Abstract
The waiting time for deceased donor kidney transplants (DDKT) is increasing. We evaluated DDKT prognosis according to the pretransplant dialysis vintage. A total of 4117 first-time kidney transplant recipients were enrolled from a prospective nationwide cohort in Korea. DDKT recipients were divided into tertiles according to pretransplant dialysis duration. Graft failure, mortality, and composite were compared between DDKT and living donor kidney transplant (LDKT) recipients. Pretransplant dialysis vintage was longer annually in DDKT recipients. In the subdistribution of the hazard model for the competing risk, the first tertile did not show an increased risk of graft failure compared with LDKT recipients; however, the second and third tertile groups had an increased risk of graft failure compared to LDKT recipients (adjusted hazard ratio [aHR] 3.59; 95% confidence interval [CI] 1.69–7.63; P < 0.001; aHR 2.37; 95% CI 1.06–5.33; P = 0.037). All DDKT groups showed a significantly higher risk of patient death than LDKT, with the highest risk in the third tertile group (aHR 11.12; 95% CI 4.94–25.00; P < 0.001). A longer pretransplant dialysis period was associated with a higher risk of the composite of patient death and graft failure in DDKT recipients. DDKT after a short period of dialysis had non-inferior results on graft survival compared with LDKT.
Similar content being viewed by others
Introduction
Kidney transplantation (KT) is the best treatment option with many advantages over dialysis in patients with end-stage kidney disease (ESKD). Although deceased donor kidney transplant (DDKT) has a much better prognosis than maintenance dialysis patients1,2, it has a worse prognosis than living donor kidney transplant (LDKT). The waiting time for DDKT is steadily increasing because of the growing gap between the rapidly increasing demand for organs and the slowly increasing donated organs3.
The pretransplant dialysis duration affects the outcomes after KT. Some differences have been found in the safety window of the dialysis period before KT. The shorter the pretransplant dialysis period, the better the prognosis after KT4,5,6,7. Our recent study of a Korean LDKT cohort also confirmed that pretransplant dialysis longer than 6 months increases the risk of graft failure8. However, most studies that evaluated the association of pretransplant duration and prognosis analyzed LDKT and DDKT recipients without separating them. Few studies have involved DDKT recipients, and no comparison with LDKT has been made4,9,10.
Pretransplant dialysis duration might not be a major obstacle due to recent progress in dialysis technology and methods11,12,13. However, many patients with ESKD included in the studies on the association between pretransplant dialysis duration and posttransplant prognosis had received KT in the last century or decades ago, which does not reflect the recent dialysis trend9,14,15,16. In addition, differences among countries exist in the proportion of each dialysis method, preferred dialysis membrane, and detailed dialysis settings such as blood flow rate11,17. Different ethnic backgrounds and adherence may also affect the association between dialysis duration and transplant outcome18,19.
This study evaluated the effect of pretransplant dialysis vintage on the clinical outcomes of DDKT recipients using a nationwide Korean cohort of KT recipients who had recently received the transplant. It will allow us to ascertain the impact of the waiting period on the prognosis after KT.
Results
Baseline characteristics
The distribution and annual change in pretransplant dialysis vintage are shown in Fig. 1. The median pretransplant dialysis period was 83.1 months (interquartile range [IQR] 50.4–114.8 months) in DDKT recipients. The trend analysis shows the pretransplant dialysis duration gradually increased every year (P = 0.049).
A comparison of baseline characteristics between LDKT and DDKT patients stratified by pretransplant dialysis vintage is shown in Table 1. The first tertile was 648 patients with pretreatment dialysis for up to 60.5 months (median 38.3 months), the second tertile was 651 patients between 60.5 and 101.8 months (median 83.1 months), and the third tertile was 647 patients over 101.8 months (median 131.7 months). The DDKT patients were older on average than the LDKT patients (P < 0.001). The proportion of patients with pretransplant desensitization was almost one-third of the LDKT group but was rare in the DDKT groups (P < 0.001). Deceased donors had diabetes and hypertension more frequently compared with living donors (both P < 0.001). The proportion of induction immunosuppressant of anti-thymocyte globulin was higher in DDKT recipients than in LDKT recipients (P < 0.001), and it was the highest in the third tertile group (33.0%). The proportion of maintenance use of tacrolimus was also higher in DDKT recipients (P < 0.001).
