Abstract
The anti-TNF-α chimeric monoclonal antibody infliximab is the first biologic to be approved for rheumatoid arthritis (RA) in Japan, and post-marketing surveillance of all of the Japanese cases treated with infliximab has been conducted to explore the safety of infliximab therapy. In addition, a retrospective clinical study on the notable efficacy and related factors of infliximab therapy in an RA management group in Japan (RECONFIRM and RECONFIRM-2) has demonstrated clinical responses. However, information on the effect of infliximab on joint destruction in Japanese RA patients remains insufficient. In this study, we retrospectively analyzed X-ray data from 67 patients in whom both hand and foot X-rays at baseline and at 54 weeks had been available among the 410 cases in the RECONFIRM-2 study. By scoring the X-rays according to the modified van der Heijde (vdH)–Sharp method, we found that the total vdH–Sharp score in the RA patients before infliximab therapy was 104.40 ± 87.34 and the yearly progression was 21.33, indicating relatively rapid progression. After infliximab therapy for 54 weeks, the total vdH–Sharp score at 54 weeks was 104.37 ± 86.87 and the estimated yearly progression was −0.03, indicating the almost complete inhibition of progression. The RECONFIRM-2J study confirmed the significant ability of infliximab to halt joint destruction in Japanese RA patients, and showed that joint destruction was significantly associated with disease activity and the dose of MTX in the patients with moderate and advanced disease durations, respectively, before infliximab therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disorder, characterized by chronic, destructive and deforming arthritis [1], resulting in significant morbidity and mortality [2–5]. Since TNF-α plays a pivotal role in the pathogenesis of RA [6–8], biological agents targeting TNF-α are highly effective at not only reducing clinical signs and symptoms but also retarding structural damage to the joints; thus, these agents have had a great impact on the management of RA [9]. The abovementioned results were obtained in double-blind, randomized, clinical trials in which methotrexate (MTX; mean weekly doses ranging between 15 and 17 mg) and infliximab were administered concomitantly [10–12]. In Japan, however, the maximal approved dose of MTX is 8 mg/week; this difference raises the question of whether infliximab can suppress joint destruction when a lower dose of MTX is being administered concomitantly, as is the case in daily clinical practice in Japan.
The anti-TNF-α chimeric monoclonal antibody infliximab is the first biologic to be approved for the treatment of RA in Japan [13], and the approved dose is 3 mg/kg every eight weeks after zero, two, and six weeks of induction with MTX. The safety of infliximab use in Japan has now been explored in a post-marketing surveillance of all of the cases that have been treated with infliximab [14]. Furthermore, a retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a RA management group in Japan (RECONFIRM) has demonstrated clinical responses, with significant improvements observed in 258 cases after 22 weeks [15] and extended observations from 22 to 54 weeks completed in 410 cases (RECONFIRM-2) [16]. The RECONFIRM-2 study has shown that the retention rate at 54 weeks was 75.6% and that European League Against Arthritis (EULAR) good, moderate and no responses to infliximab were 37.0, 41.7 and 21.2%, respectively, with EULAR remission achieved in 27.6% of patients; thus, these data confirmed the excellent clinical efficacy of infliximab when administered over a period of one year [16]. However, information on the effect of infliximab on joint destruction in Japanese RA patients remains insufficient.
In a previous study, we demonstrated that the estimated yearly progression rate was extremely high in Japanese RA patients before the start of infliximab therapy [17], and that the progression rate was suppressed by treatment with infliximab for one year [17]. This data was collected at a single center, and the patients who were recruited for the study were longstanding RA patients who had been waiting for a long time for anti-TNF inhibitors to be approved. Consequently, any interpretation of these results is limited. In the present RECONFIRM-2J study, we recruited 67 of the 410 cases reported in the RECONFIRM-2 study [16]; these patients were recruited from three centers, and 19.4% had early RA (≤2 years). Both hand and foot X-rays at baseline and at 54 weeks were available for all of the recruited patients. We then retrospectively analyzed the X-ray data from these 67 patients before and 54 weeks after the start of infliximab therapy using a modified van der Heijde (vdH)–Sharp score. The factors contributing to the progressive features of joint destruction at baseline as well as those at 54 weeks after infliximab therapy were then analyzed.
