Abstract
Background
To date, a multi-country review evaluating the cost-of-illness (COI) studies from the Central and Eastern European (CEE) region has not yet been published. Our main objective was to provide a general description about published COI studies from CEE.
Methods
A systematic search was performed between 1 January 2006 and 1 June 2017 in Medline, EMBASE, The Cochrane Library, CINAHL, and Web of Science to identify all relevant COI studies from nine CEE countries. COI studies reporting costs without any restrictions by age, co-morbidities, or treatment were included. Methodology, publication standards, and cost results were analysed.
Results
We identified 58 studies providing 83 country-specific COI results: Austria (n = 9), Bulgaria (n = 16), Croatia (n = 3), the Czech Republic (n = 10), Hungary (n = 24), Poland (n = 11), Romania (n = 3), Slovakia (n = 3), and Slovenia (n = 4). Endocrine, nutritional, and metabolic diseases (18%), neoplasms (12%), infections (11%), and neurological disorders (11%) were the most frequently studied clinical areas, and multiple sclerosis was the most commonly studied disease. Overall, 57 (98%) of the studies explicitly stated the source of resource use data, 45 (78%) the study perspective, 34 (64%) the costing method, and 24 (58%) reported at least one unit costs. Regardless of methodological differences, a positive relationship was observed between costs of diseases and countries’ per capita GDP.
Conclusions
Cost-of-illness studies varied considerably in terms of methodology, publication practice, and clinical areas. Due to these heterogeneities, transferability of the COI results is limited across Central and Eastern European countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cost-of-illness (COI) studies provide information on the economic burden of a specific disease from a societal, public payer, family or individual perspective. They aim to evaluate not only the disease-related healthcare costs but also the overall costs to society, including both medical and non-medical costs. COI studies can aid the understanding of the importance of a health problem, estimate the main cost components and the cost structure, and, thus, provide valuable cost estimates for use in full economic evaluations [1]. As a result, COI studies are an important type of health economic analysis aiming to support health policy and financing decision-making processes [2]. Over the past decade, health technology assessment has been implemented in most Central and Eastern European (CEE) countries, which, in turn, necessitates reliable, local country-specific COI studies [3,4,5].
There are no gold standard methods for calculating COI estimates [6,7,8]. Although standardization of the methods used in COI studies is becoming more and more important to allow comparability, studies apply different designs, methodologies, perspectives, and costing approaches [9, 10]. Until now, several systematic reviews of COI studies have been conducted; however, most of them were focusing on one specific disease. Few reviews targeted a single specific cost item or component, such as informal care, direct medical costs, productivity loss, a specific geographic area, or a specific methodological aspect [10,11,12,13]. Nonetheless, COI studies from CEE countries have not been reviewed to date, with the exception of Austria [13].
This review has been undertaken to provide a description of the COI studies in nine CEE countries, namely Austria, Bulgaria, the Czech Republic, Croatia, Hungary, Poland, Romania, Slovakia, and Slovenia, in the past 10 years. The main objectives were to describe study characteristics, methodology, and the COI estimates reported. First, we provide an overview of applied methods. Then, we present and compare the COI estimates across CE countries.
Methods
Search strategy
We conducted a systematic review following the PRISMA statement [14]. A literature search was performed using Medline, EMBASE, The Cochrane Library, CINAHL, and Web of Science databases to identify studies that report data on the cost of a disease. The search strategy was based on the keyword “cost of illness” and the name of the given CEE country (online Appendix 1). The search was limited to studies published in the past 10 years (1 January 2006—31 October 2016) and was updated on 30 June 2017 to shorten the time between the end of the search period and publication date. No language restrictions were applied. A complementary, non-systematic literature search was conducted in three countries. Three authors (SM, KT, and ZB) hand-searched for further papers in selected, peer-reviewed, non-indexed local journals in Austria, Bulgaria, and Hungary. The review protocol was not registered.
Study selection
After removing duplicates, titles and abstracts of studies were reviewed independently by ZB, VB, and LG, and were retrieved if at least one of the reviewers considered the study to be relevant. First, abstracts (publication type) and reviews (publication type) were excluded. Full-text papers of the remaining studies were reviewed and included (ZB, VB, and LG). Any disagreement between reviewers was solved by discussions among the authors to reach consensus.
Studies were selected for further analysis if they met the following inclusion criteria: (i) COI data included for a specific disease without major restriction on the patient population, e.g., by age, co-morbidity, complication, or treatment, (ii) full-text paper, (iii) original research, and (iv) the study population was recruited in Austria, Bulgaria, the Czech Republic, Croatia, Hungary, Poland, Romania, Slovakia, or Slovenia. Studies were not selected for further analysis if they represented clinical trials, reviews, cost-effectiveness studies, budget impact analyses, treatment-related (drug) studies, costs of health programs (e.g., screening), or studies enrolling a patient population with co-morbidities (e.g., diabetic patient with depression).
Data extraction
A Microsoft Excel spreadsheet was developed to extract data from the identified studies, including general characteristics of the study (year of publication, geographical location, language, and funding source), methodological details of the study (disease, data collection method, study design, setting, costing year, currency, and perspective and costing methods), and results (direct costs, indirect costs, and total costs in euros). The list of extracted variables was created based on health economic checklists and adjusted by screening of six (10%) random articles [6, 15]. Costs reported in currencies other than euro were converted to euro at a mean annual exchange rate, and all costs were inflated to 2017 prices using the harmonised consumer price index extracted from Eurostat [16]. To facilitate cross-country comparisons, costs were also described as a percentage of 2017 GDP per capita. Diseases were categorised according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10 Version:16) [17]. Data extraction was conducted by ZB and respective authors for national languages and double-checked.
Results
Study selection
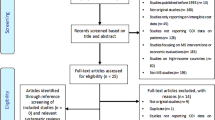
As can be seen from Fig. S1 (online Appendix), after removing 246 duplicates, the search in the electronic databases resulted in 607 potentially relevant papers. Of these studies, 55 were not full-text papers and 98 were reviews. Furthermore, 282 papers did not report disease-related costs, 54 focused on costs of multiple diseases, and 67 focused on the cost of a certain treatment. Overall, 50 articles from the electronic search fulfilled the inclusion criteria. The supplementary local search resulted in another eight relevant articles in non-indexed, peer-reviewed journals (Austria: n = 2, Bulgaria: n = 5, and Hungary: n = 1).
Altogether, we included 58 articles (involving also multi-country studies) that reported results for Hungary (n = 24), Bulgaria (n = 16), Poland (n = 11), Czech Republic (n = 10), Austria (n = 9), Slovenia (n = 4), Croatia (n = 3), Slovakia (n = 3), and Romania (n = 3).
Thirteen additional COI studies did not meet to our eligibility criteria (e.g., involved samples restricted by age, co-morbidity, complication, or treatment), but we found their results worthy of attention, and hence, a summary of their characteristics and main results is presented in online Appendix 1.
