Abstract
Background
The prevalence of rheumatoid arthritis (RA) in persons 60 years or older is estimated to be 2%. Late-onset rheumatoid arthritis (LORA) is traditionally defined as the onset of RA after the age of 60 years. Compared to younger-onset rheumatoid arthritis (YORA) which occurs before the age of 60 years, LORA has unique characteristics and disease manifestations. To date, few reports have addressed LORA and the prognosis of LORA patients remains unclear. We compared the clinical characteristics, time to remission and treatment regimen at remission between LORA and YORA patients.
Methods
This prospective cohort study used a registry database in Ontario, Canada from 2008 to 2020. Patients were included if they had active rheumatoid arthritis (RA) disease (≥1 swollen joint) and were enrolled within 1 year of diagnosis. LORA was defined as a diagnosis of RA in persons 60 years and older and YORA as a diagnosis of RA in persons under the age of 60. Remission was defined by Disease Activity Score 28 (DAS28) ≤2.6. A multivariable Cox proportional hazards model was used to estimate time to remission.
Results
The study included 354 LORA patients and 518 YORA patients. The mean (standard deviation) baseline DAS28 score was 5.0 (1.3) and 4.8 (1.2) in LORA and YORA patients, respectively (p=0.0946). Compared to YORA patients, the hazard ratio for remission in LORA patients was 1.10 (95% confidence interval 0.90 to 1.34 p=0.36) after adjusting for other prognostic factors. For patients who reached remission, LORA patients were less likely to be on a biologic or Janus kinase (JAK) inhibitor (16% vs. 27%) and more likely to be on a single conventional synthetic disease-modifying anti-rheumatic drugs (csDMARD) (34% vs. 27%) than YORA patients (p=0.0039).
Conclusion
LORA and YORA patients had similar prognosis in terms of time to remission. At remission, LORA patients were more likely to be on a single csDMARD without a biologic or JAK inhibitor.
Similar content being viewed by others
Introduction
The prevalence of rheumatoid arthritis (RA) in persons 60 years or older is estimated to be 2% [1]. Late-onset rheumatoid arthritis (LORA) is traditionally defined as the onset of RA after the age of 60 years [2]. It is estimated that the incidence and prevalence of RA increase with age up to the age of 80 to 85 years [3, 4]. Therefore, in an ageing population with an increasingly longer life expectancy, the number of LORA patients will continue to increase.
Compared to younger-onset rheumatoid arthritis (YORA), which presents before the age of 60 years, LORA has unique characteristics and disease manifestations such as a higher proportion of males, more comorbidities, less frequent positivity for rheumatoid factor or anti-cyclic citrullinated peptide (anti-CCP) antibody, higher C-reactive protein (CRP), and higher erythrocyte sedimentation rate (ESR) [5].
The prognosis of LORA patients has been unclear in previous studies. Compared to YORA, the prognosis of LORA was better in some studies [6, 7] and worse in others [8,9,10,11]. However, knowledge of prognosis is important when counselling a patient with LORA. Clinicians can use this knowledge when weighing the benefits and risks of different RA treatment regimens. This treatment decision is more complex in LORA patients, because age, comorbidities, and frailty can increase the risk of toxicity of disease-modifying anti-rheumatic drugs (DMARDs) [12].
Using data from a large registry of RA patients in Ontario, Canada, we have previously described the clinical characteristics of LORA and YORA patients [13]. The objective of this study was to compare the remission rate and treatment regimen at remission between LORA and YORA patients.
Methods
Study design
This was a prospective multicenter cohort study. The Ontario Best Practices Research Initiative (OBRI) is a multicenter provincial registry in Ontario, Canada, that prospectively follows RA patients who are under the routine care of rheumatologists [14]. Patients provided written informed consent prior to enrolment in the registry. Research ethics approval was obtained at the institution (University Health Network Research Ethics Board 07-0729 AE) as well as at each participating site.
