Abstract
Background
Donors bravely donate their kidneys because they expect that living donor kidney transplantation (LKT) confers benefits to recipients. However, the magnitude of the survival benefit of LKT is uncertain.
Methods
This prospective cohort study used two Japanese nationwide databases for dialysis and kidney transplantation and included 862 LKT recipients and 285,242 hemodialysis (HD) patients in the main model and 5299 LKT recipients and 151,074 HD patients in the supplementary model. We employed time-dependent model in the main model and assessed the hazard ratio and the difference in the restricted mean survival time (RMST) between LKT recipients and HD patients. In the main analysis of the main model (LKT, N = 675; HD, N = 675), we matched LKT recipients with HD patients by age, sex, dialysis vintage, and cause of renal failure and excluded HD patients with dementia or performance status grades 2, 3, or 4.
Results
The median observational period was 8.00 (IQR 3.58–8.00) years. LKT was significantly associated with a lower risk of mortality (hazard ratios (95% confidence interval (CI)), 0.50 (0.35–0.72)) and an increase in life expectancy (7-year RMST differences (95% CI), 0.48 (0.35–0.60) years) compared with HD. In subgroup analysis, the survival benefit of LKT was greater in female patients than in male patients in the Cox model; whereas older patients gained longer life expectancy compared with younger patients.
Conclusions
LKT was associated with better survival benefits than HD, and the estimated increase in life expectancy was 0.48 years for 7 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Living donor kidney transplantation (LKT) has been performed for over six decades [1]. Since LKT requires kidneys from living donors, potential risks for donors cannot be completely eliminated; however, medical professionals make the utmost effort to minimize the physical, psychological, and social risks to individual donors [2, 3]. Nevertheless, living donors donate their kidneys because they expect favorable outcomes for recipients. Therefore, it is important to accurately assess the outcomes of recipients. However, most previous studies have targeted deceased donor kidney transplantation in assessing the survival benefit of kidney transplantation [4], and studies targeting LKTs are scarce. Since the outcomes of LKT recipients could differ from those of recipients of deceased donor kidney transplantation [5, 6], the results of deceased donor kidney transplantation may not be extrapolated to LKT recipients. Although several papers have compared LKT to hemodialysis (HD), most papers targeted a specific population of HD patients or recipients who did not receive modern immunosuppressive agents [7,8,9,10,11]. A study conducted in Denmark, which targeted HD patients registered in the Danish Nephrology Registry, has been reported recently [12]. However, since mortality rates among patients on HD vary across countries [13], these findings may not be generalizable to other countries.
Recently, the restricted mean survival time (RMST) has been used as an alternative measure of treatment effect in some randomized controlled trials with a time-to-event outcome [14]. The RMST is the mean survival time from time 0 to a specific time point. One advantage of the RMST is that it is readily interpretable. Therefore, analyses performed using the RMST may provide LKT donors with additional useful information when they decide to donate their kidneys. Although a recent paper reported the treatment effect of deceased donor kidney transplantation assessed using the RMST [15, 16], to date, no paper has used the RMST for LKT recipients to our knowledge.
This study aimed to assess the survival benefit of LKT in HD patients and determine which subgroup received greater benefit via analyses involving the RMST.
Methods
Data sources, study population, and study design
We used the databases of two Japanese nationwide registries, which are the Japanese Society for Dialysis Therapy Renal Data Registry (JRDR) and the Japan Renal Transplantation Registry (JARTRE). Details of the JRDR survey and the JARTRE survey have been described elsewhere [17,18,19,20,21]. Briefly, the Japanese Society for Dialysis Therapy (JSDT) prospectively surveyed the demographic and clinical data of dialysis patients by sending questionnaires to all dialysis facilities in Japan at the end of each year. The response rate exceeded 95% every year. In the JARTRE survey, the Japan Society for Transplantation (JST) and the Japanese Society for Clinical Renal Transplantation (JSCRT) surveyed the demographic and clinical data of kidney transplant recipients and donors in all Japanese kidney transplantation facilities every year. Data from 2009 to 2011 were collected with a flash drive; since 2012, they have been collected online. This survey covers more than 90% of recipients of kidney transplantation after 2008 in Japan. In this survey, we selected HD patients who had the required data at the end of 2009 in the JRDR survey and LKT recipients who received kidney transplantation from 2009 to 2017 in the JARTRE survey.