Primary composite outcome of both patient death and graft failure
A total of 142 composite events (7.3%) were observed in DDKT recipients (Table 2). In the Kaplan–Meier curve for the composite outcome, all tertile groups showed lower event-free survival compared with the LDKT group (log-rank P < 0.001) (Fig. 2A). In addition, the results of the multivariable Cox regression models for composite outcome showed a consistently higher risk in increasing tertile order (model 3; first tertile: adjusted hazard ratio [aHR] 3.24, 95% confidence interval [CI] 1.87–5.62, P < 0.001; second tertile: aHR 3.25, 95% CI 1.85–5.73, P < 0.001; third tertile: aHR 5.31, 95% CI 3.12–9.03, P < 0.001) (Table 3). Among the DDKT recipients, third tertile group independently showed increased risk of composite outcome and second tertile group showed non-inferior results compared with first tertile group (Supplementary Table 1). A longer pretransplant dialysis vintage in DDKT was also consistently associated with an increased risk of composite outcome compared with LDKT in subgroups defined by age, sex, body mass index (BMI), Biopsy-proven acute rejection (BPAR), donor age, and comorbid diabetes (Supplementary Fig. 1).
Patient death
Overall, 77 deaths (3.9%) among DDKT recipients occurred within the study period (Table 4). Eleven patients died from cardiovascular disease (14.3% of total deaths), and 33 died from infectious causes (42.9% of total deaths). The Kaplan–Meier curve showed that patient survival in DDKT was significantly lower than in LDKT (P < 0.001) (Fig. 2B). After accounting for confounding factors, the rate of patient death was significantly higher in all DDKT groups compared with the LDKT group (model 3; first tertile: aHR 5.29, 95% CI 2.30–12.16, P < 0.001; second tertile: aHR 3.10, 95% CI 1.21–7.99, P = 0.019; third tertile: aHR 11.12, 95% CI 4.94–25.00, P < 0.001). Older age, pretransplant desensitization, comorbid diabetes, and cardiac disease were independent risk factors for mortality in the multivariable Cox regression model (all P < 0.05). Among the DDKT recipients, only third tertile group independently showed increased mortality compared with first tertile group (Supplementary Table 2).
Graft failure
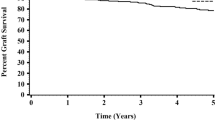
Sixty-five DDKT recipients (3.3%) lost graft function during the study period (Table 2). The Kaplan–Meier curve showed that death-censored graft survival was significantly lower in DDKT, with the lowest in the second tertile groups (log-rank P = 0.011) (Fig. 2C). For a more accurate estimation of graft failure, we applied a competing risk analysis model. The Aalen–Johansen method revealed a significantly increased cumulative incidence for graft failure in second tertile DDKT patients compared with LDKT patients (P < 0.001) (Fig. 3). The multivariable proportional hazards model for the subdistribution of the competing risk showed that the first tertile group consistently showed no increased risk of graft failure compared with the LDKT group. However, the second and third tertiles of pretransplant dialysis vintage were associated with a significantly higher risk of graft failure (model 3; second tertile: aHR 3.59, 95% CI 1.69–7.63, P < 0.001; third tertile: aHR 2.37, 95% CI 1.06–5.33, P = 0.037) (Table 5). Recipient BMI and donor age were independent risk factors of graft failure in DDKT recipients (both P < 0.05). In the subgroup competing risk analysis for graft failure, the first tertile group show no increased risk of graft failure in most subgroups compared with the LDKT group (Fig. 4). Among the DDKT recipients, there was no increase of graft failure risk in second and third tertile groups compared with first tertile group (Supplementary Table 3).
Supplementary Fig. 2 shows the serial changes in tacrolimus trough level across the groups. Overall, the mean tacrolimus trough levels were similar after transplant, and the level was significantly higher 4 years after KT in the second and third tertiles compared with the LDKT group.
Biopsy-proven acute rejection
BPAR incidence did not differ among LDKT and DDKT groups during the study period (Table 2). The Kaplan–Meier curve showed BPAR-free survival within 1 and 5 years after transplant also did not vary among the groups (Supplementary Fig. 3). In the univariable and multivariable Cox regression models for BPAR, pretransplant dialysis vintage in DDKT was not associated with the risk of BPAR compared with the LDKT (all P > 0.05) (Supplementary Table 4).