Study characteristics
The majority of publications reported costs from one country (74%), but 15 studies presented results from multiple countries, and hence, altogether, 83 country-specific results were provided by 58 studies (Table 1). Three-quarters of the studies were published in English (n = 44), and except for five papers [18,19,20,21,22], all non-English papers had an English abstract. Most of the publications (n = 45, 78%) presented costs in euro. In 37 studies, the national currency was converted to euro; of them, 17 (46%) studies stated explicitly exchange rate, 5 (14%) studies reported only the source of exchange rate, and 15 (40%) studies did not mention conversion at all. Among countries outside the euro zone, reporting costs in national currency was most common in Romania (67%). Overall, 47 (81%) studies stated the source of funding. The lack of a funding statement was most prevalent in Romania (n = 2, 67%) and in Bulgaria (n = 5; 31%). Only two studies received funds from two different sources, both of them were funded by the European Union (EU) and the local government. Regarding clinical areas, endocrine, nutritional, and metabolic diseases were the most common, in which costs were analysed (n = 15 country-specific results), followed by neoplasms (n = 12), and certain infectious and parasitic diseases (n = 10) (Fig. 1). Altogether 48 different diseases were analysed in the 58 included articles.
Methods
Analyses by countries are presented in Table 1. The most frequently used data source was a retrospective, self-completed resource use questionnaire (48%), followed by retrospective claims data analysis (14%) and prospective diary (14%). Sample sizes ranged from n = 2 (small cohorts) to n = 127,512 (large population-based study). Of the 58 studies included in the review, 26 (45%) presented aggregated results for each main cost category (i.e., direct medical, direct non-medical, and indirect). The majority of studies applied the societal perspective (52%), followed by the public payer perspective (17%). If reported, bottom–up (38%) and top–down (21%) methods were used for estimating the costs in the studies. Productivity losses were estimated in 47 (81%) studies; of them, the human capital approach and friction cost method were used in 34 (72%) and 11 (23%) studies, respectively, and the method was not specified in 11 (23%) studies. Studies that reported costs of informal care (n = 29) applied the proxy-good method (17%) or the opportunity cost method (10%), but the name of the applied method was not stated in most of them (69%). Unit costs were not reported at all in 58% of the studies.
Cost-of-illness: comparison across countries in one disease
Eighty-three COI estimates were reported for 48 different diseases. Apart from rare diseases, multiple sclerosis caused the highest economic burden in terms of average total annual cost per patient in three countries (Austria €50,599, the Czech Republic €14,777, and Poland €12,343) [23,24,25]. In Hungary, schizophrenia (€15,187), and in Bulgaria, gestational diabetes (€32,263) were the most costly diseases [22, 26].
Multi-country studies were conducted in nine diagnoses (rotavirus gastroenteritis, pneumonia, bladder cancer, hypoglycaemia, Duchenne muscular dystrophy, epidermolysis bullosa, Prader–Willi syndrome, cystic fibrosis, and haemophilia). One multi-country study (bladder cancer) was conducted in nine countries and another (hypoglycaemia) in six countries. Two studies were conducted (rotavirus gastroenteritis and pneumonia) in four countries and four studies (Duchenne muscular dystrophy, epidermolysis bullosa, Prader–Willi, and haemophilia) in two countries. The bladder cancer study involving nine countries resulted in mean total costs of €7421; however, costs differed significantly among countries, as the total cost was between €2320 (Bulgaria) and €16,479 (Slovenia). The direct medical cost ranged between €1090 (Bulgaria) and €8050 (Slovenia), and indirect cost varied between €912 (Bulgaria) and €6398 (Slovenia). The hypoglycaemia study was conducted in six countries, and the total overall societal cost per patient with diabetes was €11 and ranged between €5 (Bulgaria) and €18 (Slovenia) [27]. Rotavirus gastroenteritis and pneumonia studies were conducted in four countries and the average total costs were €541 and €764, respectively. Costs varied between €494 (Czech Republic) and €747 (Poland) in rotavirus gastroenteritis, and between €472 and €1111 in pneumonia. Duchenne muscular dystrophy, epidermolysis bullosa, Prader–Willi syndrome, cystic fibrosis, and haemophilia were studied in two countries (Hungary and Bulgaria) applying the same methodology in a European Commission founded rare disease study (BURQOL-RD project). Prader–Willi syndrome was the least costly (Bulgaria: €3842 Hungary: €12,532) and mucopolysaccharidosis was the most costly rare disease (Bulgaria: €77,414; Hungary: €25,326) [28, 29].
Unique studies in more than one country were conducted in eight diagnoses, namely multiple sclerosis, dementia, Parkinson’s disease, rheumatoid arthritis, osteoporosis, chronic obstructive pulmonary disease (COPD), systemic sclerosis, and diabetes. Multiple sclerosis and diabetes were studied most often (four studies each), while three unique studies in three different countries were conducted in Parkinson’s disease and two unique studies in three different countries were conducted in cystic fibrosis. Two unique studies on both dementia and COPD were conducted in two different countries. In multiple sclerosis, there was a 4.1 times difference in total costs between Austria (€50,599) and Poland (€12,343) [24, 30]. In diabetes, the highest direct cost was observed in Hungary (€1309) and the lowest total cost was observed in Bulgaria (€472) [31, 32]. In Parkinson’s disease, there was a 3.3 times difference in total costs between Austria (€22,984) and the Czech Republic (€6970) [33, 34]. In dementia, we found a 3.5 times difference in total costs between the Czech Republic (€2013) and Hungary (€671) [35, 36]. The costs of COPD were similar in Bulgaria (€1839) and Romania (€2103) [21, 37].
Adjusting costs for GDP per capita level, differences between countries decreased (Table 2). For instance, a 7.1-fold difference in bladder cancer and a 4.1-fold difference in multiple sclerosis were reduced to 2.4- and 1.5-fold, respectively. Comparing diseases with available cost estimates from more than one country (Fig. 2), a positive relationship was identified between costs and GDP per capita.
Total costs (euro 2017) and GDP per capita (2017): comparison of single-country and multi-country studies. a Single-country studies: each line represents one disease, and each dot represents one study and one country. b Multi-country studies: each line represents one study and one disease, and each dot represents one country
Discussion
A systematic search was conducted to provide a review of the COI studies in nine CEE countries. The diffusion of the new technologies to the health scare systems is enormous, prices, and technologies, and professional guidelines are changing; therefore, our search was limited for the past 10 years. The included papers covered a broad range of clinical areas and showed notable cross-country differences in terms of methodology and publication standards as well as the average yearly costs per patient.
