Abstract
Background
The effect of low serum uric acid (sUA) levels on kidney function is unclear. This study aimed to clarify the relationship between low sUA levels and the rapid decline in kidney function.
Methods
We examined the relationship between sUA levels and kidney function decline in health check-up examinees. A total of 10,547 participants were enrolled using data from the Yuport Medical Checkup Center Study between 1998 and 2002 for baseline and data from 2002 to 2006 as the follow-up period in Japan. According to sUA level (mg/dL), we classified the participants into the following six groups: (1) 2.0–2.9 (n = 247), (2) 3.0–3.9 (n = 1457), (3) 4.0–4.9 (n = 2883), (4) 5.0–5.9 (n = 2899), (5) 6.0–6.9 (n = 2010), and (6) 7.0–7.9 (n = 1,051). The relationship between sUA level and rapid decline in estimated glomerular filtration rate (ΔeGFR ≥ 3 mL/min/1.73 m2/year) was examined using a logistic regression model.
Results
During study period (5.4 ± 1.6 years), the incidence of rapid eGFR decline for the respective sUA groups (2.0–2.9, 3.0–3.9, 4.0–4.9, 5.0–5.9, 6.0–6.9, 7.0–7.9) were as follows: 4.5%, 4.0%, 2.4%, 3.3%, 3.1%, 3.4%. The crude and adjusted odds ratios (OR) for rapid eGFR decline were significantly higher in the 2.0–2.9 (OR:1.93 and 1.86) and 3.0–3.9 (OR:1.72 and 1.73) groups than in the 4.0–4.9 groups (reference). Stratified analysis of age differences revealed that the detrimental effect of low sUA was not evident in older adults (age ≥ 65 years).
Conclusion
A lower normal sUA level is related to an increased risk for a rapid decline in kidney function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a worldwide clinical issue, because it is not only potential end-stage kidney disease, but it is also an independent risk factor for death and/or cardiocerebrovascular events in general populations. [1,2,3]. CKD prevention is a critical target for investigation from a public health point of view. CKD is primarily caused by non-communicable, lifestyle-related diseases, such as diabetes or hypertension. As CKD is asymptomatic in the early stages, early detection of potential CKD risk factor(s) through annual checkups is a necessity.
Among potential CKD risk factors, excess serum uric acid (sUA) level, or hyperuricemia, has recently emerged as a risk factor [4,5,6,7]. Hyperuricemia causes not only gouty arthritis but also gout kidney—kidney dysfunction due to the deposition of uric acid-sodium (MSU) on the kidney interstitium [8, 9].
On the other hand, uric acid could exhibit beneficial aspects through antioxidative properties [10]. We formerly reported that slightly lower level which is within normal range (≤ 4.6 mg/dL) of serum albumin related to rapid decline in kidney function [11]. The reason of such relationship is unclear, however, antioxidative property of serum albumin might contribute to renoprotection, for serum albumin is the most abundant and thus important antioxidative substance of the extracellular space [12]. Therefore, the lack of sUA could have a detrimental effect on kidney function through different pathophysiology from that of the excess of sUA or hyperuricemia. However, previous studies on this are generally insufficient and diverse [13,14,15,16].
The present observational study examined CKD occurrence in participants with low but within the normal range of sUA levels using the large sample size (n = 10,547) health follow-up cohort data.
Methods and subjects
Study design and participants ( Fig. 1 )
This was a retrospective cohort study, that used data from health check-up examinees acquired from the health screening program conducted by the Yuport Medical Checkup Center in Tokyo. For the current study, we set a 4-year baseline period between April 1998 and March 2002, and a 4-year follow-up period between April 2002 and March 2006. During the baseline period, 21,885 individuals underwent checkups at least once. Only the first checkup was used as the baseline data for participants who underwent multiple checkups during the baseline period. Of the 11,129 patients who had been examined during both the baseline and follow-up periods, 129 diabetic participants on treatment at baseline were excluded to avoid their potential influence of diabetic kidney disease. Of the remaining 11,000 individuals, participants with hypouricemia (sUA < 2.0 mg/dL; n = 16) and hyperuricemia requiring medical treatment (sUA ≥ 8.0 mg/dL; n = 437) were also excluded. Accordingly, 10,547 participants were enrolled in this study.