In this study, we employed the model in which LKT was treated as a time-varying covariate. The time zero was the end of 2009, all patients at time zero were HD patients in the JRDR database, and we matched the data of patients receiving kidney transplantation from 2010 to 2017 in the JRDR database (N = 2589) to the data of kidney transplantation recipients in the JARTRE database (N = 3317) in this model. The details of method to match patients in the JRDR database to those in the JARTRE database, as follows. First, we created a new identification code based on sex, birth year, year of onset of HD, month of onset of HD, year of reception of kidney transplantation, and month of reception of kidney transplantation. In this method, 99.9% (N = 2585) of the subjects in the JRDR database and 99.8% (N = 3311) of the subjects in the JARTRE database had unique identification codes. Then, we defined the subjects who had similar identification codes as similar subjects, and 1455 subjects were matched. The other subjects who did not have unique identification codes were matched by defining the subjects who had a similar cause of renal failure as similar subjects, and three subjects were matched. Finally, 1458 subjects were matched.
The patient flowchart is shown in Fig. 1. In the HD group, we enrolled those patients on maintenance HD or hemodiafiltration, whose dialysis vintage lasted three months or longer, and who were aged 20 years or older. We excluded those patients who did not have data on age, sex, or dialysis vintage, those who received combined therapy with peritoneal dialysis, and those whose year of death, kidney transplantation, or dialysis withdrawal was unknown. Then, the patients who received kidney transplantation from 2010 to 2017 were moved to the LKT group.
In the LKT group, we enrolled those patients who received HD before undergoing LKT, those whose dialysis vintage was three months or longer, and those who were aged 20 years or older. We excluded those patients who received LKT in 2009 or did not have data on renal replacement therapy before LKT, age, sex, or dialysis vintage; then, we matched the LKT recipients to the HD patients. After matching, we also excluded those patients who received deceased donor kidney transplantation, kidney re-transplantation, or simultaneous transplantation and did not have data on simultaneous transplantation or date of last observation. Simultaneous transplantation included simultaneous pancreas and kidney transplantation and simultaneous liver and kidney transplantation.
In the main analysis of the main model, we conducted two-step selection to minimize the differences of baseline characteristics. First, we excluded patients whose performance statuses were grades 2, 3, or 4 or who had dementia because most HD patients who received kidney transplantation had a grade 0 or grade 1 performance status and no dementia at baseline (Table 1). Performance status was graded according to the Eastern Cooperative Oncology Group Performance Status classification [22]. Dementia was defined by doctors at each facility. After exclusion, we selected HD patients and LKT recipients matched by birth year, sex, dialysis vintage, and cause of renal failure (glomerulonephritis, diabetes, hypertension, polycystic kidney disease, and other/unknown causes). In the matching, we allowed differences in dialysis vintage of less than 1 year.
All patients were followed up until death, the end of the study (December 31, 2017), or loss to follow-up. Patients without death were censored at the end of the study or loss to follow-up. Since the month of the start of dialysis, death, or loss to follow-up was unknown for some HD patients, we assigned the month of June in such cases for these patients. Since there were no data on the day of death or loss to follow-up in the HD group (though the LKT group had the required data), we defined the day of death or loss to follow-up in the HD group as the midpoint of the days in the month of the death or loss to follow-up. The study protocol was approved by the Ethics Committee of the JSDT (approval number: 49), the Ethics Committee at Kobe University Graduate School of Medicine (approval number: B200241), and the ethics committee of the JST, and was conducted per the principles of the Helsinki Declaration. The need for participants’ informed consent was waived. The studies were in accordance the Declaration of Istanbul.
Statistical analysis
Data are presented as the mean and standard deviation for continuous variables with a normal distribution, the median and interquartile range for continuous variables with a skewed distribution, or the number and percentage for categorical variables.
