Abstract
Settlement of benthic marine invertebrates is determined by the interaction between physical factors and biological processes, in which the tide, wind, and predation can play key roles, especially for species that recruit within estuaries. This complexity promotes high variability in recruitment and limited predictability of the size of annual cohorts. This study describes the settlement patterns of megalopae of the commercially important crab Cancer edwardsii at three locations (one in the center and two at the mouth of the estuary) within the Valdivia River estuary (~39.9°S), over three consecutive years (2006–2008). At each location, 12 passive benthic collectors with a natural substratum were deployed for 48 h at 7-day intervals, over a lunar cycle. Half of the collectors were covered with mesh to exclude predators. The main findings were as follows: (1) circulation changes due to upwelling relaxation or onshore winds controlled crab settlement at sites within the mouth of the estuary, (2) at the internal estuarine site, settlement was dominated by tidal effects, and (3) the effect of predation on settlement was negligible at all scales. The results show that the predominant physical factor controlling the return of competent crab larvae to estuarine environments varies spatially within the estuary. The lack of tidal influence on settlement at the mouth of the estuary can be explained by the overwhelming influence of the intense upwelling fronts and the micro-tidal regime in the study area.
Similar content being viewed by others
Introduction
For crab species that recruit within estuarine habitats but have larvae that develop offshore, a sequence of events is required to ensure that competent larvae (sensu Forward et al. 2001) settle in a suitable habitat. The two-step process for the immigration of megalopae into the estuary (Miller and Shanks 2004) involves larval concentration in the onshore shelf zone (Jones and Epifanio 1995a; Pineda 2000, but see Tilburg et al. 2007) and the subsequent entry into nearby estuaries. These steps are normally controlled by the interaction between wind-driven circulation and tidal currents but are regulated by larval behavior (Miller and Shanks 2004; Queiroga et al. 2006; Ogburn and Forward 2009). The output of this process is the local pattern of settlement within the estuary, which is the primary input that determines temporal and spatial variation in recruitment.
Along the open coastlines of areas dominated by upwelling regimes (Mackas et al. 2006), a high degree of association has been found between periods of relaxation of wind stress that force upwelling conditions and peaks in the abundance of competent larvae and in the settlement rates of mytilids, cirripeds, and crabs (Roughgarden et al. 1991; Wing et al. 1995a; Shanks et al. 2000; Navarrete et al. 2005; Lagos et al. 2008; but see Shanks and Shearman 2009). Similar associations have also been found in estuaries located along upwelling coastlines, where wind-driven transport related to upwelling/downwelling has been identified as contributing to the cross-shelf transport and estuarine immigration of organisms (Miller and Shanks 2004; Queiroga et al. 2006).
On the other hand, once the larval pool has been formed in the onshore shelf area, larvae can be transported toward the upper estuary by selective tidal stream transport, specifically flood tide transport (FTT; Forward et al. 2001). FTT closely depends on tidal regime, where larvae migrate upwards in the water column during the flood tide and downwards during ebbing tidal currents (Epifanio et al. 1988). Studies carried out with Carcinus maenas and Callinectes sapidus in European and American estuaries have provided evidence for this transport strategy in crabs (Tankersley et al. 2002; Queiroga et al. 2006). Environmental factors such as light, salinity (Hasek and Rabalais 2001), and turbulence (Welch and Forward 2001) seem to influence larval swimming behavior, aiding them in avoiding the net seaward flow of the estuary during ebb tides by keeping them close to the bottom.
Depending on local conditions, the relative balance of wind and tide controls the larval entry into estuaries. Ogburn et al. (2009) proposed a conceptual model to identify the environmental conditions under which entry of Callinectes sapidus megalopae is most likely to occur in the Newport River estuary. In that model, megalopae inflow depends on the degree to which tides alter wind-driven immigration of larvae into a particular estuary. Thus, in micro-tidal estuaries facing upwelling fronts, wind-driven larval transport should prevail over tidal currents in regulating settlement in the outer estuary, but tides may become more important as megalopae move further into the estuary.
Secondary biological factors, such as larval substratum preference and predation, interact with physical control of larval settlement. Predation, which is one of the most significant sources of mortality for marine invertebrate larvae (Morgan 1995), could overwhelm other factors that determine settlement rates. This could be particularly true for larvae exhibiting benthic behaviors, as with competent megalopae, which suffer higher mortalities (12–28 times) in a benthic environment than in a planktonic environment (Allen and McAlister 2007). However, the evaluation of predation and its effect on local settlement rates is uncommon in experimental designs assessing settlement and recruitment in crabs (but see Moksnes 2002; Pardo et al. 2007).