In accordance with the Private Information Protection Law, information that might identify participants was kept private by the center. Informed consent was obtained at every checkup for anonymous participation in epidemiological research.
Measurements
All checkup procedures, including blood tests, were performed in the same manner at both the baseline and follow-up visits. Blood samples were obtained after overnight fasting and analyses were performed in the center’s laboratory. Uric acid and creatinine levels were measured using enzymatic methods (reagents supplied by Mitsubishi Kagaku Iatron, Tokyo, Japan). Other hematological and biochemical parameters were measured using standard laboratory techniques. Of note, hemoglobin A1c was expressed as the Japan Diabetes Society (JDS) value, and statistical analysis was performed after translation from the JDS value to the National Glycohemoglobin Standardization Program value according to the translation formula published elsewhere [17]. Body mass index (BMI) was defined as weight divided by height squared (kg/m2), and it was calculated from the patient’s measured height and weight. Trained nurses measured blood pressure using a sphygmomanometer.
The kidney function
Kidney function was shown as an estimated glomerular filtration rate (eGFR) using the CKD Epidemiology Collaboration (CKD-EPI) modified for the Japanese population [18]. The CKD-EPI equation, which was estimated by the coefficient-modified equation, was more closely related to the CVD incidence than that estimated by the Japanese GFR equation [19]. The CKD-EPI equation for the Japanese population is as follows:
mCKDEPI-eGFR (mL/min/1.73 m2) = 141 × min (Cr/κ, 1)α × max (Cr/κ, 1)−1.209 × 0.993Age × 1.018 (if female) × 0.813 (Japanese coefficient), κ:0.7 in female and 0.9 In the male, α was -0.329 in female and -0.411 in male.
Kidney function declines with age. A previous study showed that the mean rate of kidney function decline in people aged 40 years and older was 0.36 mL/min/1.73 m2/year [20]. In our study, we defined an “abnormal decline in kidney function” as the difference in the eGFR (ΔeGFR) of ≥ 3 mL/min/1.73 m2/year between baseline and follow-up visits for each participant as a cut-off value. According to previous studies, this cut-off value was associated with clinically deleterious outcomes [21, 22]. We also performed a sensitivity analysis that regarded a ΔeGFR of ≥ 5 mL/min 1.73 m2/year as an abnormal decline.
Statistical analysis
Statistical analyses were performed using EZR Version 1.33 (Saitama Medical Center, Jichi Medical University), a graphical user interfaces for R (The R Foundation for Statistical Computing, Vienna, Austria) [23]. EZR is a modified version of the R commander, which is designed to add statistical functions frequently used in biostatistics.
Numeric data were presented as mean ± standard deviation (normally distributed data) or median within the 25th and 75th percentiles (non-normally distributed data) (Appendix 1). Categorical data were expressed as numbers and percentages. P < 0.05 (two-tailed) was considered statistically significant.
First, as baseline characteristics, we compared those who met the criteria for an abnormal decline in kidney function with those who did not. Second, we classified participants into six groups (2.0–2.9, 3.0–3.9, 4.0–4.9, 5.0–5.9, 6.0–6.9, and 7.0–7.9 mg/dL) according to their sUA levels. The abnormal decline in kidney function and odds ratio (ORs) for each subject group were estimated using the logistic regression model. Multivariable logistic analyses were used to calculate the odds ratio (OR) for an abnormal decline in kidney function after adjusting for age, sex, BMI, systolic blood pressure, eGFR at baseline, hemoglobin level, serum albumin level, serum alanine aminotransferase level (using logarithmic transformed data), serum non-high-density lipoprotein cholesterol level, serum triglyceride level (using logarithmically transformed data), serum C-reactive protein level (≥ 0.3 mg/dL or not), potential diabetes mellitus suspected from baseline data (fasting plasma glucose ≥ 126 mg/dL or hemoglobin A1c ≥ 6.5%, or both), hypertension on treatment, dyslipidemia on treatment, history of stroke, and ischemic heart disease (angina pectoris or myocardial infarction, or both).