Study characteristics and methodology
Reporting cost results in euros was dominant over national currencies, suggesting that researchers in the CEE region find it important to make their results available for the international scientific community and allow for comparability with other studies. To assess study quality, we selected some quality indicators, such as those are used in health economics checklists. Reporting study perspective, reference year, costing method (top–down vs. bottom–up), source of resource use, valuation of informal care, valuation of productivity loss, and funding source were considered as quality indicators. We find it noteworthy to mention that whilst the source of data on resource utilization and reference year of costing were stated in nearly every paper (98% and 95%, respectively), other important quality indicators were less often reported. The study perspective was reported in 78%, the approach to valuing indirect costs in 77%, costing method in 64%, at least one unit cost in 42%, and method for valuing informal care in 31% of the studies. A recent review of economic evaluations in Austria found that the study perspective and reference year were not reported by 60% and 25% of the studies, respectively [13]. Differences may be explained by inclusion of non-peer-reviewed or grey literature (e.g., economic evaluation reports from national health technology assessment agencies) and of other forms of economic evaluations in the study by Mayer et al. The review by Mayer et al. included 93 (partial and full) economic evaluations, 14 of which were cost-of-illness analyses. Out of the 93 included studies, 23 were not indexed according to the Journal Citation Reports (Social) Sciences Edition and 12 were non-peer-reviewed reports [13].
Clinical areas
A large variety of diseases was covered by the studies, and most of them occurred in a one study. Each disease was studied by, on average, 1.3 papers. Considering country-specific results by ICD categories, endocrine, nutritional, and metabolic diseases (18%), neoplasms (14%), infectious (12%), neurologic (11%), and musculoskeletal diseases (11%) represented the five main fields of COI research in CEE. It is difficult to judge the drivers of the selection of clinical fields. The public health importance of a disease might be an important factor as, for instance, all the studies in the ‘Endocrine, nutritional and metabolic diseases’ ICD category were related to diabetes, and among neoplasms studies, the most prevalent malignancies (breast, colorectal, lung, and prostate cancer) were present (Table 2). According to the Global Burden of Disease study, the leading three causes of total Disability-Adjusted Life Years (DALY) included ischaemic heart disease, cerebrovascular disease, and lower respiratory infection, comprising 16% of all DALYs [38]. Leading causes of DALYs were represented only in six (10%) studies (cerebrovascular disease: n = 1, ischaemic heart disease: n = 2, and lower respiratory infection: n = 3) in our review, questioning public health importance as a driver of topic selection in COI studies. The need for COI data to support decision-making on reimbursement of highly effective but costly new drugs seems to be another relevant issue, and this hypothesis is supported by the relatively high rate of studies in inflammatory rheumatic diseases, where biological drugs were introduced in the CEE countries in the observed period. Multiple sclerosis is another disorder where biologicals revolutionized the treatment that partly explains the relatively high rate of neurological studies in the region. Moreover, when counting papers, neurologic diseases were most frequently studied (19%). A possible explanation could be that neurologic conditions in the CEE region were priorities for state-funded or EU-funded research. Eight out of the ten COI studies focusing on neurologic diseases received funding from the local governments or EU organisations. It is interesting that neurologic diseases were found also the most frequently studied clinical area according to a recently published systematic review of EQ-5D studies in the CEE region [39]. These results suggest that neurologic diseases have a high priority in health economics research in the CEE.
Comparison of costs across countries
With respect to diseases for which cost estimates were present in multiple countries, costs varied substantially across countries. However, there are apparent differences in the level of comparability between studies. There were multi-country studies following a standardized methodology in which more than one CEE country together with Western European countries was participated. We also identified single-country studies in various diseases using very different methods. Both multi-country and single-country studies reported significant cost differences in diseases across countries.
For the interpretation of data, it is important to take into consideration that the number of patients, sample characteristics (e.g., age, gender, disease duration, and disease severity), and the availability of costly treatments at the time of the study (e.g., biological drugs for inflammatory diseases) varied a great deal across studies that may strongly influence the COI results and their comparability. Large differences in unit costs can also cause significant variations in costs. In bladder cancer, for example, the cost of an inpatient day was seven times higher in Austria (€495) than in Romania (€67). Methodological differences, such as prevalence- and incidence-based costing, form an obstacle for the comparison of costs. Therefore, the incidence-based prostate cancer study by Brodszky et al. cannot be compared with the prevalence-based prostate cancer study by Inotai et al., although both studies were conducted in Hungary [40, 41]. It should also be noted that differences in health care systems (private/public, financing, etc.) might have a significant impact on costs; for instance, global budget, fee-for-service or DRG financing mechanisms, the presence of co-payments, minor or major share of private services, and many more aspects might influence the actual costs, access to health care, and, finally, the COI figure [42].
According to the literature, one might expect a higher COI in a country with a higher GDP [43,44,45]. In many diseases (multiple sclerosis, bladder cancer, Parkinson’s disease, rheumatoid arthritis, Prader–Willi syndrome, haemophilia, diabetes, and hypoglycaemia), there was a clear positive association between total costs and GDP per capita. As opposed to this, cost estimates, sometimes, inversely correlated with the per capita GDP. For instance, GDP per capita in Bulgaria is almost half of that in Hungary; nevertheless, costs of mucopolysaccharidosis were threefold higher in Bulgaria. Thus, in some cases, adjusting costs for the GDP further increased the inter-country differences. On the other hand, the 3.5-fold higher GDP per capita in Austria decreased the cross-country differences (from 4- to 1.3-fold) in costs of multiple sclerosis. In spite of the considerable heterogeneity observed in the studies included in this review, some trends could be identified. The magnitude of costs increased with the level of per capita GDP. In other words, cross-country differences decreased or even vanished when the costs were adjusted. In contrast, higher costs with lower GDP per capita could be observed only in some rare diseases (cystic fibrosis, epidermolysis bullosa, and mucopolysaccharidosis) and rotavirus gastroenteritis. Moreover, methodological differences did not seem to affect this relationship. Comparing multi-country studies in a disease applied the same methodology for more than one country and single-country studies analysed costs in the same disease, the relationship between cost-of-illness and GDP per capita showed similar pattern in these two groups of studies (see Fig. 2).
Quality, publication standards, and the assessment of transferability
Cost-of-illness studies varied considerably both in methods and in cost estimates, and serve many purposes. Methodological deficiencies, such as the lack of reporting either on the three distinct phases of costing (identifying the relevant cost items, measuring the use of the identified resources, and placing a value on these cost items) [46], or other important characteristics such as the perspective of the study, related to the production function (direct and indirect costs) were the leading causes of shortcomings in comparability. However, no specific costing guidelines for health care interventions are available in these countries, and except in Austria, there is no national cost database available, providing some kind of unit cost data in a collected form [13, 47, 48]. Another important difficulty in costing relates to the different Managed Entry Agreements (MEA), such as price volume agreements, discounts, outcome guarantees, and many more, in the reimbursement of the health technologies in the different countries [49, 50]. Due to the MEAs, for instance, the real purchasing price of the medicinal products is not publicly available.