Study population
To be included in the OBRI registry, a patient must be 18 years or older at enrolment, with disease onset after 16 years of age and a rheumatologist-confirmed RA diagnosis.
This study used data from patients who were enrolled in the OBRI registry from January 17, 2008, to January 1, 2020. Patients were included in this study if they were enrolled in the OBRI registry the same or next calendar year relative to their RA diagnosis, not in remission as per the Disease Activity Score 28 joint count (DAS28) score [15] at enrolment and followed for a minimum of 6 months in the OBRI registry.
Data collection
At each routine clinic visit, the rheumatologist completed a case report form. Data was also collected from the patient via a telephone interview every 6 months. Data collection included age, sex, family history, smoking history, comorbidities, disease activity (DAS28), Health Assessment Questionnaire Disability Index (HAQ DI) [16] and medications.
Definition of variables
Patients who were diagnosed with RA at the age of 60 years or older were classified as late-onset RA (LORA), whereas patients diagnosed with RA at an age younger than 60 years were classified as younger-onset RA (YORA).
RA medications were classified into conventional synthetic (csDMARD), or biologic/Janus kinase (JAK) inhibitors. The DMARD regimen was classified based on the number of csDMARDs used and the need for biologic or JAK inhibitors.
The primary outcome was time to remission as defined by a DAS28 score of ≤2.6 [15]. Persistent remission was defined as remission for at least in two visits. We also compared the DMARD regimen of LORA and YORA patients when they first reached remission.
Statistical analysis
Continuous variables were described using mean and standard deviation, or median and interquartile range (IQR) when appropriate. Categorical variables were described using numbers and percentages. Comparison between LORA and YORA patients were done with Student’s T-test for continuous variables and chi-squared test for categorical variables.
A Cox proportional hazards model was used to estimate hazard ratio (HR) for time to the first remission defined as DAS28 ≤2.6. In the univariate analysis, potential predictors included age, gender, smoking history, family history, comorbidities, RA disease characteristics, baseline DAS28 score and treatment. Treatment with a biologic or JAK inhibitor was entered as a time-dependent variable. Most of these predictors were selected based on a prior systemic review of important predictors for remission in RA [17]. Selection of predictors for the multivariable Cox proportional hazards model was based on p-value <0.2 in the univariate analysis. As well, predictors that did not satisfy the p-value <0.2 but were deemed clinically important prognostic factors were also entered into the multivariable model along with LORA.
We performed two sensitivity analyses. First, we performed a subgroup analysis, where only seropositive patients with positive rheumatoid factor or anti-CCP were included in the Cox proportional hazards model for time to remission. Second, we used remission as defined by simplified disease activity index (SDAI) ≤3.3 [18] instead of the DAS28 score as the dependent variable for the Cox proportional hazards model.
All reported confidence intervals (CIs) were two-sided 95% intervals and all tests were two-sided with a p<0.05 significance level. All analyses were done using SAS 9.4.
Results
In total, 872 participants in the OBRI registry had early and active RA. On enrolment, 734 (84%) patients had definite RA based on the American College of Rheumatology / European League Against Rheumatism 2010 criteria [19]. Of the 872 patients, 354 (41%) patients had LORA and 518 (59%) patients had YORA (Fig. 1).
Baseline characteristics
Baseline characteristics of patients at enrolment are described in Table 1. LORA and YORA patients had a mean age of 69.8 and 47.2 years, respectively (p<0.0001). Compared to YORA patients, LORA patients were more likely to be male (34% versus 20%, p<0.0001), and less likely to have a positive rheumatoid factor or anti-CCP (63% vs. 75%, p=0.0003). LORA patients had a higher number of comorbidities (mean of 1.5 vs. 0.8, p<0.0001). Although the DAS28 score was similar between the two groups, LORA patients had significantly higher inflammatory markers (ESR and CRP).