Results
Table 1 presents the baseline characteristics of the participants according to an abnormal decline in kidney function. Of the 10,547 participants, 333 had a rapid eGFR decline during the study period (5.4 ± 1.6 years). The baseline eGFR (mg/min/1.73 m2) of those with rapid decline was significantly higher than that of those with a non-rapid decline (84.3 ± 12.8 vs. 83.1 ± 9.97, p < 0.05, t-test). Of note, irrespective of the baseline exclusion of known diabetic individuals, still 501 (4.8%) potential diabetic subjects were newly identified.
Table 2 presents the baseline characteristics of the participants according to their sUA levels. The prevalence of males and hypertension on treatment was increased, in sUA elevated group. (p < 0.05) in the Cochran-Armitage test. Similarly, BMI, systolic blood pressure, hemoglobin, serum albumin, alanine transferase, creatinine, non-HDL cholesterol, triglyceride, and C-reactive protein levels were also elevated in the group that had an elevation of sUA (p < 0.05). These positive findings in trend tests (Cochran-Armitage and Jonckheere-Terpstra tests) suggest the presence of a confounding relationship between each parameter and the sUA level.
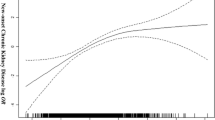
Figure 2 shows the percentage of participants with significantly reduced kidney function (ΔeGFR ≥ 3 mL/min 1.73 m2/year) according to serum uric acid level. The incidence of each subject group was significantly different (p < 0.05, in the Chi-square test). Of all the participants’ groups, the 4.0–4.9 group exhibited the lowest incidence (2.4%). Sensitivity analysis also showed the same trend. This finding was similar to that of an analysis which showed incremental increases of 0.5 in sUA levels, the ratios of rapid decline in 4.0–4.4 and 4.5–4.9 were 2.4% and 2.3%, respectively, and those of all the other groups were higher than 2.5% (Appendix 2). Therefore, we regarded the 4.0–4.9 group as the reference group in the following logistic regression analyses.
Percentage of participants with significantly reduced kidney function (ΔeGFR of ≥ 3 mL/min 1.73 m2/year) by serum uric acid level. The percentage of rapid decliner (ΔeGFR of ≥ 3 mL/min 1.73 m2/year) was the lowest with serum uric acid levels of 4.0–4.9 mg/dL (A). This tendency was similar in the sensitivity analysis using ΔeGFR of ≥ 5 mL/min 1.73 m2/year as the criteria for rapid kidney function decline (B)
Logistic regression analysis was used to calculate the OR for the rapid decline in kidney function according to the sUA level (Fig. 3, Table 3). The OR of rapid eGFR decline was higher in both higher sUA groups and lower normal sUA groups (the so-called J-curve phenomenon). This tendency was also observed after adjusting for multiple confounding factors.
The odds ratio for the rapid decline in kidney function by serum uric acid level classification. Reference level 4.0–4.9 mg/dL. Adjusted for age, sex, BMI, systolic blood pressure, hemoglobin, alanine aminotransferase (logarithmically transformed value), serum albumin, kidney function at baseline, high-density lipoprotein cholesterol, triglyceride (logarithmically transformed value), C-reactive protein (elevated or not), potential diabetes, and other comorbidities (hypertension on treatment, dyslipidemia on treatment, stroke history, and ischemic heart disease history)
Because 501 participants met the criteria of potential diabetes mellitus at baseline notwithstanding they had not diagnosed as diabetes before (Table 1), we performed logistic regression analysis using data set without potential diabetes at baseline to omit profound effect of early-stage diabetes on eGFR decline. As a result, same trend as analysis including the “potentially diabetic” participants was obtained (Appendix 3).
Then, we performed stratified analyses for sex and age. In the stratified analysis after classification on the basis of sex (Fig. 4), a similar trend was observed in both males and females. Likewise, we stratified the analysis regarding age, in which participants were divided into the following two groups: young and middle-aged adults (ages 19–64 years; n = 8660) and older adults (aged 65 years and over; n = 1887) (Fig. 5). Unlike in young and middle-aged adults, the OR of the lower normal sUA (2.0–3.9) group was not elevated [crude OR 1.03 (95% confidence interval [CI] 0.643–1.72) and adjusted OR was 1.19 (95% CI 0.752–2.01)] in older adults. A similar tendency was observed when participants were divided into the following three groups: young adults (ages 19–44 years; n = 2512), middle-aged adults (ages 45–64 years; n = 6148), and older adults (aged 65 years and over; n = 1887) (Fig. 6). The OR for rapid eGFR decline in the lower normal sUA group (2.0–3.9) in older adults was not elevated in either crude or adjusted analyses.