Survival curves in each group were drawn using the Simon and Makuch method [23] with statistical comparison using the Mantel–Byar method [24, 25]. The time-dependent Cox proportional hazard regression analysis was also used to compare mortality rates between groups. In addition, we assessed differences in the RMST and ratios of the restricted mean time lost (RMTL), which is the area above the survival curve [26], between the HD and LKT groups. The following three time points were used for this analysis: 3 years, 5 years, and 7 years.
Furthermore, we performed similar analysis in the model to simply compare LKT recipients in the JARTRE database to HD patients in the JRDR database as a supplementary model having larger sample size compared with the main model. In this model, the time zero in the HD group was the end of 2009, whereas the time zero in the LKT group was the moment LKT was performed. The patient flow chart in the model is shown in Supplementary Fig. 1. In main analysis of this model, we targeted patients who received LKT from 2009 to 2013; however, we also performed the analysis targeting LKT recipients from 2009 to 2010 or those from 2009 to 2017 as sensitivity analysis. In this model, survival curves were drawn using Kaplan–Meier methods with statistical comparison using the log-rank test.
We also performed other sensitivity analyses. First, we performed a conventional multivariable Cox proportional hazard regression analysis in the unmatched population. In this analysis, the adjusted variables were all the baseline characteristics listed in Table 1. If serum albumin levels were under 4 g/dL, serum calcium levels were corrected using the following formula: corrected calcium = serum calcium (mg/dL) + [4 − serum albumin (g/dL)]. If the parathyroid hormone (PTH) assay was whole, whole PTH levels were multiplied by 1.7 to obtain equivalent values measured by an intact PTH assay [27]. Dialysis vintage, intact PTH, and C-reactive protein (CRP) were log-transformed to normalize the distribution. Second, we also performed a conventional multivariable Cox proportional hazard regression analysis after multiple imputations were conducted because some covariates had missing data (Supplementary Table 1). We imputed the missing values using fully conditional chained equations, created five imputed datasets, and combined them using Rubin's rules [28]. Third, propensity score matching was performed using nearest-neighbor 1:1 matching. Propensity scores were created using multivariable logistic regression analyses to estimate the probability of receiving or not receiving LKT. All variables using a conventional multivariable Cox proportional hazard regression analysis were included as covariates. Fourth, the inverse probability of treatment weighting (IPTW) was used to balance differences across groups [29]. We performed a Cox proportional hazard regression analysis after each patient was weighted by the inverse of the probability of that patient being assigned to LKT. Fifth, we performed a Cox proportional hazard regression analysis for only HD patients with an HD frequency of three times per week or an HD frequency of three times per week and ≥ 4-h session length. Finally, in the supplementary model, we performed a Cox proportional hazard regression analysis for only HD patients with a grade 0 performance status and a hazard ratio of death was estimated for each of the two intervals of time following the baseline: less than 1 year and at least 1 year.
Subgroup analyses were performed for age, sex, dialysis vintage, and causes of renal failure in a Cox proportional hazard regression analysis and the assessment of differences in the RMST and ratios of the RMTL. In the assessment of differences in the RMST and ratios of the RMTL, interaction terms were estimated using Tian et al.’s method [30, 31].
In the comparison of each baseline variable, a standardized mean difference of < 10% was considered to denote a negligible difference between the groups. A p value of < 0.05 was considered statistically significant. All statistical analyses were performed using Stata/MP 14.2 software for Windows (Stata, College Station, TX, USA).
Results
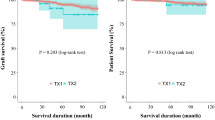
Table 1 shows the baseline characteristics for each group before and after matching and restriction in the main model. Most variables were balanced after matching and restriction, except for the dialysis time, history of cerebral infarction, and serum creatinine levels. The median observational periods were 8.00 (IQR 3.58–8.00) years. The survival curve using the Simon and Makuch method showed that the survival rate in the LKT group was significantly higher than that in the HD group (Fig. 2). The annual mortality rates for HD patients and LKT recipients were 2.03 and 1.20 per 100 patient-year, respectively. At 5 years, patient survival in the HD and the LKT groups was 87.2% and 98.0%, respectively.
