Abstract
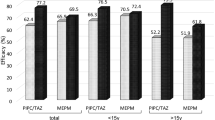
A multi-institutional study was conducted to assess efficacy and safety of biapenem (BIPM), a carbapenem antibiotic, as an initial-stage therapeutic agent for febrile neutropenia (FN) in patients with hematopoietic diseases. A total of 216 patients from 25 medical institutions were enrolled in this study; of these, 204 were included in the safety analysis and 178 in the efficacy analysis. The combined (excellent and good) response rate was 67.9%, and antipyretic effect (subsidence + tendency to subsidence) was achieved within 3 and 5 days of treatment in 67.3 and 75.9% of patients, respectively. Thus, the clinical responses were gratifying. A response rate of 61.7% (37/60) was observed even in high-risk FN patients in whom neutrophil counts prior to and at 72 h after the start of BIPM were ≤100/μl. BIPM is considered to be a highly promising drug, with prompt onset of clinical benefit, as an initial-stage therapeutic agent for the treatment of FN in patients with hematopoietic diseases.



Similar content being viewed by others
Notes
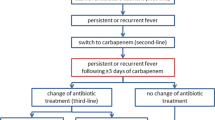
Patients with fever obviously not attributable to infection were excluded.
References
Hughes WT, Armstrong D, Bodey GP, Feld R, Mandell GL, Meyers JD, From the Infectious Diseases Society of America, et al. Guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. J Infect Dis. 1990;161:381–96.
Hughes WT, Armstrong D, Bodey GP, Brown AE, Edwards JE, Feld R, et al. 1997 guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. Infectious Diseases Society of America. Clin Infect Dis. 1997;25:551–73.
Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730–51.
Masaoka T. Conclusions and recommendations: evidence-based recommendations for antimicrobial use in febrile neutropenia in Japan. Int J Hematol. 1998;68:S39.
Hikida M, Kawashima K, Yoshida M, Mitsuhashi S. Inactivation of new carbapenem antibiotics by dehydropeptidase-1 from porcine and human renal cortex. J Antimicrob Chemother. 1992;30:129–34.
Sakai H, Sanada M, Shimamoto K, Azuma R, Harada H, Mori H, et al. Clinical evaluation of biapenem for febrile neutropenia in patients with hematological disorders. Jpn J Antibiot. 2007;60:125–31 (Japanese).
Kasahara S, Hara T, Tsurumi H, Goto N, Kanemura N, Yoshikawa T, et al. Clinical effects of biapenem on febrile neutropenia in patients with hematological malignancy. Jpn J Antibiot. 2008;61:115–21 (Japanese).
Sasada M. Anti-infection measures. Nippon Rinsho. 2007;65:S445–9 (Japanese).
Yamaguchi K, Ishii Y, Iwata M, Watanabe N, Uehara N, Yasujima M, et al. Nationwide surveillance of parenteral antibiotics containing meropenem activities against clinically isolated strains in 2006. Jpn J Antibiot. 2007;60:344–77 (Japanese).
Matsumoto F, Inoue M, Sakurai I, Ishida Y, Osonoi T, Itoh H, et al. A comparative study of biapenem and imipenem/cilastatin in lower respiratory infections. Jpn J Chemother. 2000;48:45–67 (Japanese).
Shibata H, Yamane T, Sakamoto E, Nakamae H, Ohta K, Hino M. Clinical analysis of antibiotic treatment for febrile neutropenia. Jpn J Antibiot. 2005;58:382–7 (Japanese).
Urabe A, Mutoh Y, Mizoguchi H, Toyama K, Oshimi K, Suzuki K, et al. Efficacy and safety of panipenem/betamipron (PAPM/BP) in the treatment of infections accompanying hematologic diseases. Antibiot Chemother. 1998;14:119–26 (Japanese).
Sawae Y, Niho Y, Okamura T, Gondo H, Suehiro Y, Kumakawa M, et al. Comparison between monotherapy with imipenem/cilastatin sodium (IPM/CS) and combinations of IPM/CS and other drugs for treating bacterial infections in patients with hematopoietic disorders. Jpn J Antibiot. 1996;49:1049–61 (Japanese).
Bodey GP, Rodriguez V, Chang HY, Narboni G. Fever and infection in leukemic patients: a study of 494 consecutive patients. Cancer. 1978;41:1610–22.
Craig WA. Interrelationship between pharmacokinetic and pharmacodynamic in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis. 1995;22:89–96.
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12.
Craig WA. The role of pharmacodynamics in effective treatment of community-acquired pathogens. Adv Stud Med. 2002;2:126–34.
Drusano GL. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin Infect Dis. 2003;36:S42–50.
Takata T, Aizawa K, Shimizu A, Sakakibara S, Watabe H, Totsuka K. Optimization of dose and dose regimen of biapenem based on pharmacokinetic and pharmacodynamic analysis. J Infect Chemother. 2004;10:76–85.
Tamura K, Matsuoka H, Tsukada J, Masuda M, Ikeda S, Matsuishi E, et al. Cefepime or carbapenem treatment for febrile neutropenia as a single agent is as effective as a combination of 4th-generation cephalosporin + aminoglycosides: comparative study. Am J Hematol. 2002;71:248–55.
Tamura K, Imajo K, Akiyama N, Suzuki K, Urabe A, Ohyashiki K, et al. Randomized trial of cefepime monotherapy or cefepime in combination with amikacin as empirical therapy for febrile neutropenia. Clin Infect Dis. 2004;39:S15–24.
Klastersky J. Treatment of neutropenic infection: trends towards monotherapy? Support Care Cancer. 1997;5:365–70.
Ramphal R. Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated pathogens. Clin Infect Dis. 2004;39:S25–31.
Acknowledgments
We are indebted to all doctors involved in this group study from the institutions listed in Table 13.
Author information
Authors and Affiliations
Corresponding author
Additional information
For the Study Group for Infectious Disease involved in hematopoietic diseases.
An erratum to this article can be found at http://dx.doi.org/10.1007/s10156-010-0185-y
About this article
Cite this article
Nakagawa, Y., Suzuki, K., Hirose, T. et al. Clinical efficacy and safety of biapenem for febrile neutropenia in patients with underlying hematopoietic diseases: a multi-institutional study. J Infect Chemother 17, 58–67 (2011). https://doi.org/10.1007/s10156-010-0075-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-010-0075-3