The present study assesses the environmental control on settlement rates of the megalopae of the brachyuran crab Cancer edwardsii in one of the estuaries in southern Chile (~39.9°S). The system studied is characterized by a micro-tidal synodic regime, a large watershed, and an upwelling-dominated coastal system during the larval settlement period. C. edwardsii is the most important commercial crab species of the Chilean artisanal fishery, with a mean catch of around 5,000 tons per year (SERNAPESCA 2007–2009). It is one of the largest of the four species of Cancer present in Chile and reaches sexual maturity at 100 mm carapace width for both sexes (Pardo et al. 2009a). In southern Chile (40°S–50°S), this species intensively uses estuarine systems as areas for larval release as well as juvenile nursery grounds. After approximately 3 months and five zoea stages (Quintana 1983), megalopae reinvade the estuarine systems in late spring and recruit subtidally on both hard and soft substrata (Cardyn 2009). Advanced zoeae have not been found inside estuaries (Pardo, unpublished data), which indicates that C. edwardsii uses an exportation–importation larval development strategy in this estuarine zone.
This study examines the influence of tidal signals (i.e., lunar phases and spring–neap cycles), river runoff, wind stress, and upwelling on settlement of megalopae simultaneously at three sites within the Valdivia River estuary. We expected that the relative influence on larval settlement of the tidal cycle and the wind that promotes upwelling relaxation (or any onshore larval transport) should change in different sections of the estuary depending on their proximity to the outer coast. In order to account for possible modifications of spatial settlement patterns by predation, we also determined the variability in predation pressure on megalopae among the study sites. Settlement surveys were replicated over 3 years during the recruitment season of C. edwardsii in order to describe eventual temporal changes in the environmental control of larval settlement.
Materials and methods
Study sites
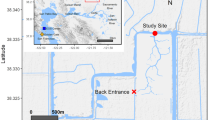
This study was carried out in the mouth of the Valdivia River estuary (Corral Bay, Fig. 1). This is one of the most important estuaries in southern Chile, the freshwater flow of which is regulated by a series of Andean lakes (Pino et al. 1994). The estuary is characterized by salt-wedge circulation with an average annual discharge close to 600 m3 s−1, with peaks during the austral winter season linked to the period of maximum precipitation (Davila et al. 2002). Tides exhibit semi-diurnal phases with a mean range of 0.8 m but vary from 1.48 m during spring tides to 0.53 m during neaps (Pino et al. 1994). Thus, the estuary is classified as micro-tidal (Dyer 2001).
In this region, the influence of the southeast Pacific subtropical anticyclone in spring time forces winds toward the equator that drive upwelling (Montecino and Lange 2009). Thus, coastal waters outside the Valdivia River estuary experience several springtime upwelling episodes (Atkinson et al. 2002).
Three sites within the Valdivia River estuary, approximately 5 km apart, were selected to evaluate the settlement rates of crab megalopae (Fig. 1): (1) San Carlos (39.862°S; 73.441°W), a semi-protected embayment on the southern shore facing the open ocean but protected from the prevailing strong southerly winds. This site is dominated by hard bottoms with extensive areas of gravel and coarse sand; (2) Los Molinos (39.847°S; 73.394°W), a wind-protected embayment on the northern shore of the estuary, where the substratum is dominated by soft bottoms, principally sand, but with some rocky reefs and boulders present, and (3) Isla Mancera (39.897°S; 73.394°W), which was selected as the inner and central site within the Valdivia River estuary. The substratum is dominated by a soft bottom of principally fine sands but with areas of a brittle sedimentary rock called ‘Cancagua’. The thermohaline conditions for each site during November–December (2008) were measured every 10 min by a mini-CTD Start Oddi® deployed on the bottom at a depth of 5–8 m. Mean values (±SD) were 11.5 ± 1.5°C and a salinity of 29.3 ± 2.1 in San Carlos, 11.3 ± 1.5°C and a salinity of 29.1 ± 2.2 in Los Molinos, and 11.8 ± 1.6°C and a salinity of 24.5 ± 2.6 in Mancera. Due to their locations and high salinities, San Carlos and Los Molinos were considered environments under marine influence and Mancera was considered a river-influenced environment. To describe the current dynamics of the estuary, the current velocity was determined close to the sites at San Carlos and Mancera, at 8 and 5 m depth, respectively. From August to December 2009, two current meters (Innovex Stream 300) were deployed at each site, one at 0.5 m and the other at 1 m above the bottom. During the sampling period, currents inside the estuary were evidently stronger within the middle section of the estuary than in the outer section. Also, surface currents displayed higher values in comparison with bottom records (Fig. 2).