In terms of treatment at enrolment, LORA patients were less likely to be on a biologic or JAK inhibitor (3% vs. 8%, p=0.0100) or non-steroidal anti-inflammatory drugs (NSAID) (32% vs. 42%, p=0.0070). Also, a significantly higher proportion of LORA patients were taking an oral glucocorticoid (23% vs. 14%, p=0.0010).
Time to remission
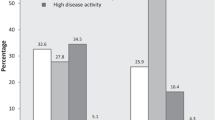
The median (IQR) follow-up time to remission was 12.2 (IQR 6.0 to 25.5) months for all patients, 13.2 (IQR 6.1 to 26.9) months for LORA patients, and 11.9 (IQR 6.0 to 24.3) months for YORA patients. The mean (SD) follow-up time to remission was 19.5 (19.9) months for all patients, 20.6 (20.5) months for LORA patients and 18.7 (19.4) months for YORA patients. The decrease in DAS28 score, over time as compared to the baseline for each patient, is described in Table 2. The survival curves of LORA and YORA patients for active disease not at remission are shown in Fig. 2. At the end of follow-up, 72% of LORA patients and 78% of YORA patients reached remission. Furthermore, 54% of LORA patients and 48% of YORA patients reached persistent remission.
Univariate and multivariable Cox proportional hazard model predicting time to remission is shown in Table 3. After adjusting for significant predictors, the adjusted hazard ratio for LORA was 1.10 (95% CI 0.90 to 1.34, p=0.36).
As a sensitivity analysis, the multivariable Cox proportional hazards model for the subgroup of seropositive patients with a positive rheumatoid factor or anti-CCP is shown in Supplementary Materials Table S1. In another sensitivity analysis, the multivariable Cox proportional hazards model using remission as defined by SDAI≤3.3 is shown in Supplementary Materials Table S2. Both sensitivity analyses showed consistent results of similar remission rates between LORA and YORA patients.
Patients at remission
The DAS28 components and medication regimen of LORA and YORA patients when they first reached remission are shown in Table 4. Compared to YORA patients, LORA patients were less likely to be on a biologic or JAK inhibitor (16% vs. 27%) and more likely to be on a single csDMARD (34% vs. 27%, p=0.0039). Furthermore, a significant proportion of LORA patients were on oral glucocorticoids at the time of remission (27% vs. 13%, p<0.0001).
Adverse events due to treatment
The rate of serious infections and new cancers stratified by use of biologic or JAK inhibitor are described for LORA and YORA patients in Supplementary Materials Table S3. LORA patients had consistently higher rates of serious infections and new cancers than YORA patients irrespective of biologics or JAK inhibitor use (Supplementary Materials Table S3).
Discussion
In this cohort study, LORA patients had a similar time to remission to YORA patients after adjusting for other prognostic factors (HR of 1.10, 95% CI 0.90 to 1.34). For those patients who reached remission, LORA patients were more likely to be on a less intensive DMARD regimen such as using a single csDMARD without a biologic or JAK inhibitor.
In our study, LORA patients had a higher proportion of males, a lower proportion of RF or anti-CCP positivity, and higher inflammatory markers compared to YORA patients. These findings were similar to other published studies [5, 8, 13].
Previous studies have shown conflicting results on the prognosis of LORA patients compared to YORA patients. LORA patients had a better prognosis in some older studies [6, 7] and a worse prognosis in more recent studies [8,9,10,11]. For example, in a large multicenter French cohort study of 698 patients with early RA, 118 LORA patients (age >60 years) had an adjusted odds ratio of 0.33 (95% CI 0.16 to 0.71) for remission at 1 year using SDAI, compared to YORA patients (age <45 years), suggesting a worse prognosis for LORA [10]. Whereas, in a US cohort study of 422 patients with RA onset within 2 years, the remission rates using the American Rheumatism Association (ARA) criteria in 214 LORA patients (age ≥65 years), and 186 YORA patients (age <65 years) were 46% and 20%, respectively, suggesting a better prognosis for LORA [6].