The odds ratio for men (A) and women (B) regarding the rapid decline in kidney function by serum uric acid level classification. Reference level 4.0–4.9 mg/dL. Adjusted for age, BMI, systolic blood pressure, hemoglobin, alanine aminotransferase (logarithmically transformed value), serum albumin, kidney function at baseline, high-density lipoprotein cholesterol, triglyceride (logarithmically transformed value), C-reactive protein (elevated or not), potential diabetes, and other comorbidities (hypertension, dyslipidemia, stroke, and ischemic heart disease)
The odds ratio for young and middle-aged adults (A) and older adults (B) regarding the rapid decline in kidney function by serum uric acid level classification. Reference level 4.0–4.9 mg/dL. Adjusted for sex, BMI, systolic blood pressure, hemoglobin, alanine aminotransferase (logarithmically transformed value), serum albumin, kidney function at baseline, high-density lipoprotein cholesterol, triglyceride (logarithmically transformed value), C-reactive protein (elevated or not), potential diabetes, and other comorbidities (hypertension, dyslipidemia, stroke, and ischemic heart disease)
The crude (A) and adjusted (B) odds ratio for age groups (young, middle-aged, or older adults) regarding the rapid decline in kidney function by serum uric acid level classification. Reference level 4.0–4.9 mg/dL. Adjusted for sex, BMI, systolic blood pressure, hemoglobin, alanine aminotransferase (logarithmically transformed value), serum albumin, kidney function at baseline, high-density lipoprotein cholesterol, triglyceride (logarithmically transformed value), C-reactive protein (elevated or not), potential diabetes, and other comorbidities (hypertension, dyslipidemia, stroke, and ischemic heart disease)
Discussion
The aim of this study was to clarify the relationship between lower normal sUA levels and rapid decline in kidney function using health check-up examinee cohort data from a large sample size. As a result, we clarified that lower normal sUA levels (2.0–3.9) were independently associated with rapid eGFR decline. Of course, the pathogenesis of such mild hypouricemia in this study might include profound insufficiency in proximal tubular function. In our analyses, the uric acid level at the lowest risk of rapid eGFR decline was 4.0–4.9 mg/dL.
One of the novelties of our present study is that the contribution of lower normal sUA to rapid decline of eGFR was ensured in a prospective manner. Although recent cohort studies showed the relationship between lower sUA and kidney dysfunction [15, 16], these studies were cross-sectional and thus cause-and-result relationship was unclear.
Regarding the clinical impact, incidence, and pathogenesis of rapid eGFR decline in general, Rifkin et al. evaluated in their cohort of community-dwelling older adults recruited individuals aged ≤ 65 years from Medicare eligibility lists in 4 US communities (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania) [21, 22]. In their cohort, the clinical impact of rapid eGFR decline was statistically significant relationship with increased risk of cardiovascular and all-cause mortality [21]. The incidence of rapid eGFR decline was 16–25% [21, 22], which was quite higher than that in present study (3.16%), suggesting the racial difference between their cohort and ours. As to the pathogenesis, rapid eGFR decline in their cohort was independently associated with systolic blood pressure (SBP) [22]. Such significant relationship between rapid decline of eGFR and SBP was also confirmed among our present study.
Another novelty of this study is that the profound effect of “metabolic” multiple confounding factors (such as obesity, diabetes, hypertension, dyslipidemia, liver disease, chronic inflammation, and serum albumin level) was minimized through study design (exclusion of participants being treated for diabetes) and statistical correction. In particular, correction of serum albumin level, one of the important predictors of renal prognosis probably through antioxidative property [11] (Appendix 4), has seldom been performed in previous studies.