Several papers were published about transferability in the past 2 decades [51,52,53,54,55,56]. At the moment, health economics and health technology assessment guidelines in CEE countries either include very limited advice or provide no guidance on the transferability or adaptation of clinical and economic data from other jurisdictions. Thus, establishing better guidelines for COI studies on transferability would be valuable for robust decision-making in the CEE countries [56]. As Gao et al. stated, confirming the transferability of COI estimates across jurisdictions would contribute significantly to resolving the issue of transferability of cost-effectiveness results [45]. Transferability is a very important issue around the world and especially in Central or Eastern Europe with limited resources to provide COI studies [53,54,55]. Data transferability and transferability of the results are not discussed in these COI studies. Both should be improved using Drummond’s check list for evaluating economic evaluations [57]. Transferability might be an important alternative to conduct local COIs. However, due to the methodological, data, and publication heterogeneity, the usefulness of the COI results in other jurisdictions is limited.
Limitations
There are a few limitations to note. A systematic approach was taken to identify studies that have considered the costs of diseases; however, the possibility that relevant studies were not identified and included in this systematic literature review remains. Some COI results might have been missed due to excluding grey literature (i.e., conference abstracts and project reports) from our search. Other limitationis that the local search in non-indexed journals was conducted only in three of the nine countries. On the other hand, no language restriction was applied in the systematic search. Adopting a Medical Subject Heading (MeSH)-based search strategy may have led to missing some studies using keywords improperly. At the same time, the PubMed search engine uses a broad range of entry terms which may minimize the number of excluded studies. Further limitation is that no comprehensive checklist was applied, because, according to our best knowledge, there is no COI study-specific checklist in English. This might bias our conclusions on study quality, but we believe that the presented study characteristics could give a good overall description of the included studies.
Conclusions
Fifty-eight COI studies were identified between 1 January 2006 and 30 June 2017 published in Austria, Bulgaria, the Czech Republic, Croatia, Hungary, Poland, Romania, Slovakia, and Slovenia, providing 83 country-specific COI results. Endocrine, nutritional, and metabolic diseases, neoplasms, infectious disease, and neurological disorders were the most frequently studied clinical areas. Transferability might be an important alternative to conduct local COIs. However, due to the methodological, data, and publication heterogeneity of these 58 COI studies, the transferability is limited across the nine Central and Eastern European Countries.
References
Drummond, M., Sculpher, M., Torrance, G.: Methods for the economic evaluation of health care programmes, 3rd edn. Oxford University PressOxford University Press, Oxford (2005)
Tarricone, R.: Cost-of-illness analysis. What room in health economics? Health Policy 77(1), 51–63 (2006). https://doi.org/10.1016/j.healthpol.2005.07.016
Gulacsi, L., Rotar, A.M., Niewada, M., Loblova, O., Rencz, F., Petrova, G., Boncz, I., Klazinga, N.S.: Health technology assessment in Poland, the Czech Republic, Hungary, Romania and Bulgaria. Eur. J. Health Econ. 15(Suppl 1), S13–S25 (2014). https://doi.org/10.1007/s10198-014-0590-8
Boncz, I., Sebestyen, A.: Financial deficits in the health services of the UK and Hungary. Lancet 368(9539), 917–918 (2006). https://doi.org/10.1016/S0140-6736(06)69369-0
Feig, C., Cheung, K.L., Hiligsmann, M., Evers, S., Simon, J., Mayer, S.: Best-worst scaling to assess the most important barriers and facilitators for the use of health technology assessment in Austria. Expert Rev. Pharmacoecon. Outcomes Res. (2017). https://doi.org/10.1080/14737167.2017.1375407
Larg, A., Moss, J.R.: Cost-of-illness studies: a guide to critical evaluation. PharmacoEconomics 29(8), 653–671 (2011). https://doi.org/10.2165/11588380-000000000-00000
Jacobs, P., Ohinmaa, A., Brady, B.: Providing systematic guidance in pharmacoeconomic guidelines for analysing costs. Pharmacoeconomics 23(2), 143–153 (2005)
Brouwer, W., Rutten, F., Koopmanschap, M.: Costing in economic evaluations. In: Drummond, M., McGuire, A. (eds.) Economic evaluations in health care: merging theory with practice. Oxford University Press, New York (2001)
Angelis, A., Tordrup, D., Kanavos, P.: Socio-economic burden of rare diseases: a systematic review of cost of illness evidence. Health Policy 119(7), 964–979 (2015). https://doi.org/10.1016/j.healthpol.2014.12.016
Onukwugha, E., McRae, J., Kravetz, A., Varga, S., Khairnar, R., Mullins, C.D.: Cost-of-illness studies: an updated review of current methods. PharmacoEconomics 34(1), 43–58 (2016). https://doi.org/10.1007/s40273-015-0325-4
Clabaugh, G., Ward, M.M.: Cost-of-illness studies in the United States: a systematic review of methodologies used for direct cost. Value Health 11(1), 13–21 (2008). https://doi.org/10.1111/j.1524-4733.2007.002
Oliva-Moreno, J., Trapero-Bertran, M., Pena-Longobardo, L.M., Del Pozo-Rubio, R.: The valuation of informal care in cost-of-illness studies: a systematic review. PharmacoEconomics 35(3), 331–345 (2017). https://doi.org/10.1007/s40273-016-0468-y
Mayer, S., Kiss, N., Laszewska, A., Simon, J.: Costing evidence for health care decision-making in Austria: a systematic review. PLoS One 12(8), e0183116 (2017). https://doi.org/10.1371/journal.pone.0183116