Patients and methods
Patients and evaluation
Data and information on RA patients fulfilling the diagnostic criteria of the American College of Rheumatology (ACR) [18] were collected from three major rheumatology centers in Japan: the Department of Rheumatology and Clinical Immunology, Saitama Medical Center, Saitama Medical University, Saitama; the First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health Japan, Kitakyushu; and the Institute of Rheumatology, Tokyo Women’s Medical University. All patients receiving infliximab treatment at one of these institutions as of December 2005 were registered in this retrospective study. Demographic data, including disease duration and concomitant therapy, were collected from the patients’ medical charts. The following parameters were evaluated before and at 54 weeks after the initial infliximab infusion: tender joint count (TJC) 28, swollen joint count (SJC) 28, patient’s assessment of pain on a visual analog scale (patient’s pain VAS), patient’s global assessment of disease activity (patient’s global VAS), physician’s global assessment of disease activity (physician’s global VAS), and C-reactive protein (CRP).
Infliximab therapy
Infliximab was infused to patients at zero, two, and six weeks followed by every eight weeks at a dose of 3 mg/kg, according to the drug labeling and the guidelines of the Infliximab Study Group of the Ministry of Health, Welfare and Labor in Japan [19]. The concomitant use of MTX was instituted in all cases, although the dose of MTX was determined by each attending physician.
Therapeutic response
Disease activity was assessed using the disease activity score, including a 28-joint count (DAS28)-CRP that was calculated according to the authorized formula (http://www.das-score.nl/www.das-score.nl/index.html). DAS28-CRP values are reportedly lower than the original DAS28 assessments using the erythrocyte sedimentation rate (ESR), and we used a threshold of 4.1, instead of the original 5.1, as the cut-off for high activity and 2.7, instead of 3.2, as the cut-off for low activity [20]. Thus, we defined DAS28-CRP values ≥4.1 as high activity, ≤2.7 to <4.1 as moderate activity, and <2.7 as low activity, with ≤2.3 defined as remission [15, 16]. The responses to infliximab therapy at 22 weeks were evaluated using the European League Against Arthritis (EULAR) response criteria, with 4.1 and 2.7 used as the thresholds for high and low disease activities, respectively.
X-ray evaluations of hand and feet joints
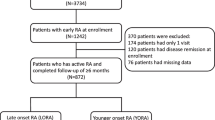
Among the 410 patients in the RECONFIRM-2 study, X-ray images of both hands and feet at 0 and 54 weeks were available for 67 patients. Two expert readers independently scored articular damage and progression in a blinded fashion according to the modified vdH–Sharp scoring method. Radiographic progression was defined as a difference that was greater than the smallest detectable difference (SDD) [21]. The SDD for the mean change from baseline using the two readers’ scores for each patient’s radiographs was 4.39 (the standard deviation of the per-patient differences between the readers divided by the square root of 2), which corresponds to nearly 1% of the maximum total modified vdH–Sharp score (TSS)—that is, 448.
Statistical analysis
Patients with radiographs of both hands and feet at baseline and at 54 weeks after the start of infliximab therapy were included in the analysis. Summary statistics of the median, mean, and interquartile ranges are presented for continuous variables. Spearman correlation analyses were performed to evaluate the association between baseline covariates and changes in the radiographic scores. For categorical response parameters, group comparisons were made using the chi-square test. Logistic regression analyses were performed to examine the significance of individual variables in predicting radiographic progression at baseline and at week 54. Patients were grouped into tertiles according to key predictors identified by the correlation and regression analyses at baseline; changes in total modified vdH–Sharp score (TSS) were summarized for the patients in each tertile.
The proportions of patients who had no progression in their TSS and joint space narrowing score were calculated. No progression was defined as less than 0.5 units of change from baseline or less than a given threshold value, such as the SDD, in their TSS.
Statistical analyses were performed using JMP software version 6.1 (SAS Institute, Cary, NC, USA). All statistical testing was two-sided. P values ≤0.05 were considered significant.