For studies that reported disease activity based on DAS28, the mean area under the curve was 47.7 in LORA patients versus 44.8 in YORA patients (P<0.01) at 1 year in a cohort of 750 RA patients [8]. In another study, LORA had a lower response to therapy based on DAS28 <3.2 at 6 months with an odds ratio of 0.28 (95% CI 0.08 to 0.98) in 140 patients with early RA [11]. Finally, in a cohort of 229 patients with early RA, although DAS28 was higher at baseline in LORA (mean of 5.0 vs. 4.0 p<0.001), the DAS28 was not significantly different between the two groups at the end of the 2-year follow-up (mean of 2.5 vs. 2.3 p=0.07) [9]. Our study results are consistent with this last study [9], which had a large sample of patients with early RA with a long follow-up.
In contrast to these studies, our study results provide an estimate showing a similar prognosis between LORA and YORA patients. The different results may be related to differences in the definition of LORA, the time point with respect to the disease course at enrolment, criteria for remission, the geographic location and sample size. Compared to other studies, our study defined LORA as being a diagnosis in the same or next calendar year and a widely accepted remission criteria based on DAS28. As well, our study has a larger sample size than the aforementioned studies allowing for a more precise estimate. The use of different measures for disease activity and remission makes comparison difficult across studies. The most commonly used measures of disease activity include DAS28, Clinical Disease Activity Index (CDAI) and SDAI [20]. The DAS28, CDAI and SDAI cut-offs do not translate into the same clinical information [20]. Even CDAI and SDAI had significant disagreements when applied to the same patients [20]. Of these three composite measures, the DAS28 is the oldest instrument that has been extensively validated and most widely used in clinical practice as well as research [20,21,22].
The results of this study have important implications to the management of LORA. When deciding on the initial treatment regimen for LORA, it is likely not necessary to start combination DMARDs or a biologic/JAK inhibitor, because many LORA patients were able to reach remission while on csDMARD. This validates the current practice pattern where LORA patients are typically not treated with combination DMARD or a biologic [23, 24]. However, the goal of treatment for LORA should be the same for YORA, given that LORA patients were just as likely as YORA patients to reach remission on follow-up in our study. Therefore, if LORA patients are still not at remission at follow-up, treatment should be escalated in the same aggressive manner as YORA patients so that they may reach remission. For example, in a study of 197 patients with LORA, patients that adhered to a treat-to-target strategy targeting low disease activity with escalation of therapy were more likely to have sustained remission by SDAI (42.2% vs. 14.5% p<0.001) as well as less progression of joint destruction and better physical function during 3 years of follow-up [25]. Based on observational cohort studies and randomized controlled trials, the effectiveness and safety of biologic DMARDs are likely similar in elderly patients [25]. Therefore, clinicians should not hesitate to escalate and add a biologic or JAK inhibitor for LORA patients who have been treated with csDMARDs and have not reached remission.
A higher proportion of LORA patients were initiated on glucocorticoids in our study. LORA patients may have more active disease at baseline based on higher inflammatory markers and joint erosions, prompting the rheumatologist to treat with glucocorticoid for early disease control. Interestingly, a higher proportion of LORA patients were maintained on glucocorticoids at remission. Similarly, in a large observational cohort of 4202 patients, prednisone use was much higher in LORA patients than in YORA patients (41.0% vs. 37.6% P=0.025) [23]. Prolonged glucocorticoid therapy can have potential adverse effects [26]. This presents an opportunity to improve the care of LORA. Once LORA patients reach remission, the rheumatologist should re-evaluate the treatment regimen and readjust the regimen if necessary to make it safer in the long term.
Our study has several strengths. First, this is one of the largest studies to date describing the prognosis of LORA patients over time. The large sample size allowed for more precise estimates. Second, this study has detailed data on clinical characteristics, treatment and outcome over a long period of follow-up. The data collection is prospective, rigorous and complete.