Moher, D., Liberati, A., Tetzlaff, J., Altman, D.G.: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6(7), e1000097 (2009). https://doi.org/10.1371/journal.pmed.1000097
Husereau, D., Drummond, M., Petrou, S., Carswell, C., Moher, D., Greenberg, D., Augustovski, F., Briggs, A.H., Mauskopf, J., Loder, E., Force, C.T.: Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health 16(2), e1–e5 (2013). https://doi.org/10.1016/j.jval.2013.02.010
Eurostat: ECU/EUR exchange rates versus national currencies. http://ec.europa.eu/eurostat/estat-navtree-portlet-prod/NodeInfoServices?lang=en&code=tec00033 Accessed 19 Sep 2017
International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10 Version:2016) (2016). http://apps.who.int/classifications/icd10/browse/2016/en Accessed 21 Jul 2017
Georgieva, V.: Pharmacoeconomic evaluation of surgical and endovascular therapy of brain aneurism. PhD thesis, Medical University of Sofia (2015)
Glogovska, P., Ivanov, Y., Hristova, P., Pavlov, P., Popova, T.E.B.: Pharmacoeconomic evaluation of the ambulatory and hospital cost of therapy of patients with community acquired respiratory tract infections. Thorasis Med. 2(3), 52–56 (2010)
Ivanova, Z., Veleva, N., Glogovska, P., Pavlov, P., Popova, T., Ivanov, Y.: Hospital therapy cost of patients with asthma. MEDINFO 14(12), 28–31 (2014)
Kyuchukov, N., Yanev, N., Krachunov, I., Ivanova, Z., Pavlov, P., Popova, T., Glogovska, P., Hristova, P., Ivanov, Y.: Evaluation of COPD cost for patients on home oxygen therapy. Thorasic Med. 7(4), 50–56 (2015)
Todorova, K.: Pharmacoeconomic analysis of the gestational diabetes therapy during pregnancy. PhD thesis, Medical University of Sofia (2007)
Blahova Dusankova, J., Kalincik, T., Dolezal, T., Kobelt, G., Havrdova, E.: Cost of multiple sclerosis in the Czech Republic: the COMS study. Mult. Scler. 18(5), 662–668 (2012). https://doi.org/10.1177/1352458511424422
Kobelt, G., Berg, J., Lindgren, P., Plesnilla, C., Baumhackl, U., Berger, T., Kolleger, H., Vass, K.: Costs and quality of life of multiple sclerosis in Austria. Eur. J. Health Econ. 7(Suppl 2), S14–S23 (2006). https://doi.org/10.1007/s10198-006-0382-x
Szmurlo, D., Fundament, T., Ziobro, M., Kruntoradova, K., Dolezal, T., Glogowski, C.: Costs of multiple sclerosis—extrapolation of Czech data to Polish patients. Expert Rev. Pharmacoecon. Outcomes Res. 14(3), 451–458 (2014). https://doi.org/10.1586/14737167.2014.906305
Pentek, M., Harangozo, J., Egerhazi, A., Kelemen, O., Gulacsi, L., Baji, P., Mattyassy, A., Erdelyi, R., Lehoczky, S., Orlewska, E., Vartokne Hever, N., Ferencz, A., Brodszky, V.: Health related quality of life and disease burden of patients with schizophrenia in Hungary. Psychiatr. Hung. 27(1), 4–17 (2012)
Jakubczyk, M., Lipka, I., Paweska, J., Niewada, M., Rdzanek, E., Zaletel, J., Ramirez de Arellano, A., Dolezal, T., Chekorova Mitreva, B., Nagy, B., Petrova, G., Saric, T., Yfantopoulos, J., Czech, M.: Cost of severe hypoglycaemia in nine European countries. J. Med. Econ. 19(10), 973–982 (2016). https://doi.org/10.1080/13696998.2016.1188823
Lopez-Bastida, J., Linertova, R., Oliva-Moreno, J., Posada-de-la-Paz, M., Serrano-Aguilar, P., Kanavos, P., Taruscio, D., Schieppati, A., Iskrov, G., Baji, P., Delgado, C., von der Schulenburg, J.M., Persson, U., Chevreul, K., Fattore, G., Network, B.-R.R.: Social/economic costs and health-related quality of life in patients with Prader-Willi syndrome in Europe. Eur J Health Econ 17(Suppl 1), 99–108 (2016). https://doi.org/10.1007/s10198-016-0788-z
Pentek, M., Gulacsi, L., Brodszky, V., Baji, P., Boncz, I., Pogany, G., Lopez-Bastida, J., Linertova, R., Oliva-Moreno, J., Serrano-Aguilar, P., Posada-de-la-Paz, M., Taruscio, D., Iskrov, G., Schieppati, A., von der Schulenburg, J.M., Kanavos, P., Chevreul, K., Persson, U., Fattore, G., Network, B.-R.R.: Social/economic costs and health-related quality of life of mucopolysaccharidosis patients and their caregivers in Europe. Eur J Health Econ 17(Suppl 1), 89–98 (2016). https://doi.org/10.1007/s10198-016-0787-0
Pentek, M., Gulacsi, L., Rozsa, C., Simo, M., Iljicsov, A., Komoly, S., Brodszky, V.: Health status and costs of ambulatory patients with multiple sclerosis in Hungary. Ideggyogy Sz 65(9–10), 316–324 (2012)
Nerat, T., Kos, M.: Burden of Type 2 Diabetes from the Healthcare Payer Perspective in Slovenia/Breme Sladkorne Bolezni Tipa 2 S Stališča Plačnika Zdravstvenega Varstva V Sloveniji. Slov. J. Public Health (2013). https://doi.org/10.2478/sjph-2013-0018
Valov, V., Doneva, M., Borisova, A.M., Tankova, T., Czech, M., Manova, M., Savova, A., Peikova, L., Petrova, G.: Regional differences in diabetic patients’ pharmacotherapy in Bulgaria. Eur Rev Med Pharmacol Sci 18(10), 1499–1506 (2014)
von Campenhausen, S., Winter, Y., Gasser, J., Seppi, K., Reese, J.P., Pfeiffer, K.P., Geiger-Gritsch, S., Botzel, K., Siebert, U., Oertel, W.H., Dodel, R., Poewe, W.: Cost of illness and health service patterns in Morbus Parkinson in Austria. Wien. Klin. Wochenschr. 121(17–18), 574–582 (2009). https://doi.org/10.1007/s00508-009-1223-6
Winter, Y., von Campenhausen, S., Brozova, H., Skoupa, J., Reese, J.P., Botzel, K., Eggert, K., Oertel, W.H., Dodel, R., Ruzicka, E.: Costs of Parkinson’s disease in eastern Europe: a Czech cohort study. Parkinsonism Relat Disord 16(1), 51–56 (2010). https://doi.org/10.1016/j.parkreldis.2009.07.005
Ersek, K., Kovacs, T., Wimo, A., Karpati, K., Brodszky, V., Pentek, M., Jonsson, L., Gustavsson, A., McDaid, D., Kenigsberg, P.A., Valtonen, H., Gulacsi, L.: Costs of dementia in Hungary. J Nutr Health Aging 14(8), 633–639 (2010)
Holmerova, I., Hort, J., Rusina, R., Wimo, A., Steffl, M.: Costs of dementia in the Czech Republic. Eur J Health Econ (2016). https://doi.org/10.1007/s10198-016-0842-x
Stambu, I., Stoicescu, I.P.: Estimation of direct medical costs of chronic obstructive pulmonary disease over 12 months. Pneumologia 62(2), 86–92 (2013)
