Abstract
Background
Midostaurin (MIDO) combined with standard chemotherapy was approved by the European Medicines Agency in 2017 for the treatment of adults with newly diagnosed FLT3-mutated acute myeloid leukemia (AML) based on results from the RATIFY trial.
Methods
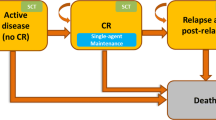
A cost-effectiveness model was developed to compare MIDO and standard-of-care (SOC) to SOC alone in France. Per Haute Autorité de Santé (HAS) guidelines, a partitioned survival model with eight health states was used: diagnosis/induction, complete remission, relapse, hematopoietic stem cell transplantation (HSCT), HSCT recovery, post-HSCT recovery (stabilized after HSCT recovery), post-HSCT relapse, and mortality. A lifetime horizon was used beginning at diagnosis with a “cure model,”, which assumed natural mortality after trial cut-off. Utility values were obtained from a systematic literature review and included disutilities. Resource utilization was based on HAS clinical guidelines and a survey of French physicians and included drugs and administration, adverse events, routine medical care, HSCT, and end-of-life care costs.
Results
In RATIFY and after extrapolation, MIDO improved survival compared to SOC, translating into MIDO-treated patients gaining 1.12 life years (LYs) and 1.23 quality-adjusted life years (QALYs) versus SOC. The incremental cost-effectiveness ratio (ICER) for MIDO versus SOC was €68,781 per LY and €62,305 per QALY. Sensitivity analyses showed consistency with base case findings.
Conclusions
MIDO represents a clinically significant advancement in the management of newly diagnosed FLT3-mutated AML. In this analysis, MIDO add-on therapy showed gains in LYs and QALYs versus SOC alone and was found to be a cost-effective option at a €100,000 per QALY threshold for end-of-life treatment.





Similar content being viewed by others
Abbreviations
- AML:
-
Acute myeloid leukemia
- FLT3:
-
FMS-like tyrosine kinase 3
- SOC:
-
Standard of care
- HSCT:
-
Hematopoietic stem cell transplantation
- MIDO:
-
Midostaurin
- EQ-5D:
-
EuroQol 5 dimensions
- QLQ-30:
-
EORTC core quality of life questionnaire
- CI:
-
Confidence interval
- QALY:
-
Quality-adjusted life year
- CR:
-
Complete remission
- HRQoL:
-
Health-related quality of life
- ICER:
-
Incremental cost-effectiveness ratio
- OS:
-
Overall survival
- CE:
-
Cost-effectiveness
- HR:
-
Hazard ratio
- EFS:
-
Event-free survival
- NOS:
-
Not otherwise specified
References
Visser, O., Trama, A., Maynadie, M., Stiller, C., Marcos-Gragera, R., De Angelis, R., Mallone, S., Tereanu, C., Allemani, C., Ricardi, U., Schouten, H.C.: Incidence, survival and prevalence of myeloid malignancies in Europe. Eur. J. Cancer 48(17), 3257–3266 (2012). https://doi.org/10.1016/j.ejca.2012.05.024
Khan, I., Altman, J.K., Licht, J.D.: New strategies in acute myeloid leukemia: redefining prognostic markers to guide therapy. Clin. Cancer Res. 18(19), 5163–5171 (2012). https://doi.org/10.1158/1078-0432.CCR-12-0313
Sant, M., Minicozzi, P., Mounier, M., Anderson, L.A., Brenner, H., Holleczek, B., Marcos-Gragera, R., Maynadie, M., Monnereau, A., Osca-Gelis, G., Visser, O., De Angelis, R.: Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol. 15(9), 931–942 (2014). https://doi.org/10.1016/s1470-2045(14)70282-7
Maynadie, M., De Angelis, R., Marcos-Gragera, R., Visser, O., Allemani, C., Tereanu, C., Capocaccia, R., Giacomin, A., Lutz, J.M., Martos, C., Sankila, R., Johannesen, T.B., Simonetti, A., Sant, M., Group, H.W.: Survival of European patients diagnosed with myeloid malignancies: a HAEMACARE study. Haematologica 98(2), 230–238 (2013). https://doi.org/10.3324/haematol.2012.064014