Results
Baseline demographics and subsequent changes in clinical parameters for 67 patients with X-ray data
Among the 410 patients enrolled in the RECONFIRM-2 study, we selected 67 patients in whom both hand and foot X-ray data were available and investigated structural damage and its progression during infliximab therapy in this RECONFIRM-2 Joint (RECONFIRM-2J) study. When the baseline characteristics of the 67 patients in the RECOMFIRM-2J study were compared with the rest of the patients in the RECONFIRM-2 study without X-ray data (n = 343), the patients in the present study were found to be younger and received higher doses of MTX than the 343 patients without X-ray data in the RECONFIRM-2 study (Table 1). Age and dose of MTX were significantly different between the patients in the RECONFIRM-2J study and the rest of the patients in the RECONFIRM-2 study (age: 50.8 ± 11.4 vs. 53.7 ± 12.9 years, respectively, P = 0.0411; MTX dose: 8.3 ± 2.4 vs. 7.7 ± 1.9 mg, respectively, P = 0.0349). As shown in Table 1, the clinical parameters of the 67 patients in the present study responded dramatically to infliximab therapy (DAS28 at 0 week: 5.64 ± 1.03, and DAS28 at 54 weeks: 3.02 ± 1.25; 46.5% reduction), and the response rate was slightly better than that of the 343 patients in the RECONFIRM-2 study (DAS28 at 0 week: 5.48 ± 1.14, and DAS28 at 54 weeks: 3.34 ± 1.41; 39.1% reduction).
Baseline X-ray data
Joint destruction was assessed using the modified vdH–Sharp score of hand and foot X-rays at baseline (0 week) and at 54 weeks after the start of infliximab therapy. The score at 0 week ranged from 9.5 to 410. The mean ± SD of the score at baseline was 104.40 ± 87.34, and the median, upper quartile, and lower quartile values were 73.5, 147.5, and 45.0, respectively. When the estimated yearly progression from disease onset was calculated, its value ranged from 2.11 to 118.80, with a mean value of 21.33. The median, upper quartile, and lower quartile values of the estimated yearly progression in the patient cohort were 16.19, 25.50, and 9.69, respectively.
To clarify the clinical parameters related to the total modified vdH–Sharp score (TSS) before infliximab therapy, we analyzed a possible correlation between the TSS and a series of clinical parameters at 0 weeks. No significant relations between the TSS at 0 weeks and sex, age, RF titer, DAS28, MTX dose, or PSL dose at 0 weeks were found, whereas the TSS at 0 weeks was significantly correlated with the disease duration (r = 0.62, P < 0.001). Since these factors interact with each other, we analyzed the relationship between the TSS and a series of clinical parameters at 0 weeks using a multivariate analysis but found no significant correlation among the parameters, with the exception of disease duration again (data not shown). Thus, we further analyzed the possible correlation between TSS and a series of clinical parameters at 0 weeks in subgroups of the patients with variable disease durations.
Correlation between total modified vdH–Sharp score and clinical characteristics among patients with variable disease durations before infliximab therapy
Since disease duration was the only factor significantly correlated with the total modified vdH–Sharp score (TSS) at 0 weeks, we attempted to categorize the patients into three groups by the disease duration according to the lower quartile (2.5 years) and the upper quartile (11.0 years) of the distribution (Fig. 1a). The three groups of the patients, thus, subdivided into early (≤2.5 years), moderate (>2.5 to ≤11.0 years), and advanced disease (>11.0 years) clearly showed that the estimated yearly progression was most rapid in the early RA (Fig. 1b). In the early RA group (disease duration ≤2.5 years), no significant correlations between the TSS at 0 week and a series of clinical parameters were found, whereas the DAS28 at 0 weeks was significantly correlated with the TSS at 0 weeks in the moderate RA group (r = 0.416, P = 0.015); the dose of MTX was also inversely correlated with TSS at 0 weeks in the advanced RA group (r = −0.508, P = 0.045) (Fig. 2).
a Number of cases with early, moderate or advanced RA. The line in the box represents the median value, the diamond box represents the mean value, and the upper and lower ends of the box indicate the 25th and 75th percentiles of the population. b Yearly progression of TSS in patients with early RA (n = 17), moderate RA (n = 34), and advanced RA (n = 16). The line in the box represents the median value, and the upper and lower ends of the box indicate the 25th and 75th percentiles of the population
X-ray progression at 54 weeks after the start of infliximab therapy
After treatment with infliximab, the total modified vdH–Sharp score (TSS) at 54 weeks was not markedly progressed and remained unchanged from that at 0 weeks (104.40 ± 87.34 at 0 weeks vs. 104.37 ± 86.87 at 54 weeks), as shown in Fig. 3. The estimated yearly progression at 0 weeks was 21.33, while that at 54 weeks was −0.03, producing a 101.14% reduction in the rate of joint destruction. In addition, progression was completely inhibited in 39 patients (58.2%), including 25 patients (37.3%) with negative changes—suggesting the possibility of radiographic repair. No new erosions were seen in 66 patients (98.5%), and only one patient, in whom estimated yearly progression at 54 weeks was 7.5, exhibited new erosions. These results confirm that infliximab therapy strongly inhibited the progression of structural damage, even in Japanese RA patients with high estimated yearly progression rates of over 20 and mean disease durations of around ten years (13 cases, 19.4% of the total of 67 cases, were early RA with disease duration ≤2 years).