In order to assess the association between environmental variables and megalopa recruitment, settlement was compared to tidal range, wind stress, upwelling index, and river flow during spring (November–December) between 2006 and 2008. The tidal range was calculated as the difference between the average of the two daily maximum tidal heights and the average of the two daily minimum tidal heights based on data from the National Hydrographic and Oceanographic Service (SHOA). The wind stress (zonal and meridional component) and the upwelling index were calculated from satellite data. The wind stress and their zonal and meridional components were obtained from the mouth of the Valdivia River estuary (39.750°S, 73.750°W) using the QuickSCAT sensor, http://cersat.ifremer.fr/data/discovery/by_mission/quikscat/mwf_quikscat. These data were validated by correlating them with in situ data from a meteorological station located at Laboratorio Costero Calfuco, approximately 20 km east of the estuary (R = 0.78 and R = 0.77, for zonal and meridional wind stress components, respectively). Data from the Laboratorio Costero Calfuco were not available before 2008, and therefore, it was not possible to use local wind data in the analysis of settlement rates. For the upwelling index, daily sea surface temperatures (SST) between two points perpendicular to the coast of Valdivia (first point; 39.875°S; 73.625°W, second point; 39.875°S; 74.875°W) were obtained from a combination of infrared sensors AVHRR (Advanced Very High Resolution Radiometer) and the Advanced Microwave Scanning Radiometer (AMSR). These data were obtained from NOAA’s National Climatic Data Center (http://www.ncdc.noaa.gov/oa/climate/research/sst/oi-daily.php) and were validated by correlating the data with in situ values of SST from the SHOA station inside Corral Bay (Mar 08–Nov 09; R = 0.74, P < 0.01). The upwelling index was calculated using the temperature difference between these points (inshore point—offshore point). A negative difference in the index suggests upwelling. A similar index has been used by other authors (Queiroga et al. 2006) in studies off the Iberian Peninsula. Furthermore, in this research, we incorporated river runoff, which has not been taken into account in similar prior studies conducted in estuaries and could potentially affect the entry of larvae due to changes in water column stratification. The runoff data corresponded to the daily record from the Cruces Rucaco Station (39.550°S, 72.895°W) obtained from the Chilean General Water Directorate (DGA; Fig. 3).
Physical factors measured in the Valdivia River estuary during recruitment periods of Cancer edwardsii during the three study years. Flow is a proxy of river discharge, zonal stress is a measure of onshore winds, meridional stress is a measure of alongshore winds, upwelling index is a measure of upwelling intensity based on the differences in temperature between offshore and coastal estimates (see “Methods”), and tidal range is the mean of the daily difference between low and high tide height
Experimental design
In order to measure settlement rates of Cancer edwardsii, a passive megalopa collector was used. The collector consisted of a circular plastic tray (0.2 m2) fastened to an upper ring of steel rods and a 25-kg concrete disk fastened to a lower ring that served as a base for stability. Collectors self-cover with a 1-mm external mesh during retrieval to prevent the loss of organisms from the samples. Complete details of the design and performance of collectors are provided in Pardo et al. (2010). In order to test the influence of the tidal cycle in a categorical way, the collectors were deployed during each lunar phase of one complete lunar cycle, namely at Full, Quarter, New, and Waxing phase, during the recruitment season of the crabs (November–December) for each site (San Carlos, Los Molinos and Mancera, see description above). This scheme was replicated over three consecutive years (2006–2008). Nine to twelve collectors filled with 0.5 kg of coarse sand were deployed at each site every year. Half of the collectors were covered with a 5-mm mesh in order to exclude large predators such as fish and large crabs. Additionally, at one location (Los Molinos) in 2006, collectors without sand, and with and without exclusion mesh, were used to control for the effect of the collector itself on the settlement of crab megalopae. During each lunar phase, collectors were deployed underwater (between 5 and 8 m) for 48 h. Collectors without sand showed lower abundance of megalopae (around 10% of the total) than collectors with coarse sand (F 3,44 = 10,7; P < 0.01). This confirms the utility of coarse sand as settlement substratum for C. edwardsii, a result that has also been found in natural suction samples (Pardo et al. 2011).
Once the collectors were recovered, samples were carefully examined and all replicates with less than 70% of the original coarse sand were excluded from the analyses (5 of all 326 replicates taken during the entire study). Samples were sieved and all megalopae were extracted manually at the Laboratorio Costero de Recursos Acuaticos de Calfuco (Universidad Austral de Chile) and identified to the species level using locally generated keys (Pardo et al. 2009b). In 2008, during parallel sampling, 30 megalopae of Cancer edwardsii were returned alive to the laboratory and maintained in standing seawater at 14°C; all of them subsequently metamorphosed to the crab I stage within 24 h indicating their competent condition (Forward et al. 2001).