Discussion
This study demonstrated that a longer pretransplant dialysis is associated with a worse prognosis in DDKT. The risk of the composite outcome of both patient death and graft failure, indicating actual graft failure, was increased with the pretransplant dialysis vintage. Considering patient death as a competing risk, the risk of graft failure in DDKT recipients with a shorter pretransplant dialysis duration was not inferior to that of LDKT recipients. This finding supports the need for efforts to shorten the waiting time for DDKT.
A recent Austrian national cohort study confirmed that pretransplant dialysis duration did not affect the risk of graft failure in transplants performed since 200013. They suggested that erythropoiesis-stimulating agents have decreased transfusion frequency and sensitization risk, thereby improving transplant outcomes. However, the patients included in the Austrian study had undergone a relatively shorter period of pretransplant dialysis than in the present study; thus, the effect of pretransplant dialysis vintage could not be clearly identified in this study. Korean patients must start dialysis before registering on the waiting list for DDKT, and the waiting period until DDKT was much longer than in other countries. Therefore, our cohort data were enabled the analysis of the effect of long-term pretransplant dialysis on transplant outcomes, and long pretransplant dialysis resulted in decreased graft survival. This is consistent with previous studies reporting that a longer pretransplant dialysis increased the risk of sensitization and was associated with the increased risk of graft failure9,16.
For accurate analysis, we applied several competing risk analysis methods to estimate the association of pretransplant dialysis vintage with the risk of graft failure. Traditional analysis methods, such as Kaplan–Meier method and Cox regression model, for kidney allograft survival are limited in estimating long-term graft survival20. The competing risk analysis provides more accurate estimates of graft survival by correcting for the erroneous assumption that dead recipients remain at risk for graft failure11. We subsequently confirmed that DDKT recipients undergoing pretransplant dialysis up to 60.5 months did not have an increased risk of graft failure compared with LDKT recipients8. Previously, Meier-Kriesche et al.16 reported that the waiting time on dialysis was the strongest modifiable risk factor for kidney transplant outcome, and the effect was prevalent in DDKT. They found the effect of pretransplant dialysis on graft survival was equivalent between DDKT recipients undergoing pretransplant dialysis up to 6 months and LDKT recipients undergoing pretransplant dialysis up to 2 years. Our study showed DDKT recipients with a longer period of dialysis had comparable allograft outcomes with LDKT patients. The differences in the pretransplant dialysis duration might be attributable to the improvement of the dialysis technique and the decrease of the impact of dialysis in DDKT. Furthermore, we used the Aalen–Johansen estimates of incidence, which is considered a more appropriate method of controlling the competing risk in long-term graft survival after KT than the Fine–Gray competing risk model or the traditional survival analysis20.
Our cohort data revealed that infection-related death was the most common cause of patient death in DDKT. Infection-related mortality and all-cause mortality were highest in KT recipients with extended pretransplant dialysis. One possible explanation could be the immunosuppression status. Recent advances in immunosuppressive agents have made KT possible in sensitized patients with extended dialysis, and strong immunosuppressive therapy is needed to prevent rejection in patients13,21. As Lenihan et al.22 reported the changing complications trend in KT recipients in the United States, the incidence of infectious disease remained similar over 10 years despite the improvements in overall transplant outcomes and serious cardiovascular events. A longer pretransplant dialysis duration was a risk factor for infection-related mortality in DDKT recipients23,24. We confirmed that a similar or higher tacrolimus concentration was prescribed up to 5 years in DDKT patients at high risk of infection. Therefore, for KT recipients who underwent an extended dialysis period, the intensity of immunosuppression needs to be individually tailored and modulated to consider the risk of serious infectious complications. This suggestion can be supported by the similarity in BPAR frequency among DDKT patients regardless of pretransplant dialysis duration.
This study features several strengths. We conducted the study using data on patients who underwent KT in recent years, during which advanced immunosuppression protocols such as a monitoring of donor-specific antibodies were applied to all patients, and pretransplant dialysis was performed using the latest methods. The results of previous studies might be limited in this respect because they included the patients who received transplants several decades ago4,11,13,25. In addition, we evaluated the effect of dialysis duration only in the DDKT recipients except for LDKT because DDKT often has more vulnerable recipients or allograft status than LDKT. Finally, this is the first study to evaluate the effect of pretransplant vintage on the clinical outcomes of DDKT in Asian cohorts. Differences were noted in the prevalence of ESKD, distribution of dialysis modality, healthcare resources, health insurance system, and ethnic characteristics between Western and Asian countries, which affect transplant outcomes13.