Finally, we investigated the possible correlation between the estimated yearly progression at 54 weeks and a series of clinical parameters and found that sex, age, disease duration, RF titer, MTX dose, and PSL dose were not significantly correlated with the progression of joint destruction (data not shown), suggesting that infliximab therapy suppressed the progression of structural damage remarkably in the vast majority of the patients, irrespective of the clinical characteristics of the patients. In addition, the estimated yearly progressions at baseline for the patients with early, moderate, or advanced RA were significantly suppressed at 54 weeks, as shown in Fig. 3 (0.21 ± 0.60, −0.11 ± 0.42, and −0.09 ± 0.62, respectively), indicating that infiximab effectively halted the progression of structural damage not only among patients with advanced diseases but also among patients with early or moderate disease.
Discussion
The RECONFIRM-2J study analyzed structural damage in Japanese RA patients who had been treated with infliximab in addition to low doses of MTX and examined the clinical parameters correlated with joint destruction. Although the clinical efficacy and safety of infliximab therapy in Japanese patients with RA has now been explored [15, 16], only a few reports have examined the effect of infliximab on structural damage [17]. In this report, we studied 67 RA patients among the 410 patients enrolled in the RECONFIRM-2 study and analyzed the structural damage in these patients using the modified vdH–Sharp scoring method at 0 weeks as well as at 54 weeks after the start of infliximab therapy. The average total modified vdH–Sharp score (TSS) at baseline was 104.40 and the estimated yearly progression was 21.33 prior to the start of infliximab therapy, whereas the average TSS was markedly suppressed to 104.37 and the estimated yearly progression was −0.03 at 54 weeks after the start of infliximab therapy.
This study has several limitations. First, among the 410 enrolled RA patients in the RECONFIRM-2 study, we retrospectively analyzed 67 patients for whom hand and foot X-rays taken at 0 and 54 weeks after the start of infliximab therapy were available. So, a possible selection bias should be seriously considered. Although the patients in this study were younger and the doses of MTX were greater than those without X-ray analysis, the other baseline characteristics and clinical parameters were comparable among two groups. On the other hand, the response to infliximab was slightly better among the 67-patient cohort in the present study, introducing the possibility that we may have selected patients with a better response to therapy. Nevertheless, when the responses were assessed using the ACR and EULAR criteria, the differences in the responses of the two patient populations were not significantly different. Second, the dose of MTX used in Japan is lower than those used in other countries, including the US, many European countries and even other Asian countries, because of a Japanese regulatory restriction stating that the maximum allowable dose of MTX is 8 mg/week; nevertheless, higher doses of MTX are sometimes used in daily clinical practice. Indeed, the mean MTX dose in the present study was 8.3 mg/week, which was still almost half of that used in double-blind, randomized clinical trials for infliximab [10–12, 22]. However, these circumstances provide an interesting opportunity to examine X-ray progression during infliximab therapy administered concomitantly with low-dose MTX. In addition, the present study appears to reflect daily clinical practice, whereas most studies showing the efficacy of anti-TNF biologics on joint destruction have been derived from randomized controlled trials [10–12, 22].