Meyers, J., Yu, Y., Kaye, J.A., Davis, K.L.: Medicare fee-for-service enrollees with primary acute myeloid leukemia: an analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl. Health Econ. Health Policy 11(3), 275–286 (2013). https://doi.org/10.1007/s40258-013-0032-2
Almeida, A.M., Ramos, F.: Acute myeloid leukemia in the older adults. Leukemia Res. Rep. 6, 1–7 (2016). https://doi.org/10.1016/j.lrr.2016.06.001
Erba, H.P.: Prognostic factors in elderly patients with AML and the implications for treatment. Hematology. American Society of Hematology. Education Program, pp. 420–428 (2007). https://doi.org/10.1182/asheducation-2007.1.420
Patel, J.P., Gonen, M., Figueroa, M.E., Fernandez, H., Sun, Z., Racevskis, J., Van Vlierberghe, P., Dolgalev, I., Thomas, S., Aminova, O., Huberman, K., Cheng, J., Viale, A., Socci, N.D., Heguy, A., Cherry, A., Vance, G., Higgins, R.R., Ketterling, R.P., Gallagher, R.E., Litzow, M., van den Brink, M.R., Lazarus, H.M., Rowe, J.M., Luger, S., Ferrando, A., Paietta, E., Tallman, M.S., Melnick, A., Abdel-Wahab, O., Levine, R.L.: Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl. J. Med. 366(12), 1079–1089 (2012). https://doi.org/10.1056/NEJMoa1112304
Schiller, G.J.: High-risk acute myelogenous leukemia: treatment today and tomorrow. Hematology. American Society of Hematology. Education Program 2013, pp. 201–208 (2013). https://doi.org/10.1182/asheducation-2013.1.201
Milligan, D.W., Grimwade, D., Cullis, J.O., Bond, L., Swirsky, D., Craddock, C., Kell, J., Homewood, J., Campbell, K., McGinley, S., Wheatley, K., Jackson, G.: Guidelines on the management of acute myeloid leukaemia in adults. Br. J. Haematol. 135(4), 450–474 (2006). https://doi.org/10.1111/j.1365-2141.2006.06314.x
Zeiser, R., Blazar, B.R.: Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N. Engl. J. Med. 377(26), 2565–2579 (2017). https://doi.org/10.1056/NEJMra1703472
Kuba, A., Raida, L.: Graft versus host disease: from basic pathogenic principles to DNA damage response and cellular senescence. Mediat. Inflamm. 2018 (2018). https://doi.org/10.1155/2018/9451950
Jagasia, M., Arora, M., Flowers, M.E., Chao, N.J., McCarthy, P.L., Cutler, C.S., Urbano-Ispizua, A., Pavletic, S.Z., Haagenson, M.D., Zhang, M.J., Antin, J.H., Bolwell, B.J., Bredeson, C., Cahn, J.Y., Cairo, M., Gale, R.P., Gupta, V., Lee, S.J., Litzow, M., Weisdorf, D.J., Horowitz, M.M., Hahn, T.: Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 119(1), 296–307 (2012). https://doi.org/10.1182/blood-2011-06-364265
El Fakih, R., Rasheed, W., Hawsawi, Y., Alsermani, M., Hassanein, M.: Targeting FLT3 mutations in acute myeloid leukemia. Cells 7(1) (2018). https://doi.org/10.3390/cells7010004
Serrano-Blanco, A., Rubio-Valera, M., Aznar-Lou, I., Baladon Higuera, L., Gibert, K., Gracia Canales, A., Kaskens, L., Ortiz, J.M., Salvador-Carulla, L.: In-patient costs of agitation and containment in a mental health catchment area. BMC Psychiatry 17(1), 212 (2017). https://doi.org/10.1186/s12888-017-1373-4
Stone, R.M., Manley, P.W., Larson, R.A., Capdeville, R.: Midostaurin: its odyssey from discovery to approval for treating acute myeloid leukemia and advanced systemic mastocytosis. Blood Adv 2(4), 444–453 (2018). https://doi.org/10.1182/bloodadvances.2017011080
Lin, T.L., Levy, M.Y.: Acute myeloid leukemia: focus on novel therapeutic strategies. Clinical Medicine Insights. Oncology 6, 205–217 (2012). https://doi.org/10.4137/cmo.s7244
Stone, R.M., Mandrekar, S., Sanford, B.L., Geyer, S., Bloomfield, C.D., Dohner, K., Thiede, C., Marcucci, G., Lo-Coco, F., Klisovic, R.B., Wei, A., Sierra, J., Sanz, M.A., Brandwein, J.M., de Witte, T., Niederwieser, D., Appelbaum, F.R., Medeiros, B.C., Tallman, M.S., Krauter, J., Schlenk, R.F., Ganser, A., Serve, H., Ehninger, G., Amadori, S., Larson, R.A., Dohner, H.: The multi-kinase inhibitor midostaurin (M) prolongs survival compared with placebo (P) in combination with daunorubicin (D)/cytarabine (C) induction (ind), high-dose C consolidation (consol), and as maintenance (maint) therapy in newly diagnosed acute myeloid leukemia (AML) patients (pts) age 18–60 with FLT3 mutations (muts): an international prospective randomized (rand) P-controlled double-blind trial (CALGB 10603/RATIFY [Alliance]). Blood 126(23), 6 (2015)