This study has some limitations. First, this was an observational study and inevitably has unmeasured confounding factors. However, we tried to reduce the residual confounders by adjusting for multivariable factors and using competing risk analysis methods. Second, the transplant centers varied in clinical practice quality. The quality of kidney transplant centers has a significant association with mortality26. However, the Data from the Korean Organ Transplant Registry (KOTRY) database included most of the major transplant centers in Korea, minimizing the center effect.
In conclusion, pretransplant dialysis duration is an independent risk factor for patient death and graft failure in DDKT recipients. DDKT recipients with shortened pretransplant dialysis periods had good graft survival comparable with that of LDKT recipients. Therefore, efforts to reduce the waiting time for DDKT are needed, such as timely enrollment to the waiting list and maintaining healthy recipient conditions. A national policy that emphasizes the pretransplant dialysis period on allocation should be prioritized to improve outcomes in DDKT.
Methods
Study design and patient population
Data from the KOTRY were used in this study. The KOTRY has prospectively collected Korean transplant data from nationwide 59 transplant centers since 201427. All first-time single-organ LDKT recipients and DDKT recipients between 2014 and 2019 in the KOTRY database were included. Recipients younger than 19 years or those who underwent simultaneous multiorgan transplantation were excluded from the KOTRY. The detailed design and methods for the KOTRY are presented in a previously published article28. We divided DDKT recipients into three groups according to the pretransplant dialysis period then compared their clinical outcomes with those of the LDKT recipients who underwent pretransplant dialysis less than 6 months. The control group, LDKT recipients with pretransplant dialysis less than 6 months, showed the best graft survival in our previous study8.
Data collection
A total of 6,118 newly kidney transplanted patients were registered in the KOTRY database during the study period. Among them, 1,529 LDKT recipients with pretransplant dialysis longer than 6 months and 472 recipients of second KT were excluded. The remaining 4,117 KT recipients were included in the analysis. The baseline demographic characteristics of the recipients and donors, laboratory data, graft failure, patient death, and occurrence of delayed graft function and rejection were obtained. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease Equation29.
Outcomes
The main outcomes were graft failure, all-cause mortality, and the composite of both. Graft survival time was defined as the time from KT to the initiation of permanent dialysis, second KT, or end of follow-up. Patient survival time was defined as KT to death from any cause or end of follow-up. A competing risk analysis was applied to avoid censoring for patient death in analyzing the risk of graft failure. BPAR was also compared and diagnosed based on the Banff 07 classification30.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median (IQR) depending on their distribution, and categorical variables are presented as number and percentage. One-way analysis of variance or the Kruskal–Wallis test was used to determine the differences in continuous variables, as appropriate, whereas for categorical variables, Pearson’s chi-square test or Fisher’s exact test was used. The Cochran–Armitage trend test was performed to analyze the time trend of pretransplant dialysis vintage. Kaplan–Meier curves and the log-rank test were used to compare the differences in graft survival, patient survival, composite event-free survival, and early BPAR-free survival among the KT groups. The competing risk method using the Aalen–Johansen estimate was used to compare the cumulative incidence rates of graft failure among the groups31,32, and patient death was set as a competing event. The association between pretransplant dialysis vintage and clinical outcomes, including composite outcomes, was further determined using multivariable Cox proportional hazard regression models. The adjustment factors selected were baseline characteristics and clinically relevant variables. Patient death can be a competing event on graft failure; thus, we used the Fine and Gray competing risk model for subdividing a competing risk (patient death) to compare the risk of graft failure33. Subgroup analyses by age, sex, body mass index, early BPAR, donor age, and comorbid diabetes were performed for patient death and graft failure. The graft failure was also analyzed by competing risk analysis in subgroup analysis. Statistical analyses were performed with SPSS version 22.0 (IBM Corp., Armonk, NY, USA) and R (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). P values less than 0.05 were considered statistically significant.