DALYs G, Collaborators, H.: Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390(10100), 1260–1344 (2017). https://doi.org/10.1016/s0140-6736(17)32130-x
Rencz, F., Gulacsi, L., Drummond, M., Golicki, D., Prevolnik Rupel, V., Simon, J., Stolk, E.A., Brodszky, V., Baji, P., Zavada, J., Petrova, G., Rotar, A., Pentek, M.: EQ-5D in Central and Eastern Europe: 2000–2015. Qual. Life Res. 25(11), 2693–2710 (2016). https://doi.org/10.1007/s11136-016-1375-6
Brodszky, V., Varga, P., Gimesi-Orszagh, J., Fadgyas-Freyler, P., Boncz, I., Nyirady, P., Riesz, P., Baji, P., Pentek, M., Rencz, F., Gulacsi, L.: Long-term costs and survival of prostate cancer: a population-based study. Int. Urol. Nephrol. 49(10), 1707–1714 (2017). https://doi.org/10.1007/s11255-017-1669-9
Inotai, A., Abonyi-Tóth, Z., Rokszin, G., Vokó, Z.: Prognosis, cost, and occurrence of colorectal, lung, breast, and prostate cancer in hungary. Value Health Reg Issues 7, 1–8 (2015). https://doi.org/10.1016/j.vhri.2015.03.020
European Observatory on Health Systems and Policies: Health system reviews (HiT series) http://www.euro.who.int/en/about-us/partners/observatory/publications/health-system-reviews-hits/full-list-of-country-hits. Accessed 03 Nov 2018
Seuring, T., Archangelidi, O., Suhrcke, M.: The economic costs of type 2 diabetes: a global systematic review. Pharmacoeconomics 33(8), 811–831 (2015). https://doi.org/10.1007/s40273-015-0268-9
Zhao, F.L., Xie, F., Hu, H., Li, S.C.: Transferability of indirect cost of chronic disease: a systematic review and meta-analysis. Pharmacoeconomics 31(6), 501–508 (2013). https://doi.org/10.1007/s40273-013-0053-6
Gao, L., Hu, H., Zhao, F.L., Li, S.C.: Can the direct medical cost of chronic disease be transferred across different countries? Using cost-of-illness studies on type 2 diabetes, epilepsy and schizophrenia as examples. PLoS One 11(1), e0147169 (2016). https://doi.org/10.1371/journal.pone.0147169
Brouwer, W., Rutten, F., Koopmanschap, M.: Costing in economic evaluations, chapter 4 p. 68-94. In: Drummond, M., McGuire, A. (eds.) Economic Evaluation in Health Care. OHE Oxford University Press, Oxford (2001)
Mayer, S., Kiss, N., Laszewska, A., Simon, J.: Health economic costing methods and reporting in Austria. Value Health 19(7), A363 (2016)
DHE Unit Cost Online Database: Cost Collection from Existing Studies. Version 2.2/2017. Vienna: Department of Health Economics (DHE), Center for Public Health, Medical University of Vienna (2017)
Rotar, A.M., Preda, A., Löblová, O., Benkovic, Zawodnik, V.S., Gulacsi, L., Niewada, M., Boncz, I., Petrova, G., Dimitrova, M., Klazinga, N.: Rationalizing the introduction and use of pharmaceutical products: the role of managed entry agreements in Central and Eastern European countries, 2018, Health Policy accepted for publication (2018)
Ferrario A, Kanavos P. Managed entry agreements for pharmaceuticals: the European experience. 2013. EMiNet, Brussels, Belgium. http://eprints.lse.ac.uk/50513/1/__Libfile_repository_Content_Ferrario%2C%20A_Ferrario_Managed_%20entry_%20agreements_2013_Ferrario_Managed_%20entry_%20agreements_2013.pdf. Accessed 02 Nov 2018
Nixon, J., Rice, S., Drummond, M., Boulenger, S., Ulmann, P., de Pouvourville, G.: Guidelines for completing the EURONHEED transferability information checklists. Eur. J. Health Econ. 10(2), 157–165 (2009). https://doi.org/10.1007/s10198-008-0115-4
de Pouvourville, G., Ulmann, P., Nixon, J., Boulenger, S., Glanville, J., Drummond, M.: The diffusion of health economics knowledge in Europe: the EURONHEED (European Network of Health Economics Evaluation Database) project. Pharmacoeconomics 23(2), 113–120 (2005)
Drummond, M., Brown, R., Fendrick, A.M., Fullerton, P., Neumann, P., Taylor, R., Barbieri, M., Force, I.T.: Use of pharmacoeconomics information–report of the ISPOR task force on use of pharmacoeconomic/health economic information in health-care decision making. Value Health 6(4), 407–416 (2003)
Barbieri, M., Drummond, M., Rutten, F., Cook, J., Glick, H.A., Lis, J., Reed, S.D., Sculpher, M., Severens, J.L., Force, I.G.R.P.E.D.T.T.: What do international pharmacoeconomic guidelines say about economic data transferability? Value Health 13(8), 1028–1037 (2010). https://doi.org/10.1111/j.1524-4733.2010.00771.x
Mandrik, O., Knies, S., Kalo, Z., Severens, J.L.: Reviewing transferability in economic evaluations originating from Eastern Europe. Int. J. Technol. Assess. Health Care 31(6), 434–441 (2015). https://doi.org/10.1017/S0266462315000677
Gulacsi, L., Rencz, F., Pentek, M., Brodszky, V., Lopert, R., Hever, N.V., Baji, P.: Transferability of results of cost utility analyses for biologicals in inflammatory conditions for Central and Eastern European countries. Eur. J. Health Econ. 15(Suppl 1), S27–S34 (2014). https://doi.org/10.1007/s10198-014-0591-7
Drummond, M.F., Sculpher, M.J., Torrance, G.W., O’Brien, B.J., Stoddart, G.L.: Methods for the Economic Evaluation of Health care Programmes, 4th edn. Oxford University Press, Oxford (2015)
Grabmeier-Pfistershammer, K., Rieger, A., Schrock, T., Schlag, M.: Economic burden of late presentation in HIV disease in Austria: a comparison of the initial costs imposed by advanced HIV disease vs. non-late presentation. Wien Klin Wochenschr 125(13-14), 402–407 (2013). https://doi.org/10.1007/s00508-013-0392-5
Leal, J., Luengo-Fernandez, R., Sullivan, R., Witjes, J.A.: Economic burden of bladder cancer across the European union. Eur. Urol. 69(3), 438–447 (2016). https://doi.org/10.1016/j.eururo.2015.10.024
Prast, J., Oppelt, P., Shamiyeh, A., Shebl, O., Brandes, I., Haas, D.: Costs of endometriosis in Austria: a survey of direct and indirect costs. Arch. Gynecol. Obstet. 288(3), 569–576 (2013). https://doi.org/10.1007/s00404-013-2793-0