Our study also has several weaknesses. First, there may be residual confounding due to the observational nature of the study. However, we used a Cox proportional hazards model to adjust for other important prognostic factors. The significant predictors and correlation with prognosis in the final multivariable models were consistent with the important predictors of remission in a previous systematic review [17]. One significant predictor was the number of comorbidities, which may bias the assessment of disease activity. It is not possible to ascertain how much of disease activity is attributable to comorbidities versus RA. Thus, patients may have persistent symptoms due to comorbidities when their RA is actually in remission. This would underestimate the remission rate in LORA patients who had more comorbidities. Second, in an ideal inception cohort, all patients should be recruited at the onset of symptoms. This is not feasible in a cohort study. Nevertheless, our criteria of enrolment date at the same or next calendar year relative to the diagnosis of RA was more stringent than previous studies [6, 27] and should be representative of patients with early and active RA. Third, there may be variations in the treatment of LORA patients across centres and over time. In the 2011/2012 Canadian Rheumatology Association guidelines on pharmacotherapy of RA [15], there is no special consideration for LORA and this serves as general guidance for Canadian rheumatologists. Our study findings are representative of real-world data on the management of LORA patients.
Conclusions
Our study findings suggest that LORA patients have a similar prognosis as YORA patients, however, LORA patients who reached remission were less likely to be on combination DMARDs or a biologic/JAK inhibitor. This suggests that LORA patients likely do not require combination DMARD or biologic on initiation. Future studies should evaluate if a standardized treatment protocol tailored to LORA patients improves the safety of RA treatment and remission rate.
Availability of data and materials
The data underlying this article will be shared on reasonable request to the corresponding author.
Abbreviations
- RA:
-
Rheumatoid arthritis
- LORA:
-
Late-onset rheumatoid arthritis
- YORA:
-
Younger-onset rheumatoid arthritis
- Anti-CCP:
-
Anti-cyclic citrullinated peptide
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- DMARD:
-
Disease-modifying anti-rheumatic drug
- CsDMARD:
-
Conventional synthetic disease-modifying anti-rheumatic drug
- OBRI:
-
Ontario Best Practices Research Initiative
- DAS28:
-
Disease Activity Score 28 joint count
- SDAI:
-
Simplified disease activity index
- CDAI:
-
Clinical Disease Activity Index
- HAQ-DI:
-
Health Assessment Questionnaire Disability Index
- JAK:
-
Janus kinase
- NSAID:
-
Non-steroidal anti-inflammatory drug
References
Rasch EK, Hirsch R, Paulose-Ram R, Hochberg MC. Prevalence of rheumatoid arthritis in persons 60 years of age and older in the United States: effect of different methods of case classification. Arthritis Rheum. 2003;48(4):917–26.
Deal CL, Meenan RF, Goldenberg DL, Anderson JJ, Sack B, Pastan RS, et al. The clinical features of elderly-onset rheumatoid arthritis. A comparison with younger-onset disease of similar duration. Arthritis & Rheumatism: Official Journal of the American College of. Rheumatology. 1985;28(9):987–94.
Tutuncu Z, Kavanaugh A. Rheumatic disease in the elderly: rheumatoid arthritis. Rheum Dis Clin N Am. 2007;33(1):57–70.
Safiri S, Kolahi AA, Hoy D, Smith E, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78(11):1463–71.
Kobak S, Bes C. An autumn tale: geriatric rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2018;10(1):3–11.
Pease CT, Bhakta BB, Devlin J, Emery P. Does the age of onset of rheumatoid arthritis influence phenotype?: a prospective study of outcome and prognostic factors. Rheumatology (Oxford). 1999;38(3):228–34.
Glennås AN, Kvien TK, Andrup OD, Karstensen BE, Munthe EI. Recent onset arthritis in the elderly: a 5 year longitudinal observational study. J Rheumatol. 2000;27(1):101–8.
Innala L, Berglin E, Möller B, Ljung L, Smedby T, Södergren A, et al. Age at onset determines severity and choice of treatment in early rheumatoid arthritis: a prospective study. Arthritis Res Ther. 2014;16(2):1–9.