Ethics declarations
The data do not contain personal information and do not infringe on the privacy of patients. This study was approved by the Institutional Review Board of the Kyungpook National University Hospital (2020-11-057). All patients provided written informed consent before participation, and the study was conducted according to the tenets of the 2013 Declaration of Helsinki and the Declaration of Istanbul 2008.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Davis, C. L. & Delmonico, F. L. Living-donor kidney transplantation: A review of the current practices for the live donor. J. Am. Soc. Nephrol. 16, 2098–2110. https://doi.org/10.1681/asn.2004100824 (2005).
Wolfe, R. A. et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 341, 1725–1730. https://doi.org/10.1056/nejm199912023412303 (1999).
Korea Organ Donation Agency. KODA Annual Report 2019 (Korea Organ Donation Agency, 2020).
Goldfarb-Rumyantzev, A. et al. Duration of end-stage renal disease and kidney transplant outcome. Nephrol. Dial Transplant. 20, 167–175. https://doi.org/10.1093/ndt/gfh541 (2005).
Gill, J. S., Rose, C., Joffres, Y., Landsberg, D. & Gill, J. Variation in dialysis exposure prior to nonpreemptive living donor kidney transplantation in the United States and Its association with allograft outcomes. Am. J. Kidney Dis. 71, 636–647. https://doi.org/10.1053/j.ajkd.2017.11.012 (2018).
Jay, C. L., Dean, P. G., Helmick, R. A. & Stegall, M. D. Reassessing preemptive kidney transplantation in the United States: Are we making progress?. Transplantation 100, 1120–1127. https://doi.org/10.1097/tp.0000000000000944 (2016).
Grams, M. E., Chen, B. P., Coresh, J. & Segev, D. L. Preemptive deceased donor kidney transplantation: Considerations of equity and utility. Clin. J. Am. Soc. Nephrol. 8, 575–582. https://doi.org/10.2215/cjn.05310512 (2013).
Lim, J. H. et al. Declining trend of preemptive kidney transplantation and impact of pretransplant dialysis: A Korean nationwide prospective cohort study. Transpl. Int. https://doi.org/10.1111/tri.14135 (2021).
Meier-Kriesche, H. U. et al. Effect of waiting time on renal transplant outcome. Kidney Int. 58, 1311–1317. https://doi.org/10.1046/j.1523-1755.2000.00287.x (2000).
Prezelin-Reydit, M. et al. Prolonged dialysis duration is associated with graft failure and mortality after kidney transplantation: Results from the French transplant database. Nephrol. Dial. Transplant. 34, 538–545. https://doi.org/10.1093/ndt/gfy039 (2019).
Helanterä, I. et al. Pretransplant dialysis duration and risk of death after kidney transplantation in the current era. Transplantation 98, 458–464. https://doi.org/10.1097/tp.0000000000000085 (2014).
Locatelli, F. & Canaud, B. Dialysis adequacy today: A European perspective. Nephrol. Dial. Transplant. 27, 3043–3048. https://doi.org/10.1093/ndt/gfs184 (2012).
Haller, M. C., Kainz, A., Baer, H. & Oberbauer, R. Dialysis vintage and outcomes after kidney transplantation: A retrospective cohort study. Clin. J. Am. Soc. Nephrol. 12, 122–130. https://doi.org/10.2215/cjn.04120416 (2017).
Cosio, F. G. et al. Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int. 53, 767–772. https://doi.org/10.1046/j.1523-1755.1998.00787.x (1998).
Mange, K. C., Joffe, M. M. & Feldman, H. I. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N. Engl. J. Med. 344, 726–731. https://doi.org/10.1056/nejm200103083441004 (2001).
Meier-Kriesche, H. U. & Kaplan, B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: A paired donor kidney analysis. Transplantation 74, 1377–1381. https://doi.org/10.1097/00007890-200211270-00005 (2002).
Foley, R. N. & Hakim, R. M. Why is the mortality of dialysis patients in the United States much higher than the rest of the world?. J. Am. Soc. Nephrol. 20, 1432–1435. https://doi.org/10.1681/asn.2009030282 (2009).
Keith, D. S. Preemptive deceased donor kidney transplant not associated with patient survival benefit in minority kidney transplant recipients. Clin. Transplant. 26, 82–86. https://doi.org/10.1111/j.1399-0012.2011.01398.x (2012).
Weng, F. L. et al. Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. J. Am. Soc. Nephrol. 16, 1839–1848. https://doi.org/10.1681/asn.2004121059 (2005).