The X-ray progression of the Japanese RA patients was considerably rapid. Although the mean duration of the disease in these patients was 7.9 years, the estimated yearly progression, which was calculated using the total modified vdH–Sharp score at baseline divided by the disease duration, was 21.33. Since we were utilizing a 448 full-scale scoring system, 21.33 correspond to 4.76% of the full scale. The estimated yearly progression in the ATTRACT study was 7.06, corresponding to 1.61% of a 440 full-scale scoring system. These results suggest that structural damage progressed three times more rapidly in the present study than in previous American and European studies. To interpret the above results carefully, we compared the clinical characteristics of the RECONFIRM-2J and ATTRACT studies (Table 2) and found several differences in patient background characteristics, such as female dominance (91 vs. 78%), a shorter disease duration (7.94 vs. 10.84 years), earlier stage RA (% of patients with a disease duration of less than 3 years: 34.3 vs. 13.4%), and a lower dose of MTX (8.27 vs. 16.19 mg/week). Since joint destruction progresses rapidly during the initial 1–2 years after onset when patients are treated using conventional DMARD therapy [23, 24], these data imply that the patients enrolled in the RECONFIRM-2J study, who had a relatively short disease duration, likely exhibited a more rapid disease progression than those in the ATTRACT study. Nevertheless, we should seriously consider the possibility that Japanese RA patients may exhibit rapid X-ray progression. In the Japanese SAMURAI study, which compared the efficacy of tocilizumab—a humanized anti-IL-6 receptor mAb—and MTX for inhibiting the progression of joint destruction [25], the mean TSS of the 306 patients at baseline was 29.4; this figure was extremely high despite the mean disease duration of 2.3 years. The mean estimated yearly progression of TSS for the 306 Japanese RA patients recruited was 13.3, supporting the above hypothesis. In this regard, the lower dose of MTX may be responsible, in part, for the rapid progression of joint destruction, in addition to possible genetic and environmental factors unique to Japanese RA patients.
No significant relationships were noted between the total modified vdH–Sharp score at baseline and most clinical parameters, whereas the total modified vdH–Sharp score was significantly correlated with the disease duration (r = 0.62, P < 0.001), as confirmed using a multivariate analysis. This conclusion is consistent with the results of a long list of previous reports. Although whether the rate of progression decreases as the disease duration increases continues to be discussed, joint destruction certainly progresses rapidly during the first 1–2 years after disease onset [23, 24]. Our results support the hypothesis that patients with a shorter duration exhibit a higher estimated yearly progression, although this result might reflect our method of calculating progression using the score divided by disease duration since diagnosis of RA, but not initial manifestation [3].
Obviously, structural damage occurs rapidly during the early stage of the disease in caucasian RA patients, and this is also true in Japanese RA patients. The mean estimated yearly progression in patients with early disease was 41.11; this rate was faster than that reported in the PREMIER study, which had the most aggressive structural damage reported so far [26]. In addition, the mean estimated yearly progression in the moderate and advanced RA groups showed similar aggressive features of structural damage in Japanese RA patients, confirming previous results [17].
While we found no unique characteristics among patients with early RA, higher disease activity in patients with moderate RA and a lower doses of MTX in those with advanced RA were correlated with more rapid progression of joint destruction. These results may suggest that patients with distinct disease durations may have different disease features regarding the progression of structural damage. To lessen joint destruction, it is important to realize that infliximab therapy should be started much earlier, before disease activity reaches a high level at which the induction of remission in early RA becomes unlikely. In addition, tight control over disease activity in patients with moderate RA and MTX dose escalation in patients with advanced RA should be seriously considered before starting infliximab therapy.
Compared with the X-ray data at 0 weeks, the effect of infliximab on joint destruction was remarkable in Japanese patients with RA. The change in the total modified vdH–Sharp score was −0.03 out of a full-scale score in the present study, whereas that in the ATTRACT study was 1.3 out of a 440 full-scale score for the arm receiving 3 mg/kg of infliximab every eight weeks. Although 38% of the patients in the present study showed some improvement (whereas 44% of the patients in the 3-mg/kg every eight weeks arm of the ATTRACT study showed improvement), the percentage of patients with major progression was lower in the present study than in the ATTRACT study (1.5 vs. 5.5%). The average reduction from the total score at baseline at 54 weeks after the start of infliximab therapy was 101.14% in the present study, whereas that in the 3-mg/kg every eight weeks arm of the ATTRACT study was 98.35%, indicating a similarly remarkable effect on the suppression of structural damage progression.
Taken together, the present RECONFIRM-2J study demonstrated that joint destruction before the start of infliximab therapy was considerably rapid in Japanese RA patients and confirmed the significant ability of infliximab to halt joint destruction. By analyzing the factors related to joint destruction, we found a significant correlation between disease duration and the TSS at baseline before the start of infliximab therapy. In patients with advanced RA, TSS was negatively correlated with the MTX dose at 0 weeks, while it was significantly correlated with the DAS28 at 0 weeks. However, none of the factors were correlated with joint destruction at 54 weeks after the start of infliximab therapy, suggesting that infliximab remarkably suppressed the progression of structural damage in the vast majority of the patients, irrespective of the clinical characteristics of the patients.