Data analyses
To test for the influence of lunar phases on settlement rates of C. edwardsii, a nested ANOVA was performed. The independent variables were year, location, lunar phase, and predator exclusion, with lunar phase nested in year. The factor year was considered as a random variable in order to extrapolate the results to any year rather than to one particular year. This nested design deals with differences in yearly larval supply, exploring differences in settlement rates between lunar phases within years rather than comparing specific lunar phase between years. Sampling dates were during new moon phase (20-Nov-06, 9-Dec-07, 27-Nov-08), quarter phase (28-Nov-06, 17-Nov-07, 5-Dec-08), full moon phase (5-Dec-06, 24-Nov-07, 12-Dec-08), and waxing phase (13-Dec-06, 3-Dec-07, 20-Dec-08). Predator exclusion and any of its interactions were not significant in the full model nested ANOVA, and therefore, this factor was removed from further analyses and a reduced model was used. Because 12 collectors were lost at San Carlos and Mancera during the waxing lunar phase in 2006 due to a strong storm, the ANOVA was not orthogonal and Type IV sum of squares was therefore used. To test the effect of the tidal range on settlement rates, another nested ANOVA was used by pooling data from lunar phases: full and new moon = spring tide and quarter and waxing phases = neap tide. The independent variables were year, location and tide, with tide nested in year. Prior to both ANOVAs, heterogeneous data were transformed using ln (x + 1) before further analyses were carried out.
To account for the effect of environmental factors on settlement rates, a multiple regression was performed with tidal range, upwelling index, wind, and runoff as independent variables. Given that environmental variables could not have an immediate influence on settlement of megalopae due to known decoupling between larval supply and settlement (Miller and Shanks 2004), we tested the possibility of the effect of environmental factors on larval settlement rates using a lag of −5 to −1 days in the regression analysis. To avoid co-linearity among environmental variables, these were selected using a stepwise routine with a tolerance of 0.3 to construct the final reduced regression model. Because larval supply was expected to vary each year, settlement data were normalized relative to the mean and standard deviation of abundance of megalopae collected during each recruitment season at each location.
Results
During recruitment seasons (2006–2008), a total of 1,029 Cancer edwardsii megalopae were collected by the passive collectors. Overall, the highest settlement rates were observed in 2007, when up to 81 megalopae collector−1 day−1 (around 405 megalopae m−2 day−1) were recorded at San Carlos. The exclusion of large predators did not affect the settlement rates for any of the years or sites (Fig. 4). The exclusion factor and any of the interactions of which it was a part were not significant in the ANOVA (F 1,253 = 0.55; P = 0.9), and similar settlement rates were found between collectors open and closed to predators, for each year and site (Fig. 4). Therefore, the effects of large predators on settlement rates were neglected in subsequent analyses, regardless of the year under consideration.
In general, the settlement of C. edwardsii megalopae was highly variable depending on the lunar phase, year, and site (Fig. 5). In all years, settlement peaked during different lunar phases at the three different sites, despite the proximity of the sites. Moreover, contrasting values were found in different years. For example, at Los Molinos, the highest settlement rates were observed during the new moon in 2006 and 2007, but in 2008, settlement during the new moon had one of the lowest rates. Again, at San Carlos, the highest settlement rates were observed during the waxing moon phase in 2007, but in 2008 during the same phase, fewer settlers were found in comparison with other phases. On the other hand, larval settlement over the course of the lunar phase also differed at each individual site among years (Fig. 5). Thus, in 2007, the highest settlement rates of megalopae were recorded during maximum waxing at San Carlos, new moon at Los Molinos, and full moon phase at Mancera. Consequently, a lunar pattern in larval settlement rates was not detected. The high variability exhibited at both temporal and spatial scales was reflected by a significant interaction in the ANOVA (lunar phase (year) * Site; Table 1).
The ANOVA testing of neap and spring tide influences on crab settlement rates revealed a significant interaction between tidal phases and location for each year (F 6,303 = 8.9, P < 0.01). This result emerged because only Mancera exhibited a consistent tidal pattern across years, where the maximum settlement rates were always detected during the spring tidal phase, whereas San Carlos and Los Molinos showed peak settlement during contrasting tidal phases between years, or no differences in larval settlement (Fig. 6).
Mean (±SD) settlement rates of Cancer edwardsii megalopae during the spring–neap tidal cycle at three locations in the Valdivia River estuary. Total replicates: 326. Due to the fact that 24 collectors were lost in a storm, no data were available for one of the neap tide periods in San Carlos and Mancera 2006; during the other neap tide period in 2006, no larvae were recorded at these two sites
Multiple regressions between normalized settlement rates and environmental variables included in the model exhibited a spatial dependence of the relative influence on settlement. The stepwise regression model only selected tidal range as a significant factor explaining settlement for the river-ward location (Mancera), but for seaward locations, upwelling index and wind stress were selected by the model (Table 2). The time lags at which the environmental variables were associated with the settlement of C. edwardsii also differed between locations. At Mancera and San Carlos, tidal range and upwelling index, respectively, were highly correlated with settlement rates after at a lag of −2 days. However, at Los Molinos, zonal wind stress exhibited a weaker, but significant, relationship with a lag of 5 days (Fig. 7).