It was already known in the early 1980s that uric acid has a strong antioxidant effect [24]. Such antioxidant effect of uric acid could contribute to longevity of hominoids—which abolished the activity of uricase (an enzyme that breaks down uric acid to allantoin) 15,400,000 years ago, and thus which sUA level is higher than other primates [25]. The antioxidant activity of uric acid surpasses that of vitamin C [10], and clinically, uric acid has been proposed to have a neuroprotective effect due to its antioxidant effect in the field of the central nervous system, especially in multiple sclerosis, Parkinson’s disease, and acute cerebral infarction [26,27,28,29]. In addition, a low level of uric acid causes oxidative stress in endothelial cells, leading to the induction of oxidative apoptosis, expression of adhesion molecules, and reduction of microvessels [30,31,32].
Animal study regarding the effect of hypouricemia on kidney function is not present, because mammalian species except for Hominidae have uricase—an enzyme that promotes oxidation of uric acid to allantoin—and thus almost all animals (except for ape and human) are “hypouricemic” fundamentally. On the other hand, Cutler evaluated the relationship between serum and brain urate level (indexed for specific metabolic rate) and the maximum lifespan potential of various animals (22 primate and 17 non-primate mammalian species) and found the positive correlation between them [33]. This finding from inter-species comparison suggests organoprotective effect of uric acid, and accordingly, individual with lower sUA might be vulnerable to organic free radicals constantly caused by living activity.
However, high sUA levels also exhibit detrimental effects on kidney function, as is widely acknowledged [4,5,6,7]. Uric acid is involved in promoting oxidation within cells. This is because reactive oxygen species (superoxide and hydrogen peroxide) are produced in the process of uric acid synthesis by xanthine oxidase (XO) (reactive oxygen species producing-type isoform of uric acid synthase xanthine oxidoreductase (XOR)) leading to vascular endothelial damage and organ damage [34]. This might be the reason why high uric acid levels increase the risk of various complications including hypertension, diabetes, cerebrovascular and cardiovascular disease, metabolic syndrome, and CKD, even if the uric acid level is 7.0 mg/dL or less which could not cause MSU deposition in organs. We previously ensured among CKD patients that the oxidized serum albumin ratio (a marker of oxidative stress) showed an independent positive correlation with serum UA level [35] and XO / XOR ratio [36]. Furthermore, Uedono et al. clarified in a study of kidney transplant donor candidates that even if the serum uric acid levels were within the normal range, both mildly low (< 3.5 mg/dL) or high (> 6.0 mg/dL) sUA levels were associated with increased afferent arteriole resistance and decreased blood flow and glomerular filtration rate [37]. To summarize, sUA levels that are "not too low nor too high" may provide the greatest benefit for kidney function protection. Further clinical investigations using kidney biopsy specimens might offer more precise findings regarding the pathophysiology.
It is widely acknowledged that sUA levels in men are generally higher than those in women. However, the statistical relationship between sUA and the clinical characteristics of metabolic syndrome is stronger in women than in men [38]. Thus, we performed a stratified analysis regarding sex, considering the differences in sUA conditions between females and males. As a result, we could not find evident differences in the relationship between sUA levels and rapid eGFR decline, suggesting that such a relationship did not differ between the sexes.
In the present study, regarding the rapid decrease of kidney function, the detrimental effect of low UA was evident not in older adults (aged 65 years and over) but in young and middle-aged adults (ages 19–64 years)—“under 65” generation (Fig. 6). To the best of our knowledge, such a clear “generation gap” regarding the effect of lower sUA level on kidney function has not been pointed out in any previous reports. The need for social and physiological activity is higher in the “under 65” generation than in the “over 65” generation, for most of the “over 65” person retires from a social role such as working or child-rearing. In “under 65” generation, the demand for uric acid to combat oxidative stress caused by high activity [39] could be more earnest than in the “over 65” generation.
This study had some limitations. First, this study targeted health check-up examinees—such a population might have high health literacy and might be at a lower risk of developing the disease when compared to the general population. Second, information regarding regular medication is not known in this cohort, although it was confirmed that participants had not taken oral urate-lowering drugs at baseline, we do not know whether urate-lowering drugs were started after that. Third, information regarding urinary findings is lacking in this cohort, which impedes the evaluation of albuminuria, the major influencer of future kidney damage. Finally, the present cohort consisted of Asian (mainly Japanese) individuals. Thus, future prospective studies addressing these limitations are warranted.