El Ters, M., Smith, B. H., Cosio, F. G. & Kremers, W. K. Competing risk analysis in renal allograft survival: A new perspective to an old problem. Transplantation 105, 668–676. https://doi.org/10.1097/tp.0000000000003285 (2021).
Marfo, K., Lu, A., Ling, M. & Akalin, E. Desensitization protocols and their outcome. Clin. J. Am. Soc. Nephrol. 6, 922–936. https://doi.org/10.2215/cjn.08140910 (2011).
Lenihan, C. R., Liu, S., Montez-Rath, M. E. & Winkelmayer, W. C. Trends in the medical complexity and outcomes of medicare-insured patients undergoing kidney transplant in the years 1998–2014. Transplantation 103, 2413–2422. https://doi.org/10.1097/tp.0000000000002670 (2019).
Kinnunen, S., Karhapää, P., Juutilainen, A., Finne, P. & Helanterä, I. Secular trends in infection-related mortality after kidney transplantation. Clin. J. Am. Soc. Nephrol. 13, 755–762. https://doi.org/10.2215/cjn.11511017 (2018).
Chan, S. et al. Infection-related mortality in recipients of a kidney transplant in Australia and New Zealand. Clin. J. Am. Soc. Nephrol. 14, 1484–1492. https://doi.org/10.2215/cjn.03200319 (2019).
Kasiske, B. L. et al. Preemptive kidney transplantation: The advantage and the advantaged. J. Am. Soc. Nephrol. 13, 1358–1364. https://doi.org/10.1097/01.asn.0000013295.11876.c9 (2002).
Schold, J. D. et al. Association between kidney transplant center performance and the survival benefit of transplantation versus dialysis. Clin. J. Am. Soc. Nephrol. 9, 1773–1780. https://doi.org/10.2215/cjn.02380314 (2014).
Ahn, C. et al. Initial report of the Korean organ transplant registry: The first report of national kidney transplantation data. Transplant. Proc. 46, 425–430. https://doi.org/10.1016/j.transproceed.2013.11.083 (2014).
Yang, J. et al. Design and methods of the Korean organ transplantation registry. Transplant. Direct. 3, e191. https://doi.org/10.1097/txd.0000000000000678 (2017).
Levey, A. S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation: Modification of diet in renal disease study group. Ann. Intern. Med. 130, 461–470. https://doi.org/10.7326/0003-4819-130-6-199903160-00002 (1999).
Solez, K. et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am. J. Transplant. 8, 753–760. https://doi.org/10.1111/j.1600-6143.2008.02159.x (2008).
Aalen, O. O. & Johansen, S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand. J. Stat. 5, 141–150 (1978).
Odd, A. Nonparametric estimation of partial transition probabilities in multiple decrement models. Ann. Stat. 6, 534–545. https://doi.org/10.1214/aos/1176344198 (1978).
Fine, J. P. & Gray, R. J. A proportional hazards model for the subdistribution of a competing risk. JASA 94, 496–509. https://doi.org/10.2307/2670170 (1999).
Acknowledgements
We thank to the KOTRY study group for their contribution to this study. The full list of KOTRY study group is shown below.
Funding
This research was supported by a fund (2014-ER6301-00, 2014-ER6301-01, 2014-ER6301-02, 2017-ER6301-00, 2017-ER6301-01, 2017-ER6301-02) by Research of Korea Centers for Disease Control and Prevention Agency and supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI15C0001, HR22C1832). The research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1I1A3068253, 2021R1I1A3047973, 2021R1I1A3059702). This research was supported by a grant of Patient-Centered Clinical Research Coordinating Center (PACEN) funded by the Ministry of Health & Welfare, Republic of Korea (HI19C0481, HC20C0054).
Author information
Authors and Affiliations
Consortia
Contributions
J.H.L. and J.H.C. participated in research design; J.H.L. and Y.J. participated in data analysis; D.G.K., Y.H.K., J.K.K., J.Y., and M.S.K. participated in the performance of the research; J.H.L., H.Y.J., J.Y.C., S.H.P., C.D.K., Y.L.K., and J.H.C. participated in the writing of the paper; J.H.L. and J.H.C. participated in the review of the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lim, JH., Jeon, Y., Kim, D.G. et al. Effect of pretransplant dialysis vintage on clinical outcomes in deceased donor kidney transplant. Sci Rep 12, 17614 (2022). https://doi.org/10.1038/s41598-022-20003-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20003-2
- Springer Nature Limited