Willich, S.N., Nocon, M., Kulig, M., Jaspersen, D., Labenz, J., Meyer-Sabellek, W., Stolte, M., Lind, T., Malfertheiner, P.: Cost-of-disease analysis in patients with gastro-oesophageal reflux disease and Barrett’s mucosa. Aliment. Pharmacol. Ther. 23(3), 371–376 (2006). https://doi.org/10.1111/j.1365-2036.2006.02763.x
Dimai, H.P., Redlich, K., Schneider, H., Siebert, U., Viernstein, H., Mahlich, J.: Direct and indirect costs of fractures due to osteoporosis in Austria. Gesundheitswesen 74(10), e90–e98 (2012). https://doi.org/10.1055/s-0031-1301274
Wagner, E.: Direct costs of osteoarthritis. Wien. Med. Wochenschr. 161(1–2), 44–52 (2011). https://doi.org/10.1007/s10354-010-0858-2
Wagner, E.: Costs of non-specific low back pain in Austria. Wien. Med. Wochenschr. 162(5–6), 92–98 (2012). https://doi.org/10.1007/s10354-011-0050-3
Angelis, A., Kanavos, P., Lopez-Bastida, J., Linertova, R., Oliva-Moreno, J., Serrano-Aguilar, P., Posada-de-la-Paz, M., Taruscio, D., Schieppati, A., Iskrov, G., Brodszky, V., von der Schulenburg, J.M., Chevreul, K., Persson, U., Fattore, G., Network, B.-R.R.: Social/economic costs and health-related quality of life in patients with epidermolysis bullosa in Europe. Eur. J. Health Econ. 17(Suppl 1), 31–42 (2016). https://doi.org/10.1007/s10198-016-0783-4
Cavazza, M., Kodra, Y., Armeni, P., De Santis, M., Lopez-Bastida, J., Linertova, R., Oliva-Moreno, J., Serrano-Aguilar, P., Posada-de-la-Paz, M., Taruscio, D., Schieppati, A., Iskrov, G., Gulacsi, L., von der Schulenburg, J.M., Kanavos, P., Chevreul, K., Persson, U., Fattore, G., Network, B.-R.R.: Social/economic costs and quality of life in patients with haemophilia in Europe. Eur. J. Health Econ. 17(Suppl 1), 53–65 (2016). https://doi.org/10.1007/s10198-016-0785-2
Cavazza, M., Kodra, Y., Armeni, P., De Santis, M., Lopez-Bastida, J., Linertova, R., Oliva-Moreno, J., Serrano-Aguilar, P., Posada-de-la-Paz, M., Taruscio, D., Schieppati, A., Iskrov, G., Pentek, M., von der Schulenburg, J.M., Kanavos, P., Chevreul, K., Persson, U., Fattore, G., Network, B.-R.R.: Social/economic costs and health-related quality of life in patients with Duchenne muscular dystrophy in Europe. Eur. J. Health Econ. 17(Suppl 1), 19–29 (2016). https://doi.org/10.1007/s10198-016-0782-5
Chevreul, K., Michel, M., Brigham, K.B., Lopez-Bastida, J., Linertova, R., Oliva-Moreno, J., Serrano-Aguilar, P., Posada-de-la-Paz, M., Taruscio, D., Schieppati, A., Iskrov, G., Pentek, M., von der Schulenburg, J.M., Kanavos, P., Persson, U., Fattore, G., Network, B.-R.R.: Social/economic costs and health-related quality of life in patients with cystic fibrosis in Europe. Eur. J. Health Econ. 17(Suppl 1), 7–18 (2016). https://doi.org/10.1007/s10198-016-0781-6
Iskrov, G., Astigarraga, I., Stefanov, R., Lopez-Bastida, J., Linertova, R., Oliva-Moreno, J., Serrano-Aguilar, P., Posada-de-la-Paz, M., Schieppati, A., Taruscio, D., Pentek, M., von der Schulenburg, J.M., Kanavos, P., Chevreul, K., Persson, U., Fattore, G., Network, B.-R.R.: Social/economic costs and health-related quality of life in patients with histiocytosis in Europe. Eur. J. Health Econ. 17(Suppl 1), 67–78 (2016). https://doi.org/10.1007/s10198-016-0790-5
Iskrov, G.G., Stefanov, R.S., Lopez-Bastida, J., Linertova, R., Oliva-Moreno, J., Serrano-Aguilar, P., Network, B.-R.R.: Economic burden and health-related quality of life of patients with cystic fibrosis in Bulgaria. Folia Med (Plovdiv) 57(1), 56–64 (2015). https://doi.org/10.1515/folmed-2015-0020
Bencina, G., Buljan, M., Situm, M., Stevanovic, R., Benkovic, V.: Health and economic burden of skin melanoma in croatia—cost-of-illness study. Acta Dermatovenerol. Croat. 25(1), 1–7 (2017)
Klimeš, J., Vocelka, M., Šedová, L., Doležal, T., Mlčoch, T., Petříková, A., Vlček, J.: Medical and productivity costs of rheumatoid arthritis in The Czech Republic: cost-of-illness study based on disease severity. Value in Health Regional Issues 4, 75–81 (2014). https://doi.org/10.1016/j.vhri.2014.07.004
Maresova, P., Zahalkova, V.: The economic burden of the care and treatment for people with Alzheimer’s disease: the outlook for the Czech Republic. Neurol Sci 37(12), 1917–1922 (2016). https://doi.org/10.1007/s10072-016-2679-6
Mlcoch, T., Klimes, J., Fila, L., Vavrova, V., Skalicka, V., Turnovec, M., Krulisova, V., Jircikova, J., Zemkova, D., Dedeckova, K.V., Bilkova, A., Fruhaufova, V., Homola, L., Friedmannova, Z., Drnek, R., Drevinek, P., Dolezal, T., Macek Jr., M.: Cost-of-illness analysis and regression modeling in cystic fibrosis: a retrospective prevalence-based study. Eur. J. Health Econ. 18(1), 73–82 (2017). https://doi.org/10.1007/s10198-015-0759-9
Tichopad, A., Mullerova, J., Jackowska, T., Nemes, E., Pazdiora, P., Sloesen, B., Stefkovicova, M.: Cost burden of severe community-acquired rotavirus gastroenteritis requiring hospitalization in the Czech Republic, Slovakia, Poland, and Hungary: a retrospective patient chart review. Value Health Reg Issues 10, 53–60 (2016). https://doi.org/10.1016/j.vhri.2016.07.005
Tichopad, A., Roberts, C., Gembula, I., Hajek, P., Skoczynska, A., Hryniewicz, W., Jahnz-Rozyk, K., Prymula, R., Solovic, I., Kolek, V.: Clinical and economic burden of community-acquired pneumonia among adults in the Czech Republic, Hungary, Poland and Slovakia. PLoS One 8(8), e71375 (2013). https://doi.org/10.1371/journal.pone.0071375
Balogh, O., Brodszky, V., Gulacsi, L., Heredi, E., Herszenyi, K., Jokai, H., Karpati, S., Baji, P., Remenyik, E., Szegedi, A., Hollo, P.: Cost-of-illness in patients with moderate to severe psoriasis: a cross-sectional survey in Hungarian dermatological centres. Eur. J. Health Econ. 15(Suppl 1), S101–S109 (2014). https://doi.org/10.1007/s10198-014-0599-z
Brodszky, V., Balint, P., Geher, P., Hodinka, L., Horvath, G., Koo, E., Pentek, M., Polgar, A., Sesztak, M., Szanto, S., Ujfalussy, I., Gulacsi, L.: Disease burden of psoriatic arthritis compared to rheumatoid arthritis, Hungarian experiment. Rheumatol Int 30(2), 199–205 (2009). https://doi.org/10.1007/s00296-009-0936-1