Stone, R.M., Mandrekar, S.J., Sanford, B.L., Laumann, K., Geyer, S., Bloomfield, C.D., Thiede, C., Prior, T.W., Dohner, K., Marcucci, G., Lo-Coco, F., Klisovic, R.B., Wei, A., Sierra, J., Sanz, M.A., Brandwein, J.M., de Witte, T., Niederwieser, D., Appelbaum, F.R., Medeiros, B.C., Tallman, M.S., Krauter, J., Schlenk, R.F., Ganser, A., Serve, H., Ehninger, G., Amadori, S., Larson, R.A., Dohner, H.: Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med. (2017). https://doi.org/10.1056/NEJMoa1614359
Choix méthodologiques pour l’évaluation économique à la HAS. http://www.has-sante.fr/portail/upload/docs/application/pdf/2011-11/guide_methodo_vf.pdf (2011)
Husereau, D., Drummond, M., Petrou, S., et al.: Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluations Publication Guidelines Good Reporting Practices Task Force. Value Health. 16(2), 231–250 (2013)
Taux de mortalité par sexe et groupe d’âges en 2014. http://www.insee.fr/fr/themes/detail.asp?ref_id=ir-irsocsd2014&page=irweb/irsocsd2014/dd/irsocsd2014_mortalite.htm (2016). 2018
Lebègue D, B.L., Hirtzman P: Révision du taux d’actualisation des investissements publics. In. Commissariat general au Plan (2005)
Base des Médicaments et Informations Tarifaires. http://www.codage.ext.cnamts.fr/codif/bdm_it/index.php (2018). 2018
Cancer Mpact AML Drug Regimen- France 2016. In. Kantar Health, (2016)
Treatment Architecture: Western Europeleukemia, acute myeloid. In: CancerMPact®Western Europe, 2015 Kantar Health (2015)
Leucémies aiguës de l’adulte: Tumeur maligne, affection maligne du tissu lymphatique ou hématopoïétique. In: GUIDE - AFFECTION DE LONGUE DURÉE. Haute Autorité de Santé, Saint-Denis, France, (2011)
Census Profile, 2016 Census: Ontario [Province] and Canada [Country]. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/Page.cfm?Lang=E&Geo1=PR&Code1=35&Geo2=&Code2=&Data=Count&SearchText=Ontario&SearchType=Begins&SearchPR=01&B1=All&GeoLevel=PR&GeoCode=35 (2016). Accessed June 26 2018
Press, V.G., Pappalardo, A.A., Conwell, W.D., Pincavage, A.T., Prochaska, M.H., Arora, V.M.: Interventions to improve outcomes for minority adults with asthma: a systematic review. J. Gen. Intern. Med. 27(8), 1001–1015 (2012). https://doi.org/10.1007/s11606-012-2058-9
Fey, M.F., Buske, C., Group, E.G.W.: Acute myeloblastic leukaemias in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6), vi138–143 (2013). https://doi.org/10.1093/annonc/mdt320
Dohner, H., Estey, E.H., Amadori, S., Appelbaum, F.R., Buchner, T., Burnett, A.K., Dombret, H., Fenaux, P., Grimwade, D., Larson, R.A., Lo-Coco, F., Naoe, T., Niederwieser, D., Ossenkoppele, G.J., Sanz, M.A., Sierra, J., Tallman, M.S., Lowenberg, B., Bloomfield, C.D., European, L.: Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115(3), 453–474 (2010). https://doi.org/10.1182/blood-2009-07-235358
Wingard, J.R., Majhail, N.S., Brazauskas, R., Wang, Z., Sobocinski, K.A., Jacobsohn, D., Sorror, M.L., Horowitz, M.M., Bolwell, B., Rizzo, J.D., Socie, G.: Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 29(16), 2230–2239 (2011). https://doi.org/10.1200/jco.2010.33.7212
Grulke, N., Albani, C., Bailer, H.: Quality of life in patients before and after haematopoietic stem cell transplantation measured with the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire QLQ-C30. Bone Marrow Transplant. 47(4), 473–482 (2012). https://doi.org/10.1038/bmt.2011.107