Figure 3 shows the results of the Cox proportion hazard regression analyses in all patients and among the various subgroups. LKT was significantly associated with a lower risk of mortality than HD (hazard ratio (95% confidence interval (CI)), 0.50 (0.35–0.72)). In the sensitivity analyses, the association was consistent (Table 2). In most subgroups, LKT was significantly associated with a lower risk of mortality than HD. The survival benefit of LKT vs. HD was more pronounced in female patients; whereas, the benefit did not differ significantly regarding of age, dialysis vintage, or diabetic nephropathy.
Table 3 and Supplementary Tables 2–3 show the RMST and the RMTL in the LKT group and the HD group. The 7-year RMST in the LKT group was significantly higher than that in the HD group (LKT vs. HD; 6.90 (6.85–6.95) vs. 6.42 (6.31–6.54) years). The 7-year RMST differences were 0.48 (0.35–0.60) years. In a subgroup analysis using RMST, the survival benefit of LKT vs. HD was more pronounced in older patients; whereas, the benefit did not differ significantly among subgroups stratified by sex, dialysis vintage, or diabetic nephropathy. The results of the RMTL ratio among subgroups had trends similar to those in Cox proportional hazard regression analyses among subgroups.
Supplementary Table 4 shows the baseline characteristics for each group before and after matching and restriction in the supplementary model to simply compare LKT recipients to HD patients. Variables in the HD group of this model after matching and restriction did not differ significantly from those in the HD group of the main model. The median observational period was 7.87 (interquartile range (IQR) 3.20–8.00) years. In this model, the annual mortality rates for HD patients and LKT recipients were 2.72 and 1.02 per 100 patient-year, respectively. At 5 years, patient survival in the HD and the LKT groups was 87.8% and 95.9%, respectively. The Kaplan–Meier survival curve showed that the survival rate in the LKT group was significantly higher than that in the HD group (Supplementary Fig. 2). The association was consistent in Cox proportional hazard regression analyses including sensitivity analysis (Supplementary Table 5). Although the hazard ratio during the first year after the baseline was higher than that at 1 year or longer after the baseline, mortality in the LKT group was significantly lower than that in the HD group during even the first year (hazard ratio (95% CI), the first 1 year, 0.60 (0.39–0.91) and, 1 year or longer, 0.35 (0.29–0.43), respectively). In subgroup analysis using Cox proportional hazard models, the survival benefit of LKT vs. HD was more pronounced in younger patients and patients with shorter dialysis vintage in addition to female patients (Supplementary Fig. 3). In subgroup analysis using RMST, the survival benefit of LKT vs. HD was more pronounced in patients with diabetic nephropathy in addition to older patients (Supplementary Table 6–8).
Discussion
In this study, LKT was associated with a 50% reduction in mortality. A recent meta-analysis has shown that kidney transplantation was associated with a 55% reduction in mortality [4]. Although the patients in most of the populations included in the meta-analysis received deceased donor kidney transplantation, one study has reported that LKT was associated with a 70% mortality risk reduction [12]. In our study, LKT was associated with a 50% reduction in mortality, which was similar to benefit of deceased donor kidney transplantation and a little smaller than that of LKT reported in previous papers [4, 12]. In addition, our study demonstrated that annual mortality rates were 2.03 per 100 patient-years and 5-year survival rates were 87.2% in the HD group, which was better than those of HD patients on waiting lists in previous studies [12, 32, 33]. Taken together, LKT may have a significant survival benefit even in patients on HD with low mortality rates.
We assessed the survival benefit of LKT using not only the hazard ratio but also RMST. The analysis using RMST revealed that LKT was associated with an increase in life expectancy of 0.48 years during an observation period of 7 years and 0.25 years during an observation period of 5 years. Previous studies have shown that deceased donor kidney transplantation has been associated with an increase in life expectancy of 0.53 years or 0.20 years over 5 years [15, 16], the survival benefit of LKT in our study may be similar or a little smaller than that of the deceased donor kidney transplantation in terms of the RMST. The discrepancy with the results in the analysis performed using the hazard ratio may be due to the good survival of HD patients in our study. When hazard ratios are similar, the difference of RMST reduces in patients with better survival rates. Since the survival in our study was better than that reported in previous studies [15, 16], the RMST in our study could be smaller than that in previous studies.