Murata K, Ito H, Hashimoto M, Nishitani K, Murakami K, Tanaka M, et al. Elderly onset of early rheumatoid arthritis is a risk factor for bone erosions, refractory to treatment: KURAMA cohort. Int J Rheum Dis. 2019;22(6):1084–93.
Krams T, Ruyssen-Witrand A, Nigon D, Degboe Y, Tobon G, Fautrel B, et al. Effect of age at rheumatoid arthritis onset on clinical, radiographic, and functional outcomes: the ESPOIR cohort. Joint Bone Spine. 2016;83(5):511–5.
Romão VC, Humby F, Kelly S, Di Cicco M, Mahto A, Lazarou I, et al. Treatment-resistant synovitis and radiographic progression are increased in elderly-onset rheumatoid arthritis patients: findings from a prospective observational longitudinal early arthritis cohort study. Semin Arthritis Rheum. 2020;50(4):735–43.
Sugihara T, Harigai M. Targeting low disease activity in elderly-onset rheumatoid arthritis: current and future roles of biological disease-modifying antirheumatic drugs. Drugs Aging. 2016;33(2):97–107.
Ruban TN, Jacob B, Pope JE, Keystone EC, Bombardier C, Kuriya B. The influence of age at disease onset on disease activity and disability: results from the Ontario Best Practices Research Initiative. Clin Rheumatol. 2016;35(3):759–63.
Ahluwalia V, Rampakakis E, Movahedi M, Cesta A, Li X, Sampalis JS, et al. Predictors of patient decision to discontinue anti-rheumatic medication in patients with rheumatoid arthritis: results from the Ontario best practices research initiative. Clin Rheumatol. 2017;36(11):2421–30.
Bykerk VP, Akhavan P, Hazlewood GS, Schieir O, Dooley A, Haraoui B, et al. Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J Rheumatol. 2012;39(8):1559–82.
Wolfe F. A reappraisal of HAQ disability in rheumatoid arthritis. Arthritis Rheum. 2000;43(12):2751–61.
Katchamart W, Johnson S, Lin HJ, Phumethum V, Salliot C, Bombardier C. Predictors for remission in rheumatoid arthritis patients: a systematic review. Arthritis Care Res. 2010;62(8):1128–43.
Bykerk VP, Massarotti EM. The new ACR/EULAR remission criteria: rationale for developing new criteria for remission. Rheumatology (Oxford). 2012;51 Suppl 6:vi16–20. https://doi.org/10.1093/rheumatology/kes281.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. https://doi.org/10.1002/art.27584.
Martins FM, da Silva JA, Santos MJ, Vieira-Sousa E, Duarte C, Santos H, et al. DAS28, CDAI and SDAI cut-offs do not translate the same information: results from the Rheumatic Diseases Portuguese Register Reuma.pt. Rheumatology (Oxford). 2015;54(2):286–91.
Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Rheum Dis Clin N Am. 2009;35:745–57.
Weinblatt ME, Keystone EC, Furst DE, Kavanaugh AF, Chartash EK, Segurado OG. Long term efficacy and safety of adalimumab plus methotrexate in patients with rheumatoid arthritis: ARMADA 4 year extended study. Ann Rheum Dis. 2006;65(6):753–9.
Tutuncu Z, Reed G, Kremer J, Kavanaugh A. Do patients with older-onset rheumatoid arthritis receive less aggressive treatment? Ann Rheum Dis. 2006;65(9):1226–9.
Arnold MB, Bykerk VP, Boire G, Haraoui BP, Hitchon C, Thorne C, et al. Are there differences between young- and older-onset early inflammatory arthritis and do these impact outcomes? An analysis from the CATCH cohort. Rheumatology (Oxford). 2014;53(6):1075–86.
Sugihara T, Ishizaki T, Onoguchi W, Baba H, Matsumoto T, Iga S, et al. Effectiveness and safety of treat-to-target strategy in elderly-onset rheumatoid arthritis: a 3-year prospective observational study. Rheumatology. 2021;60(9):4252-61.