Partial regression between the principal explanatory physical variables and settlement rates of Cancer edwardsii megalopae for each location. Bars above and below the dots represent the standard error. Data were normalized relative to the mean and standard deviation of abundance of megalopae for each year and site
Discussion
This study provides evidence that the predominant physical factor controlling the entry of competent crab larvae into estuarine environments varies spatially within the Valdivia River estuary. The outer sites were affected by different components of wind stress (zonal versus meridional component), while the inner estuarine site was mainly influenced by the spring–neap tidal cycle (higher during spring tides). Intense predation on megalopae was not detected and assumed negligible in the estuary, which allows us to interpret the results without the confounding influence of predation effects on settlement rates.
In regions with persistent upwelling regimes, the relative importance of wind stress in promoting the settlement of meroplanktonic crustaceans in estuaries and along the open coast has been well recognized (Queiroga et al. 2007). However, field evidence indicates high variability in the type of wind control on settlement, which can occur during surface water advection by onshore winds, upwelling, downwelling, or relaxation periods (Flores et al. 2002; Narváez et al. 2006; Queiroga et al. 2007; Morgan et al. 2009), principally due to the strong regulation by larval behavior on larval transport and the influence of the coastal configuration (Palma et al. 2006; Morgan et al. 2009). Our findings reflect this variability, sites close to the mouth of the estuary were significantly influenced by wind stress but settlement at each site was influenced by different components of wind stress.
At the southern shore site (San Carlos), a direct association with the relaxation of upwelling (positive value in the index used here) was found. Upwelling relaxation is relative to the decline of equatorward winds (meridional component) and subsequent onshore advection of warm water masses associated with the offshore upwelling front. This mechanism behind larval transport has frequently been accepted (Wing et al. 1995b; Narváez et al. 2006; Queiroga et al. 2007), but sampling with high temporal frequency should be performed in order to test this hypothesis.
The effect of upwelling relaxation on larval settlement at the southern shore site of the estuary was not immediate; its signal was registered 2 days later in the settlement rates measured by the collectors. The temporal decoupling between environmental factors and settlement is caused by the time lag between the generation of the conditions needed to promote the supply of crab megalopae toward the estuary and actual settlement within estuarine habitats (Miller and Shanks 2004; Amaral et al. 2007). The state of competence of megalopae might also contribute to the observed decoupling between supply and settlement (Queiroga et al. 2006).
At the northern shore site (Los Molinos), wind stresses that drive direct advection toward the mouth of the estuary (zonal component) showed greatest association with the settlement rate. However, unambiguous environmental factors controlling the larval settlement at Los Molinos were not found (lower R 2 in partial regressions). This suggests that a more complex interaction between factors (i.e. river runoff, tide, winds, and larval behavior) is probably involved in the transport of larvae to this site. This complexity also was evidenced by the long lag between wind action (−5 days) and settlement. Given that Los Molinos is a wind-protected embayment on the northern shore of the estuary, larvae could be delivered to this section of the estuary by more local processes independent of upwelling conditions (Shanks and Shearman 2009; Shanks and Brink 2005). Other factors such as larval behavior and the upwelling shadow that forms behind coastal features could play an important role (Morgan and Fisher 2010).
In the Valdivia River estuary, tidal currents seem to have only a marginal influence on larval settlement in the most marine-influenced environments (Tables 1 and 2). The Valdivia River estuary experiences a micro-tidal regime (sensu Dyer 2001) with a maximum tidal range of 1.48 m during spring tides (Pino et al. 1994). Therefore, the tidal currents exert little control over megalopa transport and settlement at sites close to the mouth of the estuary. This is consistent with the minimal tidal current speeds observed at the outer sites (Fig. 2).
In contrast, tidal influence was evident at the inner site, where it can overwhelm wind control on crab settlement in the middle of the Valdivia River estuary. At Mancera, regardless of year, settlement was always higher during spring tides, suggesting that megalopae use the strongest flood tides to move up into the estuary. Stronger tidal currents in the middle of the estuary probably enhance the relative importance of tidal regulation on larval transport. Similar results were found by Vargas et al. (2003), who found evidence that tidal fronts and flood tides played a role in the transport of fish larvae in the Valdivia River estuary. In accordance with this, the tidal range was positively associated with settlement rates in Mancera, albeit with a 2-day lag. Longer times spent exploring for available substratum by the larvae (Moksnes et al. 2003) or higher levels of turbulence during spring tides promoting swimming activity (Welch and Forward 2001) could explain this decoupling.