Chevreul, K., Gandre, C., Brigham, K.B., Lopez-Bastida, J., Linertova, R., Oliva-Moreno, J., Serrano-Aguilar, P., Posada-de-la-Paz, M., Taruscio, D., Schieppati, A., Iskrov, G., Gulacsi, L., von der Schulenburg, J.M., Kanavos, P., Persson, U., Fattore, G., Network, B.-R.R.: Social/economic costs and health-related quality of life in patients with fragile X syndrome in Europe. Eur. J. Health Econ. 17(Suppl 1), 43–52 (2016). https://doi.org/10.1007/s10198-016-0784-3
Gulacsi, L., Majer, I., Boncz, I., Brodszky, V., Merkely, B., Maurovich, H.P., Karpati, K.: Health care costs of acute myocardial infarction in Hungary, 2003–2005. Orv. Hetil. 148(27), 1259–1266 (2007). https://doi.org/10.1556/OH.2007.28109
Lopez-Bastida, J., Linertova, R., Oliva-Moreno, J., Serrano-Aguilar, P., Posada-de-la-Paz, M., Kanavos, P., Taruscio, D., Schieppati, A., Iskrov, G., Pentek, M., Delgado, C., von der Schulenburg, J.M., Persson, U., Chevreul, K., Fattore, G., Network, B.-R.R.: Social/economic costs and health-related quality of life in patients with scleroderma in Europe. Eur. J. Health Econ. 17(Suppl 1), 109–117 (2016). https://doi.org/10.1007/s10198-016-0789-y
Minier, T., Pentek, M., Brodszky, V., Ecseki, A., Karpati, K., Polgar, A., Czirjak, L., Gulacsi, L.: Cost-of-illness of patients with systemic sclerosis in a tertiary care centre. Rheumatology (Oxford) 49(10), 1920–1928 (2010). https://doi.org/10.1093/rheumatology/keq165
Pentek, M., Bereczki, D., Gulacsi, L., Mikudina, B., Aranyi, Z., Juhos, V., Baji, P., Brodszky, V.: Survey of adults living with epilepsy in Hungary: health-related quality of life and costs. Ideggyogy Sz 66(7–8), 251–261 (2013)
Rencz, F., Kovacs, A., Brodszky, V., Gulacsi, L., Nemeth, Z., Nagy, G.J., Nagy, J., Buzogany, I., Boszormenyi-Nagy, G., Majoros, A., Nyirady, P.: Cost of illness of medically treated benign prostatic hyperplasia in Hungary. Int. Urol. Nephrol. 47(8), 1241–1249 (2015). https://doi.org/10.1007/s11255-015-1028-7
Tamas, G., Gulacsi, L., Bereczki, D., Baji, P., Takats, A., Brodszky, V., Pentek, M.: Quality of life and costs in Parkinson’s disease: a cross sectional study in Hungary. PLoS One 9(9), e107704 (2014). https://doi.org/10.1371/journal.pone.0107704
Pentek, M., Kobelt, G., Czirjak, L., Szekanecz, Z., Poor, G., Rojkovich, B., Polgar, A., Genti, G., Kiss, C.G., Brodszky, V., Majer, I., Gulacsi, L.: Costs of rheumatoid arthritis in Hungary. J. Rheumatol. 34(6), 1437 (2007)
Czech, M., Rosinska, M., Rogalska, J., Staszewska, E., Stefanoff, P.: Costs of medically attended acute gastrointestinal infections: the polish prospective healthcare utilization survey. Value Health Reg Issues 2(2), 210–217 (2013). https://doi.org/10.1016/j.vhri.2013.06.011
Dubas-Jakobczyk, K., Kocot, E., Seweryn, M., Koperny, M.: Production lost due to cervical cancer in Poland in 2012. Med. Pr. 67(3), 289–299 (2016). https://doi.org/10.13075/mp.5893.00378
Jaworski, R., Jankowska, E.A., Ponikowski, P., Banasiak, W.: Costs of management of patients with coronary artery disease in Poland: the multicenter RECENT study. Pol. Arch. Med. Wewn. 122(12), 599–607 (2012)
Kawalec, P.P., Malinowski, K.P.: The indirect costs of systemic autoimmune diseases, systemic lupus erythematosus, systemic sclerosis and sarcoidosis: a summary of 2012 real-life data from the Social Insurance Institution in Poland. Expert Rev Pharmacoecon Outcomes Res 15(4), 667–673 (2015). https://doi.org/10.1586/14737167.2015.1065733
Lesniowska, J., Schubert, A., Wojna, M., Skrzekowska-Baran, I., Fedyna, M.: Costs of diabetes and its complications in Poland. Eur. J. Health Econ. 15(6), 653–660 (2014). https://doi.org/10.1007/s10198-013-0513-0
Czech, M., Opolski, G., Zdrojewski, T., Dubiel, J.S., Wizner, B., Bolisega, D., Fedyk-Lukasik, M., Grodzicki, T.: The costs of heart failure in Poland from the public payer’s perspective. Polish programme assessing diagnostic procedures, treatment and costs in patients with heart failure in randomly selected outpatient clinics and hospitals at different levels of care: POLKARD. Kardiol Pol 71(3), 224–232 (2013). https://doi.org/10.5603/kp.2013.0032
Stoicescu, I.P., Mihaescu, T., Azoicai, D., Arama, V., Balcu, I.: Preliminary assessment of Streptococcus pneumoniae, pneumonia, economical and clinical burden in Romania. Pneumologia 56(3), 118–123 (2007)
Dzajkovska, B., Wertheimer, A.I., Mrhar, A.: The burden-of-illness study on osteoporosis in the Slovenian female population. Pharm. World Sci. 29(4), 404–411 (2007). https://doi.org/10.1007/s11096-007-9091-5
Kopcsóné-Németh, I., Kertész, A., Strbák, B., Gulácsi, L.: A Clostridium difficile fertőzések költsége magyarországi kórházakban. Egészségügyi Gazdasági Szemle 13(2), 9–15 (2013)
Ernstsson, O., Gyllensten, H., Alexanderson, K., Tinghog, P., Friberg, E., Norlund, A.: Cost of illness of multiple sclerosis—a systematic review. PLoS One 11(7), e0159129 (2016). https://doi.org/10.1371/journal.pone.0159129
Acknowledgements
Open access funding provided by Corvinus University of Budapest (BCE). This research was supported by the Higher Education Institutional Excellence Program of the Ministry of Human Capacities in the framework of the ‘Financial and Public Services’ research project (20764-3/2018/FEKUTSTRAT) at Corvinus University of Budapest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Brodszky, V., Beretzky, Z., Baji, P. et al. Cost-of-illness studies in nine Central and Eastern European countries. Eur J Health Econ 20 (Suppl 1), 155–172 (2019). https://doi.org/10.1007/s10198-019-01066-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-019-01066-x
Keywords
- Cost-of-illness
- Disease burden
- Central and Eastern Europe
- Austria
- Bulgaria
- The Czech Republic
- Croatia
- Hungary
- Poland
- Romania
- Slovakia
- Slovenia