Huscher D, Thiele K, Gromnica-Ihle E, Hein G, Demary W, Dreher R, et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009;68(7):1119–24.
Tan TC, Gao X, Thong BY, Leong KP, Lian TY, Law WG, et al. Comparison of elderly-and young-onset rheumatoid arthritis in an Asian cohort. Int J Rheum Dis. 2017;20(6):737–45.
Acknowledgements
We would like to thank the OBRI participants and the current OBRI Clinical Advisory Committee members (Drs. V. Ahluwalia, S. Aydin, E. Keystone, B. Kuriya, A. Lau, J. Pope, and C. Thorne) for the dedication, leadership, and clinical expertise they have provided to OBRI. We would also like to thank our Patient Advisory Committee members for providing their valuable patient perspective (current members: C. Hofstetter, D. Barker, J. Boyle, M. Forbes, L. Linderman, G. Major, E. McQueen, D. Morrice). This work would not be possible without our participating rheumatologists: Drs. V. Ahluwalia, Z. Ahmad, P. Akhavan, L. Albert, C. Alderdice, M. Aubrey, S. Aydin, S. Bajaj, M. Bell, W. Bensen, S. Bhavsar, R. Bobba, C. Bombardier, A. Bookman, J. Brophy, A. Cabral, S. Carette, R. Carmona, A. Chow, G. Choy, P. Ciaschini, A. Cividino, D. Cohen, R. Dhillon, S. Dixit, R. Faraawi, D. Haaland, B. Hanna, N. Haroon, J. Hochman, A. Jaroszynska, S. Johnson, R. Joshi, A. Kagal, A. Karasik, J. Karsh, E. Keystone, N. Khalidi, B. Kuriya, S. Lake, M. Larche, A. Lau, N. LeRiche, Fe. Leung, Fr. Leung, D. Mahendira, M. Matsos, H. McDonald-Blumer, E. McKeown, I. Midzic, N. Milman, S. Mittoo, A. Mody, A. Montgomery, M. Mulgund, E. Ng, T. Papneja, V. Pavlova, L. Perlin, J. Pope, J. Purvis, R. Rai, G. Rohekar, S. Rohekar, T. Ruban, N. Samadi, S. Sandhu, S. Shaikh, A. Shickh, R. Shupak, D. Smith, E. Soucy, J. Stein, A. Thompson, C. Thorne, S. Wilkinson.
Funding
Ontario Best Practices Research Initiative (OBRI) was funded by peer-reviewed grants from the Canadian Institute for Health Research, Ontario Ministry of Health and Long-Term Care, Canadian Arthritis Network, and unrestricted grants from AbbVie, Amgen, Aurora, BMS, Celgene, Gilead, Hospira, Janssen, Lilly, Medexus, Merck, Novartis, Pfizer, Roche, Sandoz, Sanofi, and UCB. Dr. Bombardier held a Canada Research Chair in Knowledge Transfer for Musculoskeletal Care and a Pfizer Research Chair in Rheumatology
Author information
Authors and Affiliations
Contributions
The corresponding author affirms that all authors (XL, AC, MM, CB) contributed to the planning, conduct, and reporting of the work described in the article. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Cox proportional hazards model predicting time to remission within subgroup of seropositive (positive rheumatoid factor or anti-CCP) patients (N=598). Table S2. Cox proportional hazards model predicting time to remission based on SDAI criteria of ≤3.3 (N=748). Table S3. Number of patients who experienced a serious infection, a new cancer or an adverse event during follow-up.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, X., Cesta, A., Movahedi, M. et al. Late-onset rheumatoid arthritis has a similar time to remission as younger-onset rheumatoid arthritis: results from the Ontario Best Practices Research Initiative. Arthritis Res Ther 24, 255 (2022). https://doi.org/10.1186/s13075-022-02952-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-022-02952-1