Thus, the relative importance of wind-driven versus tidal processes on larval settlement within an estuary is likely to vary depending on the proximity of the settlement location to the outer (coastal) parts of the estuary. In micro-tidal estuaries, upwelling events and wind transport are likely to be more important for sites close to the coast, whereas tidal range is likely to be more important within the estuary, away from the coast.
In a more general context, the relative importance of physical forcings in controlling larval settlement in estuaries could depend on the magnitude of the tidal range in each particular estuary. In estuaries with a micro-tidal regime, like the Valdivia River estuary, tidal currents appear to have marginal effects on currents relative to other components of hydrological circulation. Supporting this, Moksnes et al. (2003) did not find any tidal association of settlement of Carcinus maenas megalopae in a micro-tidal estuary on the Swedish west coast. Also, Jones and Epifanio (1995b) found no tidal effect on settlement of brachyuran megalopae in a micro-tidal estuary at the mouth of Delaware Bay. In contrast, in many meso-tidal (Paula et al. 2001; Almeida and Queiroga 2003; Forward et al. 2004; Queiroga et al. 2007) and macro-tidal estuaries (Moser and Macintosh 2001; Miller and Shanks 2004; Roegner et al. 2007), tides have been observed to significantly affect estuarine immigration of decapod larvae. Further meta-analysis studies should test whether the type of estuary, based on tidal range, can be used to predict the relative importance of winds and tides in the control of larval settlement within estuaries. For this analysis, data from multiple sites are needed to account for the spatially variable pattern of settlement within estuaries.
Intra-estuarine spatial variation in the influence of environmental forcings on larval settlement is not commonly reported, principally because the emphasis in previous studies has been on temporal rather than spatial scales. However, when multiple sites have been sampled within a single estuary to compare the relative abundance of postlarvae, high variability has been observed among sites and spatially explicit patterns of recruitment have been found in Brachyura (Etherington and Eggleston 2003; Ogburn and Forward 2009). In addition, in the present study on intra-estuarine spatial variation, it was found that settlement was controlled by physical rather than biological factors (e.g. predation on megalopae) at all study sites.
Overall, the results support the conclusion that the factors controlling settlement cannot be generalized within a particular estuary since the relative importance of factors changes spatially. Based on our results, we conclude that the relative influence of the factors that control larval settlement rates depends on the location within the estuary: in micro-tidal estuaries at an upwelling-dominated coast, settlement patterns have a weaker tidal signal in the outer section of the estuary than in the inner section, but the influence of tidal transport increases at sites further up the estuary.
References
Allen JD, McAlister JS (2007) Testing rates of planktonic versus benthic predation in the field. J Exp Mar Biol Ecol 347:77–87
Almeida MJ, Queiroga H (2003) Physical forcing of onshore transport of crab megalopae in the northern Portuguese upwelling system. Estuar Coast Shelf Sci 57:1091–1102
Amaral V, Queiroga H, Skov M, Paula J (2007) Planktonic availability and settlement of Carcinus maenas megalopae at high temporal resolution in the lower Mira Estuary (SW Portugal). Mar Ecol Prog Ser 348:239–248
Atkinson LP, Valle-Levinson A, Figueroa D, De Pol-Holz D, Gallardo VG et al (2002) Oceanographic observations in Chile and coastal waters between Valdivia and Concepción. J Geophys Res 107:1–14
Cardyn CS (2009) Dinámica espacio temporal del asentamiento larval de Cancer edwardsii (Bell, 1835), en el estuario del Rio Valdivia. Lic. Thesis, Universidad Austral de Chile, p 61
Davila PM, Figueroa D, Muller E (2002) Freshwater input into the coastal ocean and its relation with the salinity distribution off austral Chile (35–55 degrees S). Cont Shelf Res 22:521–534
Dyer K (2001) Estuarine circulation. In: Steele J, Thorpe S, Turekian K (eds) Encyclopedia of ocean science. Academic Press, London, pp 846–852
Epifanio CE, Little KT, Rowe PM (1988) Dispersal and recruitment of fiddler crab larvae in the Delaware River estuary. Mar Ecol Prog Ser 43:181–188