In conclusion, our study showed that a lower normal sUA level is independently related to the risk of a rapid decline in kidney function, especially in young and middle-aged adults.
Data availability
The dataset used in this study is under the control of the Data Management Committee of the Yuport Medical Checkup Center (YMCC) study and cannot be shared publicly. However, when the researcher needs to use the data for the individual patient level meta-analysis or the validation study between another independent cohort, the data set will be available after the review of the Data Management Committee of the YMCC study. The amended protocol will need to be approved by the ethical committee of Teikyo University School of Medicine. Send a request to Hiroyuki Terawaki, M.D., Ph.D., Teikyo University Chiba Medical Center, terawaki@med.teikyo-u.ac.jp.
References
Nakayama M, Metoki H, Terawaki H, Ohkubo T, Kikuya M, Sato T, et al. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general Japanese population⎯the Ohasama study. Nephrol Dial Transplant. 2007;22:1910–5.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305.
Ninomiya T, Kiyohara Y, Kubo M, Tanizaki Y, Doi Y, Okubo K, et al. chronic kidney disease and cardiovascular disease in a general Japanese population: the Hisayama study. Kidney Int. 2005;68:228–36.
Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642–50.
Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19:2407–13.
Kawashima M, Wada K, Ohta H, Terawaki H, Aizawa Y. Association between asymptomatic hyperuricemia and new-onset chronic kidney disease in Japanese male workers: along-term retrospective cohort study. BMC Nephrol. 2011;12:31.
Kuwabara M, Bjornstad P, Hisatome I, Niwa K, Roncal-Jimenez CA, Andres-Hernando A, et al. Elevated serum uric acid level predicts rapid decline in kidney function. Am J Nephrol. 2017;45:330–7.
Seegmiller JE, Erazier PD. Biochemical considerations of the renal damage of gout. Ann Rheum Dis. 1966;25:668–72.
Sellmayr M, Petzsche MRH, Ma Q, Krüger N, Lisapis H, Brink A, et al. Only hyperuricemia with crystalluria, but not asymptomatic hyperuricemia, drives progression of chronic kidney disease. J Am Soc Nephrol. 2020;31:2773–92.
Nakayama K, Terawaki H, Nakayama M, Iwabuchi M, Sato T, Ito S. Reduction of serum antioxidantive capacity during haemodialysis. Clin Exp Nephrol. 2007;11:218–24.
Amano H, Yoshimura K, Iijima R, Waki K, Matsumoto K, Ueda H, et al. A slight decrease in the serum albumin level is associated with the rapid progression of kidney dysfunction, even within the normal range. Intern Med. 2020;59:2679–85.
Ogasawara Y, Namai T, Togawa T, Ishii K. Formation of albumin dimers induced by exposure to peroxides in human plasma: a possible biomarker for oxidative stress. Biochem Biophys Res Commun. 2006;340:353–8.
Kojima S, Uchiyama K, Yokota N, Tokutake E, Wakasa Y, Hiramitsu S, Febuxostat for cerebral and cardiorenovascular events prevention study (FREED) investigators, et al. Optimal uric acid levels by febuxostat treatment and cerebral, cardiorenovascular risks: post hoc analysis of a randomized controlled trial. Rheumatology. 2022;61:2346–59.
Takae K, Nagata M, Hata J, Mukai N, Hirakawa Y, Yoshida D, et al. SUA as a risk factor for chronic kidney disease in a Japanese community -the Hisayama study. Circ J. 2016;80:1857–62.
Wakasugi M, Kazama JJ, Narita I, Konta T, Fujimoto S, Iseki K, et al. Association between hypouricemia and reduced kidney function: a cross-sectional population-based study in Japan. Am J Nephrol. 2015;41:138–46.
Kuwabara M, Hisatome I, Niwa K, Bjornstad P, Roncal-Jimenezet CA, Andres-Hernandoal A, et al. The optimal range of sUA for cardiometabolic diseases: a 5-year Japanese cohort study. J Clin Med. 2020;9:942.
Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanfusa T, Ito H, et al. International clinical harmonization of glycated haemoglobin in Japan: from Japan diabetes society to national glycohaemoglobin standardization program values. J Diabetes Investig. 2012;3:39–40.
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56:32–8.