References
Scott DL, Grindulis KA, Struthers GR, Coulton BL, Popert AJ, Bacon PA. Progression of radiological changes in rheumatoid arthritis. Ann Rheum Dis. 1984;43:8–17.
Pincus T. Long-term outcomes in rheumatoid arthritis. Br J Rheumatol. 1995;34 Suppl 2:59–73.
Scott DL, Pugner K, Kaarela K, Doyle DV, Woolf A, Holmes J, et al. The links between joint damage and disability in rheumatoid arthritis. Rheumatology (Oxford). 2000;39:122–32.
Pincus T, Sokka T, Wolfe F. Premature mortality in patients with rheumatoid arthritis: evolving concepts. Arthritis Rheum. 2001;44:1234–6.
Doran MF, Pond GR, Crowson CS, O’Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46:625–31.
Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440.
Ivashkiv LB. Cytokine expression and cell activation in inflammatory arthritis. Adv Immunol. 1996;63:337–76.
Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–16.
Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–96.
Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N Engl J Med. 2000;343:1594–602.
St Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–43.
Taylor PC, Steuer A, Gruber J, McClinton C, Cosgrove DO, Blomley MJ, et al. Ultrasonographic and radiographic results from a two-year controlled trial of immediate or one-year-delayed addition of infliximab to ongoing methotrexate therapy in patients with erosive early rheumatoid arthritis. Arthritis Rheum. 2006;54:47–53.
Abe T, Takeuchi T, Miyasaka N, Hashimoto H, Kondo H, Ichikawa Y, et al. A multicenter, double-blind, randomized, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritis. J Rheumatol. 2006;33:37–44.
Takeuchi T, Tatsuki Y, Nogami Y, Ishiguro N, Tanaka Y, Yamanaka H, et al. Post-marketing surveillance of the safety profile of infliximab in 5,000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:189–94.
Yamanaka H, Tanaka Y, Sekiguchi N, Inoue E, Saito K, Kameda H, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan (RECONFIRM). Mod Rheumatol. 2007;17:28–32.
Tanaka Y, Takeuchi T, Inoue E, Saito K, Sekuguchi N, Iikuni N, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan: 1-year clinical and ragiographic outcomes (RECONFIRM-II). Mod Rheum. 2008;18:146–152.
Kameda H, Sekiguchi N, Nagasawa H, Amano K, Takei H, Suzuki K, et al. Development and validation of handy rheumatoid activity score with 38 joints (HRAS38) in rheumatoid arthritis patients receiving infliximab. Mod Rheumatol. 2006;16:381–8.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24.
Miyasaka N, Takeuchi T, Eguchi K. Proposed [corrected] Japanese guidelines for the use of infliximab for rheumatoid arthritis. Mod Rheumatol. 2005;15:4–8.
Inoue E, Yamanaka H, Hara M, Tomatsu T, Kamatani N. Comparison of disease activity score (DAS)28− erythrocyte sedimentation rate and DAS28− C-reactive protein threshold values. Ann Rheum Dis. 2007;66:407–9.
van der Heijde D, Lassere M, Edmonds J, Kirwan J, Strand V, Boers M. Minimal clinically important difference in plain films in RA: group discussions, conclusions, and recommendations. OMERACT imaging task force J Rheumatol. 2001;28:914–7.
Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50:1051–65.
Strand V, Landewe R, van der Heijde D. Using estimated yearly progression rates to compare radiographic data across recent randomised controlled trials in rheumatoid arthritis. Ann Rheum Dis. 2002;61(suppl 2):ii61–4.
Wick MC, Anderwald C, Weiss RJ, Imhof H, Kainberger F, Smolen JS. Radiological progression of joint damage in a longitudinal cohort of early DMARD-treated rheumatoid arthritis patients followed for 10 years. Scand J Rheumatol. 2004;33:162–6.
Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an X-ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–7.
Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37.
Acknowledgments
The authors thank all of the medical staff at the three institutions for providing the data. This work was supported in part by a Research Grant-In-Aid for Scientific Research from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Takeuchi, T., Yamanaka, H., Inoue, E. et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan: one-year outcome of joint destruction (RECONFIRM-2J). Mod Rheumatol 18, 447–454 (2008). https://doi.org/10.1007/s10165-008-0077-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10165-008-0077-5