RMST has been recently used in the assessment of survival benefits. It may be difficult for patients and clinicians to understand the exact meaning of the hazard ratio; meanwhile, the difference in life expectancy as assessed by RMST is simple and understandable [15]. Since understandability is important for people who will donate their living kidneys, the analysis by RMST should be performed in the assessment of the survival benefit of LKT.
Per our findings, kidney transplantation provided greater survival benefits in older patients in the analysis using RMST. Since this finding is in line with those of a previous study using RMST [16], older patients may gain longer life expectancy compared to younger patients. On the other hand, younger patients may have a lower hazard ratio than older patients per the results of the analysis performed using Cox models. Since previous studies have shown that hazard ratios in younger patients were lower or equal to those in older patients [12, 33,34,35,36,37], this finding about the hazard ratio is also in line with the results of previous studies. The results assessed by RMST may sometimes be inconsistent with those obtained using the hazard ratio [38]. However, since studies that used both RMST and the hazard ratio are scarce in subgroup analyses for the survival benefit of kidney transplantation, further studies need to be conducted to pinpoint the reason for the discrepancy.
The survival benefit of LKT was greater in female patients than in male patients in the Cox model; however, the benefit did not differ significantly between the two sexes in the analysis using RMST. The results of females' greater benefit were inconsistent with those of previous studies, which have shown that the survival benefit in female patients was similar to that in male patients [33, 34, 36, 37]. This discrepancy could be due to the difference in survival rate between female patients and male patients. The survival rate did not differ significantly between the two sexes in the aforementioned studies [33, 34, 36, 37], while female patients had a significantly better survival rate than male patients in our study. When the gain in life expectancy does not differ significantly, the Cox model shows a lower hazard ratio in patients with better survival rates. Therefore, the increase in life expectancy may not differ significantly between female patients and male patients.
In our study, patients with diabetic nephropathy may gain longer life expectancy than those without diabetic nephropathy. To the best of our knowledge, no paper has compared RMSTs between patients with and without diabetic nephropathy. Previous studies using hazard ratios have reported inconsistent results [32,33,34, 36, 37]. As we mentioned above, the difference of RMST reduces in patients with better survival rates when hazard ratio does not differ significantly. Since HD patients with diabetic nephropathy have a poor prognosis [39, 40], the gained life expectancy of patients with diabetic nephropathy might be longer than that of those without diabetic nephropathy.
Our study had several limitations. First, we could not use the data of a transplant waiting list. In an assessment of the survival benefit of kidney transplantation, many papers have compared dialysis patients on a waiting list because many dialysis patients have contraindications to kidney transplantation, and not using patients on a waiting list introduces a significant selection bias [4]. Our approach of using the data of the performance and dementia statuses could minimize the selection bias because the survival rate in the HD group of our study was better than the rates in previous studies using waiting lists [12, 15, 16, 32, 33]. However, in our study, patients in the HD group had higher proportion of cerebral infarction, shorter dialysis time, and lower serum creatinine levels compared with those in the LKT group, though we performed multivariable Cox proportional hazard model adjusting these factors and the results were comparable with that in the main analysis. On the other hand, some characteristics may differ between patients receiving LKT and patients receiving deceased donor kidney transplantation. In Japan, dialysis vintage differed significantly (LKT vs. deceased donor kidney transplantation, 3.0 vs. 15.8 years in 2015) [20]. Therefore, even if HD patients on waiting lists were enrolled, the selection bias could not have been sufficiently minimized. Further studies are needed to clarify whether matching using performance status and dementia is appropriate for comparison between LKT and HD. Second, unique identifying variables to match patients in the two databases were not lacking. However, the identification code created in our study was unique for over 99% of patients, and this limitation is unlikely to render the treatment effect systematically biased in favor of either transplantation or dialysis [32]. Third, since the population in our study had good survival rate, generalizability to the population with poor survival rate was uncertain. Fourth, the data about history of malignancy is lacking in this study. Since Kidney Disease: Improving Global Outcomes guidelines recommend that patients with active malignancy be excluded from kidney transplantation [41], the number of patients with history of malignancy in LKT group will be small. Further studies are needed to clarify how this lack of data influences the benefit of LKT.