Terawaki H, Nakayama M, Asahi K, Kakamu T, Hayakawa T, Iseki K, et al. Comparison of predictive value for first cardiovascular event between Japanese GFR equation and coefficient-modified CKD-EPI equation. Clin Exp Nephrol. 2015;19:387–94.
Imai E, Horio M, Yamagata K, Iseki K, Hara S, Ura N, et al. Slower decline of glomerular filtration rate in the Japanese general population: a longitudinal 10-year follow-up study. Hypertens Res. 2008;31:433–41.
Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168:2212–8.
Rifkin DE, Katz R, Chonchol M, Shlipak MG, Sarnak MJ, Fried LF, et al. Blood pressure components and decline in the kidney function in community-living older adults: the cardiovascular health study. Am J Hypertens. 2013;26:1037–44.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical -caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–62.
Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19:640–53.
Amaro S, Soy D, Obath V, Cervera A, Plnas AM, Chamorro A. A pilot study of dual treatment with recombinant tissue plasminogen activator and uric acid in acute ischemic stroke. Stroke. 2007;38:2173–5.
Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J Neurochem. 2002;80:101–10.
Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, et al. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci U S A. 1998;95:675–80.
Spitsin SV, Scott GS, Mikheeva T, Zborek A, Kean RB, Brimer CM, et al. Comparison of uric acid and ascorbic acid in protection against EAE. Free Radic Biol Med. 2002;33:1363–71.
Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38.
George J, Struthers AD. Role of urate, xanthine oxidase and the effects of allopurinol in vascular oxidative stress. Vasc Health Risk Manag. 2009;5:265–72.
Sugihara S, Hisatome I, Kuwabara M, Niwa K, Maharani N, Kato M, et al. Depletion of uric acid due to SLC22A12 (URAT1) loss-of-function mutation causes endothelial dysfunction in hypouricemia. Circ J. 2015;79:1125–32.
Cutler RG. Urate and ascorbate: their possible roles as antioxidants in determining longevity of mammalian species. Arch Gerontol Geriatr. 1984;3:321–48.
Liu N, Xu H, Sun Q, Yu X, Chen W, Wei H, et al. The role of oxidative stress in hyperuricemia and xanthine oxidoreductase (XOR) inhibitors. Oxid Med Cell Longev. 2021;2021:1470380.
Terawaki H, Yoshimura K, Hasegawa T, Matsuyama Y, Negawa T, Yamada K, et al. Oxidative stress is enhanced in correlation with renal dysfunction: examination with the redox state of albumin. Kidney Int. 2004;66:1988–93.
Terawaki H, Hayashi T, Murase T, Iijima R, Waki K, Tani Y, et al. Relationship between xanthine oxidoreductase redox and oxidative stress among chronic kidney disease patients. Oxid Med Cell Longev. 2018;2018:9714710.
Uedono H, Tsuda A, Ishimura E, Nakatani S, Kurajoh M, Mori K, et al. U-shaped relationship between serum uric acid levels and intrarenal hemodynamic parameters in healthy participants. Am J Physiol Renal Physiol. 2017;312:F992–7.
Seki R, Kimura T, Inoue K. Serum uric acid level has stronger correlations with metabolic syndrome-related markers in women than in men in a Japanese health check-up population. Open J Epidemiol. 2020;10:399–418.
Imai H, Hayashi T, Negawa T, Nakamura K, Tomida M, Koda K, et al. Strenuous exercise-induced change in redox state of human serum albumin during intensive kendo training. Jpn J Physiol. 2002;52:135–40.
Acknowledgements
We would like to thank Dr. Kimihiko Akimoto for donating the Yuport cohort data.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception. HU, YN and HT designed the study, performed the statistical analyses. The first draft of the manuscript was written by HU and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript mainly modified by HT.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB approval number 15–205, the institutional review board of Teikyo University School of Medicine) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants for anonymous participation in epidemiological research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ueda, H., Inoue, K., Seki, R. et al. Lower range of serum uric acid level increases risk of rapid decline of kidney function in young and middle-aged adults: the Yuport Medical Checkup Center Study. Clin Exp Nephrol 27, 435–444 (2023). https://doi.org/10.1007/s10157-023-02318-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-023-02318-0