Etherington LL, Eggleston DB (2003) Spatial dynamics of large-scale, multistage crab (Callinectes sapidus) dispersal: determinants and consequences for recruitment. Can J Fish Aquat Sci 60:873–887
Flores AAV, Cruz J, Paula J (2002) Temporal and spatial patterns of settlement of brachyuran crab megalopae at a rocky coast in central Portugal. Mar Ecol Prog Ser 229:207–220
Forward RB, Tankersley RA, Rittschof D (2001) Cues for metamorphosis of brachyuran crabs: an overview. Am Zool 41:1108–1122
Forward RB, Cohen JH, Irvine RD, Lax JL, Mitchell R et al (2004) Settlement of blue crab Callinectes sapidus megalopae in a North Carolina estuary. Mar Ecol Prog Ser 269:237–247
Hasek BE, Rabalais NN (2001) Settlement patterns of brachyuran megalopae in a Louisiana estuary. Estuaries 24:796–807
Jones MB, Epifanio CE (1995a) Patches of crab megalopae in the mouth of delaware bay—an analysis of spatial scales. J Shellfish Res 24(1):261–267
Jones MB, Epifanio CE (1995b) Settlement of brachyuran megalopae in Delaware Bay—an analysis of time-series data. Mar Ecol Prog Ser 125:67–76
Lagos NA, Castilla JC, Broitman BR (2008) Spatial environmental correlates of intertidal recruitment: a test using barnacles in northern Chile. Ecol Monogr 78:245–261
Mackas D, Strub PT, Thomas AC, Montecino V (2006) Eastern ocean boundaries pan-regional overview. In: Robinson AR, Brink KH (eds) The global coastal ocean. Harvard Press Ltd, Cambridge, pp 21–59
Miller JA, Shanks A (2004) Ocean-estuary coupling in the Oregon upwelling region: abundance and transport of juvenile fish and of crab megalopae. Mar Ecol Prog Ser 271:267–279
Moksnes PO (2002) The relative importance of habitat-specific settlement, predation and juvenile dispersal for distribution and abundance of young juvenile shore crabs Carcinus maenas L. J Exp Mar Biol Ecol 271:41–73
Moksnes PO, Hedvall O, Reinwald T (2003) Settlement behavior in shore crabs Carcinus maenas: why do postlarvae emigrate from nursery habitats? Mar Ecol Prog Ser 250:215–230
Montecino V, Lange CB (2009) The Humboldt current system: ecosystem components and processes, fisheries, and sediment studies. Prog Oceanog 83:65–79
Morgan SG (1995) Life and death. In the plankton larval mortality and adaptation In: McEdwards L (ed) Larval ecology of marine invertebrates, CRC, Boca Raton, pp 279–321
Morgan SG, Fisher JL (2010) Larval behavior regulates nearshore retention and offshore migration in an upwelling shadow and along the open coast. Mar Ecol Prog Ser 404:109–126
Morgan SG, Fisher JL, Mace AJ (2009) Larval recruitment in a region of strong, persistent upwelling and recruitment limitation. Mar Ecol Prog Ser 394:79–99
Moser SM, Macintosh DJ (2001) Diurnal and lunar patterns of larval recruitment of Brachyura into a mangrove estuary system in Ranong Province, Thailand. Mar Biol 138:827–841
Narváez D, Navarrete SA, Largier J, Vargas CA (2006) Onshore advection of warm water and larval invertebrate settlement during relaxation of upwelling off central Chile. Mar Ecol Prog Ser 309:159–173
Navarrete SA, Wieters EA, Broitman BR, Castilla JC (2005) Scales of benthic-pelagic coupling and the intensity of species interactions: from recruitment limitation to top-down control. Proc Natl Acad Sci USA 102:18046–18051
Ogburn MB, Forward RB Jr (2009) Ingress of brachyuran crab post-larvae to the Newport River estuary. Estuar Coast 32:309–318
Ogburn MB, Diaz H, Forward RB Jr (2009) Mechanisms regulating estuarine ingress of blue crab Callinectes sapidus megalopae. Mar Ecol Prog Ser 389:181–192
Palma AT, Pardo LM, Veas R, Cartes C, Silva M, Manriquez K, Diaz A, Muñoz C, Ojeda FP (2006) Coastal brachyuran decapods: settlement and recruitment under contrasting coastal geometry conditions. Mar Ecol Prog Ser 316:139–153
Pardo LM, Palma AT, Prieto C, Sepulveda P, Valdivia I, Ojeda FP (2007) Processes regulating early post-settlement habitat use in a subtidal assemblage of brachyuran decapods. J Exp Mar Biol Ecol 344:10–22
Pardo LM, Fuentes JP, Olguín A, Orensanz JML (2009a) Reproductive maturity in the edible Chilean crab Cancer edwardsii: methodological and management considerations. J Mar Biol Assoc UK 89:1627–1634
Pardo LM, Ampuero D, Veliz D (2009b) Using morphological and molecular tools to identify megalopae larvae collected in the field: the case of sympatric Cancer crabs. J Mar Biol Assoc UK 89:481–490