Forsythe, A., Brandt, P.S., Dolph, M., Patel, S., Rabe, A.P.J., Tremblay, G.: Systematic review of health state utility values for acute myeloid leukemia. CEOR 10, 83–92 (2018). https://doi.org/10.2147/ceor.s153286
Crott, R., Briggs, A.: Mapping the QLQ-C30 quality of life cancer questionnaire to EQ-5D patient preferences. Eur. J. Health Econ 11(4), 427–434 (2010). https://doi.org/10.1007/s10198-010-0233-7
Tolley, K., Goad, C., Yi, Y., Maroudas, P., Haiderali, A., Thompson, G.: Utility elicitation study in the UK general public for late-stage chronic lymphocytic leukaemia. Eur. J. Health Econ: HEPAC 14(5), 749–759 (2013). https://doi.org/10.1007/s10198-012-0419-2
Peric, Z., Desnica, L., Durakovic, N., Ostojic, A., Pulanic, D., Serventi-Seiwerth, R., Prenc, E., Basak, G., Vrhovac, R., Pavletic, S.Z., Nemet, D.: Which questionnaires should we use to evaluate quality of life in patients with chronic graft-vs-host disease? Croat. Med. J. 57(1), 6–15 (2016)
Uyl-de Groot, C.A., Lowenberg, B., Vellenga, E., Suciu, S., Willemze, R., Rutten, F.F.: Cost-effectiveness and quality-of-life assessment of GM-CSF as an adjunct to intensive remission induction chemotherapy in elderly patients with acute myeloid leukemia. Br. J. Haematol. 100(4), 629–636 (1998)
Batty, N., Yin, Y., Wetzler, M.: Decitabine is more cost effective than cytarabine and daunorubicin in elderly acute myeloid leukemia patients. J. Cancer Res. Ther. 2(4), 68–73 (2014). http://dx.doi.org/10.14312/2052-4994.2014-9
Leunis, A., Redekop, W.K., Uyl-de Groot, C.A., Lowenberg, B.: Impaired health-related quality of life in acute myeloid leukemia survivors: a single-center study. Eur. J. Haematol. 93(3), 198–206 (2014). https://doi.org/10.1111/ejh.12324
Pan, F., Peng, S., Fleurence, R., Linnehan, J.E., Knopf, K., Kim, E.: Economic analysis of decitabine versus best supportive care in the treatment of intermediate- and high-risk myelodysplastic syndromes from a US payer perspective. Clin. Ther. 32(14), 2444–2456 (2010). https://doi.org/10.1016/j.clinthera.2010.12.003
Briggs, A.H., Weinstein, M.C., Fenwick, E.A., Karnon, J., Sculpher, M.J., Paltiel, A.D., Force, I.-S.M.G.R.P.T.: Model parameter estimation and uncertainty: a report of the ISPOR-SMDM modeling good research practices task force-6. Value Health 15(6), 835–842 (2012). https://doi.org/10.1016/j.jval.2012.04.014
National Institute for Health and Care Excellence. Azacitidine for treating acute myeloid leukaemia with more than 30% bone marrow blasts 27 July 2016. https://www.nice.org.uk/guidance/ta399/chapter/4-Committee-discussion. Accessed 27 Sep 2019
Acknowledgements
Some authors contributed to an analysis of the cost-effectiveness of midostaurin from a United Kingdom perspective, published in Cost-Effectiveness and Resource Allocation in October of 2018 [1]. Mapping of utilities used in this analysis was described in full in a 2018 study by Forsythe et al. [2]. We thank Jaclyn Hearnden for her contributions to writing and editing the manuscripts.
Funding
This study was funded by Novartis Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Clemence Cariou, Christian Recher, Patricia Brandt, and Anne-Sandrine Blanc are employees of Novartis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tremblay, G., Cariou, C., Recher, C. et al. Cost-effectiveness of midostaurin in the treatment of newly diagnosed FLT3-mutated acute myeloid leukemia in France. Eur J Health Econ 21, 543–555 (2020). https://doi.org/10.1007/s10198-019-01149-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-019-01149-9
Keywords
- Acute myeloid leukemia
- FMS-like tyrosine kinase 3
- Cost-effectiveness analysis
- Life years
- Quality-adjusted life years
- Incremental cost-effectiveness ratio