder dominated wetland. Hydrol Process 18:2681–2697
Korom SF (1992) Natural denitrification in the saturated zone: a review. Water Resour Res 28:1657–1668
Knowles R (1982) Denitrification. Microbiol Mol Biol Rev 46:43–70
Matson PA, Vitousek PM, Ewel JJ, Mazzarino MJ, Robertson GP (1987) Nitrogen transformations following tropical forest felling and burning on a volcanic soil. Ecology 68:491–502
McLaughlin JW, Lewin JC, Reed DD, Trettin CC, Jurgensen MF, Gale MR (1994) Soil factors related to dissolved organic carbon concentrations in a black spruce swamp, Michigan. Soil Sci 158:454–464
McLaughlin JW, Martin GL, Jurgensen MF, Gale MR (1996) Organic carbon characteristics in a spruce swamp five years after harvesting. Soil Sci Soc Am J 60:1228–1236
McLaughlin JW, Gale MR, Jurgensen MF, Trettin CC (2000) Soil organic matter and nitrogen cycling in response to harvesting, mechanical site preparation, and fertilization in a wetland with a mineral substrate. For Ecol Manag 129:7–23
Mitsch WJ, Gosselink JG (2000) Wetlands, 3rd edn. Wiley, New York
Nakamura F, Ahn YS (2006) Landscape restoration—a case practice of Kushiro Mire, Hokkaido. In: Hong SK, Nakagoshi N, Fu B, Morimoto Y (eds) Landscape ecological applications in man-influenced areas: linking man and nature systems. Springer, Dordrecht, pp 209–233
Nakamura F, Sudo T, Kameyama S, Jitsu M (1997) Influences of channelization on discharge of suspended sediment and wetland vegetation in Kushiro Marsh, northern Japan. Geomorphology 18:279–289
Nakamura F, Jitsu M, Kameyama S, Mizugaki S (2002) Changes in riparian forest in the Kushiro Mire, Japan, associated with stream channelization. River Res Appl 18:65–79
Nakamura F, Kameyama S, Mizugaki S (2004a) Rapid shrinkage of Kushiro Mire, the largest mire in Japan, due to increased sedimentation associated with land-use development in the catchment. Catena 55:213–229
Nakamura T, Yamada H, Nakagawa Y, Kasai Y, Nakamura F, Watanabe T (2004b) Ecological aspects of Hirosato restoration area in the Kushiro Mire: Impacts of artificial disturbances on the relationship between distribution of vegetation and hydrochemical environments. Ecol Civ Eng 7:53–64 (in Japanese with English summary)
Schlesinger WH (1997) Biogeochemistry: an analysis of global change, 2nd edn. Academic Press, San Diego
Spencer DR, Perry JE, Silberhorn GM (2001) Early secondary succession in bottomland hardwood forests of southeastern Virginia. Environ Manag 27:559–570
Sun G, Riekerk H, Kornhak LV (2000) Ground-water-table rise after forest harvesting on cypress-pine flatwoods in Florida. Wetlands 20:101–112
Swank WT, Vose JM, Elliott KJ (2001) Long-term hydrologic and water quality responses following commercial clearcutting of mixed hardwoods on a southern Appalachian catchment. For Ecol Manag 143:163–178
Van Miegroet H, Cole DW (1984) The impact of nitrification on soil acidification and cation leaching in a red alder ecosystem. J Environ Qual 13:586–590
Vitousek PM, Gosz JR, Grier CC, Melillo JM, Reiners WA (1982) A comparative analysis of potential nitrification and nitrate mobility in forest ecosystems. Ecol Monogr 52:155–177
Westbrook CJ, Devito KJ (2004) Gross nitrogen transformations in soils from uncut and cut boreal upland and peatland coniferous forest stands. Biogeochemistry 68:33–50
Xu Y-J, Burger JA, Aust WM, Patterson SC, Miwa M, Preston DP (2002) Changes in surface water table depth and soil physical properties after harvest and establishment of loblolly pine (Pinus taeda L.) in Atlantic coastal plain wetlands of South Carolina. Soil Tillage Res 63:109–121
Yamada H, Nakamura T, Nakagawa Y, Kamiya Y, Nakamura F, Watanabe T (2004) Ecological aspects of Hirosato restoration area in the Kushiro Mire: effect of pasture developments and river improvements on hydrochemical environments of groundwater. Ecol Civ Eng 7:37–51 (in Japanese with English summary)
Yamashita T, Kasuya N, Nishimura S, Takeda H (2004) Effects of root zone trenching on soil nitrogen dynamics in Japanese ceder and cypress plantations. J For Res 9:333–340
Zedler JB, Kercher S (2004) Cause and consequences of invasive plants in wetlands: opportunities, opportunists, and outcomes. Crit Rev Plant Sci 23:431–452
Zhu WX, Ehrenfeld JG (2000) Nitrogen retention and release in Atlantic white ceder wetlands. J Environ Qual 29:612–620
Acknowledgments
This study was supported by the Eastern Hokkaido Regional Office for Nature Conservation, Ministry of the Environment Government of Japan. Field observation and chemical analysis were greatly helped and facilitated by the many staffs of Kankyo Consultant Co., Ltd., E. Takada and N. Ishikawa, FSC Hokkaido University, also helped with chemical analysis. Y. Momose cooperated fully with us in the field observation. Dr. K. Okada, Hokkaido University, allowed us to use the unpublished data on soil temperature. Y. Shida, Wildlife Research Institute Co., Ltd., H. Shinsho, Conservation of Wetland, Kushiro City, Dr. K. Yabe, Sapporo City University, Dr. T. Uchida, Obihiro University of Agriculture and Veterinary Medicine, offered helpful and useful suggestions for the present study. Dr. K. Terasawa, Hokkaido Forestry Research Institute, gave the useful literatures on the gas exchange through wetland vegetation. The current study complies with the current Japanese laws.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Nakagawa, Y., Nakamura, T., Yamada, H. et al. Changes in nitrogen and base cation concentrations in soil water due to the tree cutting in a wetland alder forest in the Kushiro Wetland, northern Japan. Limnology 13, 27–36 (2012). https://doi.org/10.1007/s10201-011-0350-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-011-0350-8