Pardo LM, Cardyn CS, Mora P, Wahle RA (2010) A new passive collector to assess settlement rates, substrate selection and predation pressure in decapod crustacean larvae. J Exp Mar Biol Ecol 393:100–105
Pardo LM, González K, Fuentes JP, Paschke K, Chaparro OR (2011) Survival and behavioral responses of juvenile crabs of Cancer edwardsii to severe hyposalinity events triggered by increased runoff at an estuarine nursery ground. J Exp Mar Biol Ecol 404:33–39
Paula J, Dray T, Queiroga H (2001) Interaction of offshore and inshore processes controlling settlement of brachyuran megalopae in Saco mangrove creek, Inhaca Island (South Mozambique). Mar Ecol Prog Ser 215:251–260
Pineda J (2000) Linking larval settlement to larval transport: assumptions, potentials, and pitfalls. Oceanogr East Pacific I:84–105
Pino M, Perillo G, Santamarina P (1994) Residual fluxes in a cross-section of the Valdivia River estuary, Chile. Estuar Coast Shelf Sci 38:491–505
Queiroga H, Almeida MA, Alpuim A, Flores AAV, Francisco S et al (2006) Tide and wind control of megalopal supply to estuarine crab populations on the Portuguese west coast. Mar Ecol Prog Ser 307:21–36
Queiroga H, Cruz T, dos Santos A, Dubert J, Gonzalez-Gordillo I et al (2007) Oceanographic and behavioural processes affecting invertebrate larval dispersal and supply in the western Iberia upwelling ecosystem. Prog Oceanog 74:174–191
Quintana R (1983) Larval development of the edible crab, Cancer edwardsi Bell, 1835 under laboratory conditions (Decapoda, Brachyura). Rep USA Mar Biol Inst Kochi Univer 5:1–19
Roegner GC, Armstrong DA, Shanks AL (2007) Wind and tidal influences on larval crab recruitment to an Oregon estuary. Mar Ecol Prog Ser 351:377–388
Roughgarden J, Pennington JT, Stoner D, Alexander S, Miller K (1991) Collisions of upwelling fronts with the intertidal zone—the cause of recruitment pulses in barnacle populations of central California. Acta Oecol 12:35–51
SERNAPESCA (2007–2009) Anuario de estadísticas pesqueras. Ministerio de Economía, Servicio Nacional de Pesca, Gobierno de Chile, p 186
Shanks AL, Brink L (2005) Upwelling, downwelling, and cross-shelf transport of bivalve larvae: test of a hypothesis. Mar Ecol Prog Ser 302:1–12
Shanks AL, Shearman RK (2009) Paradigm lost? Cross-shelf distributions of intertidal invertebrate larvae are unaffected by upwelling or downwelling. Mar Ecol Prog Ser 385:189–204
Shanks AL, Largier J, Brink L, Brubaker J, Hooff R (2000) Demonstration of the onshore transport of larval invertebrates by the shoreward movement of an upwelling front. Limnol Oceanogr 45:230–236
Tankersley RA, Welch JM, Forward RB (2002) Settlement times of blue crab (Callinectes sapidus) megalopae during flood-tide transport. Mar Biol 141:863–875
Tilburg CE, Dittel AI, Epifanio CE (2007) Retention of crab larvae in a coastal null zone. Estuar Coast Shelf Sci 72:570–578
Vargas C, Araneda S, Valenzuela G (2003) Influence of tidal phase and circulation on larval fish distribution in a partially mixed estuary, Corral Bay, Chile. J Mar Biol Assoc UK 83:217–222
Welch J, Forward R (2001) Flood tide transport of blue crab, Callinectes sapidus, postlarvae: behavioral responses to salinity and turbulence. Mar Biol 139:911–918
Wing SR, Largier JL, Botsford LW, Quinn JF (1995a) Settlement and transport of benthic invertebrates in an intermittent upwelling region. Limnol Oceanogr 40:316–329
Wing SR, Botsford LW, Largier JL, Morgan LE (1995b) Spatial structure of relaxation events and crab settlement in the northern California upwelling system. Mar Ecol Prog Ser 128:199–211
Acknowledgments
We would like to thank Juan Pablo Fuentes and Paulo Mora for their active participation. LMP thanks the Fondo Nacional de Ciencia y Tecnología 11070024 and Dirección de Investigación de la Universidad Austral de Chile for financial support. We thank three anonymous reviewers and Martin Thiel for suggestions on an earlier version of the manuscript. All experimental work was conducted within the Republic of Chile and in compliance with its existing laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Martin Thiel.
Rights and permissions
About this article
Cite this article
Pardo, L.M., Cardyn, C.S. & Garcés-Vargas, J. Spatial variation in the environmental control of crab larval settlement in a micro-tidal austral estuary. Helgol Mar Res 66, 253–263 (2012). https://doi.org/10.1007/s10152-011-0267-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10152-011-0267-y