In conclusion, LKT was associated with better survival benefits than HD, and the estimated increase in life expectancy was 0.48 years for 7 years. Although the benefit was observed across most subgroups, the magnitude of the benefit may differ among subgroups.
Data availability
The data underlying this article were provided by the JSDT, JST, and JSCRT by permission. Data will be shared on request to the corresponding author with permission of the JSDT, JST, and JSCRT.
References
Delmonico F; Council of the Transplantation Society. A report of the Amsterdam forum on the care of the live kidney donor: data and medical guidelines. Transplantation. 2005;79:S53-66.
Mjøen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int. 2014;86:162–7.
Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579–86.
Chaudhry D, Chaudhry A, Peracha J, et al. Survival for waitlisted kidney failure patients receiving transplantation versus remaining on waiting list: systematic review and meta-analysis. BMJ. 2022;376: e068769.
Chkhotua AB, Klein T, Shabtai E, et al. Kidney transplantation from living-unrelated donors: comparison of outcome with living-related and cadaveric transplants under current immunosuppressive protocols. Urology. 2003;62:1002–6.
Gjertson DW, Cecka JM. Living unrelated donor kidney transplantation. Kidney Int. 2000;58:491–9.
Vollmer WM, Wahl PW, Blagg CR. Survival with dialysis and transplantation in patients with end-stage renal disease. N Engl J Med. 1983;308:1553–8.
Garcia-Garcia G, Deddens JA, D’Achiardi-Rey R, et al. Results of treatment in patients with end-stage renal disease: a multivariate analysis of risk factors and survival in 341 successive patients. Am J Kidney Dis. 1985;5:10–8.
Medin C, Elinder CG, Hylander B, et al. Survival of patients who have been on a waiting list for renal transplantation. Nephrol Dial Transpl. 2000;15:701–4.
Glanton CW, Kao TC, Cruess D, et al. Impact of renal transplantation on survival in end-stage renal disease patients with elevated body mass index. Kidney Int. 2003;63:647–53.
Pauly RP, Gill JS, Rose CL, et al. Survival among nocturnal home haemodialysis patients compared to kidney transplant recipients. Nephrol Dial Transpl. 2009;24:2915–9.
Sørensen VR, Heaf J, Wehberg S, et al. Survival benefit in renal transplantation despite high comorbidity. Transplantation. 2016;100:2160–7.
Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the dialysis outcomes and practice patterns study (DOPPS). J Am Soc Nephrol. 2003;14:3270–7.
Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152.
Lenain R, Boucquemont J, Leffondré K, et al. Clinical trial emulation by matching time-dependent propensity scores: the example of estimating impact of kidney transplantation. Epidemiology. 2021;32:220–9.
Strohmaier S, Wallisch C, Kammer M, et al. Survival benefit of first single-organ deceased donor kidney transplantation compared with long-term dialysis across ages in transplant-eligible patients with kidney failure. JAMA Netw Open. 2022;5: e2234971.
Nakai S, Iseki K, Itami N, et al. Overview of regular dialysis treatment in Japan (as of 31 december 2009). Ther Apher Dial. 2012;16:11–53.
Yuzawa K, Takahara S, Kanmochi T, et al. New registry and tracking system for renal transplantation in Japan. Transpl Proc. 2010;42:4010–3.
Yuzawa K, Takahara S, Kanmochi T, et al. Evolution of registry and tracking system for organ transplantation in Japan. Transpl Proc. 2012;44:828–31.
Yagisawa T, Mieno M, Yoshimura N, et al. Current status of kidney transplantation in Japan in 2015: the data of the kidney transplant registry committee, Japanese society for clinical renal transplantation and the Japan society for transplantation. Ren Replace Ther. 2016;2:68.
Yagisawa T, Mieno M, Ichimaru N, et al. Trends of kidney transplantation in Japan in 2018: data from the kidney transplant registry. Ren Replace Ther. 2019;5:3.
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–55.
Schultz LR, Peterson EL, Breslau N. Graphing survival curve estimates for time-dependent covariates. Int J Methods Psychiatr Res. 2002;11:68–74.
Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–9.
Mantel N, Byar DP. Evaluation of response-time data involving transient states; an illustration using heart-transplant data. J Am Stat Assoc. 1974;69:81–6.
Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32:2380–5.
Fukagawa M, Yokoyama K, Koiwa F, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17:247–88.
Rubin DB, editor. Multiple imputation for nonresponse in surveys. John Wiley & Sons Inc; 1987.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79.
Cronin A, Tian L, Uno H. strmst2 and strmst2pw: New commands to compare survival curves using the restricted mean survival time. Stata Journal. 2016;16:702–16.
Tian L, Zhao L, Wei LJ. Predicting the restricted mean event time with the subject’s baseline covariates in survival analysis. Biostatistics. 2014;15:222–33.
Rabbat CG, Thorpe KE, Russell JD, et al. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11:917.
Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol. 2005;16:1859–65.
Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30.
McDonald SP, Russ GR. Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991–2001. Nephrol Dial Transpl. 2002;17:2212–9.
Yoo KD, Kim CT, Kim MH, et al. Superior outcomes of kidney transplantation compared with dialysis: an optimal matched analysis of a national population-based cohort study between 2005 and 2008 in Korea. Medicine (Baltimore). 2016;95: e4352.
Kaballo MA, Canney M, O’Kelly P, et al. A comparative analysis of survival of patients on dialysis and after kidney transplantation. Clin Kidney J. 2018;11:389–93.
Trinquart L, Jacot J, Conner SC, et al. Comparison of treatment effects measured by the hazard ratio and by the ratio of restricted mean survival times in oncology randomized controlled trials. J Clin Oncol. 2016;34:1813–9.
Hayashino Y, Fukuhara S, Akiba T, et al. Diabetes, glycaemic control and mortality risk in patients on haemodialysis: the Japan dialysis outcomes and practice pattern study. Diabetologia. 2007;50:1170–7.
Rayner HC, Pisoni RL, Bommer J, et al. Mortality and hospitalization in haemodialysis patients in five European countries: results from the dialysis outcomes and practice patterns study (DOPPS). Nephrol Dial Transpl. 2004;19:108–20.
Kidney disease: improving global outcomes (KDIGO) kidney transplant candidate work group. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation. 2020; 104:S1–S103.
Acknowledgements
We thank the members of the Committee of Renal Data Registry of the JSDT and the personnel at the participating institutions involved in the survey of the JRDR or JARTRE. The data reported in this study were supplied by the JRDR and JARTRE. The interpretation and reporting of these data are the responsibilities of the authors and should in no way be seen as an official policy or interpretation of the JSDT, JST, or JSCRT. This study was supported in part by a Grant-in-Aid for Investigating New Evidence to Understand the Safety of Kidney Transplantation from Marginal Donors and Promotion of Renal Disease Control Grants from the Japan Agency for Medical Research and Development. A part of the results of the study were presented as abstracts at the 68th Annual Meeting of the Japanese Society for Dialysis Therapy. The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
Open access funding provided by Kobe University. This study was supported in part by a Grant-in-Aid for Investigating New Evidence to Understand the Safety of Kidney Transplantation from Marginal Donors and Promotion of Renal Disease Control Grants from the Japan Agency for Medical Research and Development.
Author information
Authors and Affiliations
Contributions
SG was responsible for the research idea and study design and contributed to the analysis and drafting of the manuscript. HF, MM, TY, MA, KN, and SN contributed to the concept of design, analysis, interpretation of data, and revision of the manuscript. All authors have approved the final version of the article.
Corresponding author
Ethics declarations
Conflict of interest
S.N. reports scholarship grants from Chugai Pharmaceutical. All remaining authors have nothing to disclose.
Ethical approval
The study protocol was approved by the Ethics Committee of the JSDT (approval number: 49), the Ethics Committee at Kobe University Graduate School of Medicine (approval number: B200241), and the ethics committee of the JST, and was conducted per the principles of the Helsinki Declaration. The studies were in accordance the Declaration of Istanbul.
Informed consent
The need for participants’ informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Goto, S., Fujii, H., Mieno, M. et al. Survival benefit of living donor kidney transplantation in patients on hemodialysis. Clin Exp Nephrol 28, 165–174 (2024). https://doi.org/10.1007/s10157-023-02417-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-023-02417-y